INTRODUCTION
Dicalcium phosphate is one of the most widely used sources of supplementary phosphorus (P) for all farm animal species. Although mainly used as a P source, the supplement also provides calcium (Ca). The availability of P in this supplement has been assessed by researchers in the past (Ammerman et al. Reference Ammerman, Forbes, Garrigus, Newmann, Norton and Hatfield1957; Tillman & Brethour Reference Tillman and Brethour1958; Lofgreen Reference Lofgreen1960; O'Donovan et al. Reference O'Donovan, Plumlee, Smith and Beeson1965). However, Ca availability from dicalcium phosphate has not, to the present authors’ knowledge, been evaluated sufficiently despite the known interactions between the minerals.
Most of the studies carried out previously to determine P availability from dicalcium phosphate used methods that are not currently considered to be accurate enough (France et al. Reference France, Dias, Kebreab, Vitti, Crompton, López, Vitti and Kebreab2010). For instance, some studies assessed true P availability of dicalcium phosphate by considering endogenous P as the P excreted by animals fed a low-P or P-free basal diet (Ammerman et al. Reference Ammerman, Forbes, Garrigus, Newmann, Norton and Hatfield1957; O'Donovan et al. Reference O'Donovan, Plumlee, Smith and Beeson1965), which usually leads to an erroneous estimate of true endogenous P excretion. Other studies (Long et al. Reference Long, Tillman, Nelson, Gallup and Davis1957) used the values of P in plasma to compare P availability from different supplements. Although plasma P values may provide an indication of P availability, its use as the only source of information to evaluate mineral availability is not recommended due to its susceptibility to metabolic changes (Vitti et al. Reference Vitti, Silva-Filho, Louvandini, Dias, Bueno, Kebreab, Vitti and Kebreab2010). The availability of a nutrient is more accurately established in experiments with graded intakes of the mineral source evaluated (Johnson & McClure Reference Johnson and McClure1967). Studies considering only one level of intake (Tillman & Brethour Reference Tillman and Brethour1958; Arrington et al. Reference Arrington, Outler, Ammerman and Davis1963) of the supplement may provide incomplete information on mineral availability. Current knowledge of Ca and P availability from dicalcium phosphate remains incomplete, justifying further studies for more efficient use of this supplement given the unquestionable importance of this source of P for ruminant diets. The use of isotopic tracer methods in combination with mathematical modelling offers a suitable tool to investigate metabolic transactions of minerals between the body compartments (France et al. Reference France, Dias, Kebreab, Vitti, Crompton, López, Vitti and Kebreab2010). In addition, excess of P excreted in faeces is of major environmental concern, highlighting the importance and relevance of investigating the consequences of providing dicalcium phosphate as a dietary supplement. The objective of the current study is to investigate the effects of increasing ingestion of dicalcium phosphate by growing sheep on Ca and P absorption, excretion and utilization.
MATERIALS AND METHODS
Animal and diets
The experiment was carried out at the Animal Nutrition Laboratory, Centre for Nuclear Energy for Agriculture (CENA), University of Sao Paulo (USP), Brazil, using a protocol approved by the Commission of Ethics in Experimentation with Animals (CIEEA-CENA/USP) and the Commission of Environmental Ethics (CEA-CENA/USP) of CENA, USP.
Eighteen male Santa Inês sheep, initial live weight 33·7 (s.d. 1·66) kg, aged 8 months, were randomly allocated to one of the three experimental groups (six sheep per group). The treatments consisted of a basal diet supplemented with increasing amounts of dicalcium phosphate (Serrana, Sao Paulo, Brazil). According to the supplier specifications, the commercial product (c. 0·80 g dicalcium phosphate/g product) used as a supplement contained 0·24 g Ca and 0·18 g P/g product. The experimental basal diet was composed of roughage (chopped coast cross hay), a concentrate mixture (cassava meal, soybean meal, sugarcane molasses and urea) and a mineral mixture (Table 1). The experimental treatments comprised three diets providing 3·5, 6·3 and 9·0 g Ca/kg dry matter (DM) and 1·4; 3·2 and 4·9 g P/kg DM, respectively. To achieve these Ca and P concentrations, animals ingested 0, 12·5 or 25 g of dicalcium phosphate/day for each of the experimental diets. In all groups, sheep were fed the corresponding diet in two equal meals at 08.00 and 17.00 h.
Table 1. Composition of experimental diets

DM, dry matter; OM, organic matter; CP, crude protein; NDF, neutral detergent fibre; ADF, acid detergent fibre; ME, metabolizable energy; P, phosphorus; Ca, calcium.
* Diets were supplemented with vitamin A, 1000000 IU/kg; vitamin D, 250000 IU/kg; vitamin E, 6250 IU/kg.
† Trace mineral premix composition per kilogram: 30 g magnesium, 30 g sulphur, 3 g copper, 5 g manganese, 12 g zinc, 80 mg selenium, 180 mg iodine.
‡ Calculated from NRC (2007).
After a 21-day adaptation period in ground stalls, the animals were transferred to individual metabolism cages fitted with a device for separated collection of faecal and urinary outputs. The animals were maintained in the metabolism cages for 2 weeks, 6 days of adaptation and the last 8 days constituting the collection period that started when each animal received a single dose of 7·4 MBq of 45Ca and 32P injected into the right jugular vein. Subsequently, samples of plasma were taken by venepuncture from the left jugular vein and collected into heparin every 24 h up to 7 days after injection. Feed intake and the total faecal and urinary outputs were recorded daily during the collection period. Diets and feed refusals were weighed and sampled daily. All feed refusal samples from each animal were combined, so that at the conclusion of the experimental period, feed and feed refusal composites were obtained for chemical analyses. Faeces and urine were collected and weighed daily and representative aliquots (in each case 0·10 of the total amount collected) of each output were sampled and preserved in a refrigerator at 4 °C. At the end of the collection period, the preserved daily samples were composited to obtain representative faeces and urine samples for each animal, which were frozen at −20 °C until further chemical analysis. Rumen fluid samples were taken only on days 4, 5, 6 and 7 after injection of radioactive elements to minimize stress to the animals. Rumen contents were collected using an oesophageal tube and a syringe pumping out 50 ml of rumen sample each time of collection.
On the last day of the collection period and once all sampling was completed, the animals (which had not received feed in the last 24 h) were killed by intravenous injection of 0·05 mg of xylazine (Rompum-Bayer, Sao Paulo, Brazil)/kg of body weight (BW) followed by exsanguination. Rumen volume (l) was measured and samples of the third and fourth metatarsal bones were collected.
Sample preparation
Feed and feed refusal samples were dried at 60 °C for 48 h and ground through a 1-mm screen in a Wiley mill. Dried samples (1 g) of feed and feed refusals were ashed at 500 °C for 8 h and then 5 ml of concentrated HCl (12 m) were added and heated to dissolve the ash completely. The mixture was filtered through P-free filter paper. Faecal samples (1 g) were dried at 105 °C and ashed at 500 °C for 8 h and then 5 ml of concentrated hydrochloric acid (HCl; 12 M) were added for wet ashing digestion. The mixture was filtered through P-free filter paper. The urine samples were thawed, acidified (12 M HCL), dried at 55 °C and ashed at 500 °C. Then ashes were diluted (3 M HCl), filtered and volume made up to 10 ml. The blood samples were diluted in a solution of trichloroacetic acid (TCA, 100 g TCA/l distilled water), mixing 1 ml with 9 ml TCA and then centrifuged at 1100×g for 10 min at room temperature for plasma separation and protein precipitation. Samples of rumen fluid were diluted in a solution of TCA (100 g TCA/l distilled water) for protein precipitation (0·5 ml rumen fluid+4·5 ml TCA) and then centrifuged at 1800×g for 10 min at room temperature. Samples of bone from each animal were cleaned and degreased with diethyl ether and thereafter acid-digested on a hot plate upon the addition of an aqueous solution of nitric acid (500 ml HNO3/l). Then the samples were ashed in a muffle furnace at 550 °C and the residues were further digested using 10 ml of an aqueous solution of HCl (100 ml HCl/l) and filtered into a 25 ml volumetric flask through P-free filter paper.
Chemical analyses
Feed samples were analysed for DM (method 934·01), ash (method 942·05), crude protein (method 984·13) and ADF (method 973·18) following recommendations of the Association of Official Analytical Chemists (AOAC 1995). Neutral detergent fibre (NDF) was analysed according to Mertens (Reference Mertens2002), without using amylase or sodium sulphite.
Filtrates obtained after ashing and acid digestion of feed, orts, faeces, urine and bone samples, and supernatants obtained after centrifugation of plasma and rumen fluid samples were used for P and Ca analyses. Inorganic P was determined by colorimetry using vanadate molybdate reagent (Fiske & Subbarow Reference Fiske and Subbarow1925; Sarruge & Haag Reference Sarruge and Haag1974). This colorimetric determination of P is an adaptation of method 965·17 of AOAC (1995). A vanadate solution was prepared by dissolving 2·5 g ammonium vanadate (NH4VO3) in 500 ml boiling water. After cooling, 350 ml of concentrated nitric acid (HNO3) were added and the solution was diluted to 1 litre with distilled water. For the molybdate aqueous solution, 50 g of ammonium molybdate ((NH4)6Mo7O24) were dissolved in hot distilled water. Cooled vanadate and molybdate solutions were combined (1:1, vol/vol) to obtain the vanadate–molybdate reagent. Filtered ash solution or supernatants (2 ml) were mixed thoroughly with 2 ml of vanadate–molybdate reagent and 3 ml of distilled water. The mixture was allowed to stand for 5 min and absorbance was read at 420 nm against a standard P curve (using solutions of potassium dihydrogen phosphate) to estimate the P concentration in samples. Calcium was determined by atomic-absorption spectrometry (Zagatto et al. Reference Zagatto, Krug, Bergamim Filho, Jorgensen and Reis1979).
Specific radioactivity (45Ca and 32P) was measured in faeces and plasma samples. In addition, 32P was also measured in the rumen samples. These determinations were performed in a Beckman liquid scintillation spectrometer (model ls 5000 TA, Beckman–Coulter Inc., Fullerton, CA). Aliquots (1 ml) of centrifuged (1100 ×g for 10 min) and filtered plasma and rumen fluid samples were directly dispensed into borosilicate vials with 10 ml of a scintillation solution and placed in the spectrometer for counting. Faeces (1 g fresh matter) were ashed for inorganic P and Ca determination and ashes obtained were dissolved and digested in 10 ml 18 N H2SO4 for 1 h and then 1 ml of this solution was transferred to counting vials and diluted with a scintillation solution (1 : 10 or 1 : 20 (v/v) depending on the expected radioactivity values) for radioactivity measurement. To improve the efficiency of radioactivity readings, an external standard calibration procedure of quench correction was used. Specific radioactivity (SA) in faeces, plasma or rumen fluid was expressed as MBq 45Ca/g of Ca and MBq 32P/g of P.
Calculations
Mineral intake (mg/kg BW/day) was calculated as:
where DMI is the dry matter intake (kg DM/day), CaPfeed is the Ca or P content of the diet (mg/kg DM) and BW is the body weight (kg).
Endogenous faecal losses of Ca and P were calculated according to Lofgreen & Kleiber (Reference Lofgreen and Kleiber1953) as:
 $$\eqalign{& {\rm Endogenous}\;{\rm faecal}\;{\rm loss}\;({\rm mg}/{\rm kg}\;{\rm BW/day}) \cr & \quad = ({\rm SA}_{{\rm faeces}} /{\rm SA}_{{\rm plasma}} ) \cr & \quad \;\; \times {\rm total}\;{\rm faecal}\;{\rm excretion}\;({\rm mg}/{\rm kg}\;{\rm BW/day})} $$
$$\eqalign{& {\rm Endogenous}\;{\rm faecal}\;{\rm loss}\;({\rm mg}/{\rm kg}\;{\rm BW/day}) \cr & \quad = ({\rm SA}_{{\rm faeces}} /{\rm SA}_{{\rm plasma}} ) \cr & \quad \;\; \times {\rm total}\;{\rm faecal}\;{\rm excretion}\;({\rm mg}/{\rm kg}\;{\rm BW/day})} $$where, SAfaeces and SAplasma, are the SA in faeces and plasma, respectively. An average of SAs recorded on days 6 and 7 after injection of isotopes was used for the calculation, assuming isotopic conditions to approximate steady state after such time, once the difference between successive SA values (on days 6 and 7) was of little significance. Endogenous faecal loss of Ca and P was used to calculate the true absorption of Ca and P in the gastro-intestinal tract:
 $$\eqalign{& {\rm Mineral\ true}\;{\rm absorption} \cr & \quad = {\rm Intake} -[{\rm total}\;{\rm faecal}\;{\rm excretion} \cr & \quad \;\;\;\;\;- {\rm endogenous}\;{\rm faecal}\;{\rm loss}]} $$
$$\eqalign{& {\rm Mineral\ true}\;{\rm absorption} \cr & \quad = {\rm Intake} -[{\rm total}\;{\rm faecal}\;{\rm excretion} \cr & \quad \;\;\;\;\;- {\rm endogenous}\;{\rm faecal}\;{\rm loss}]} $$Mineral retention was calculated as:
Phosphorus endogenous flow entering into the rumen (S r) and P turnover time (T) in the rumen were calculated according to Smith et al. (Reference Smith, Kleiber, Black and Baxter1955), from the total amount of P in the rumen (g of P), P intake (g/day) and SA of 32P (MBq/g P) in the rumen fluid and in plasma as described in detail in Dias et al. (Reference Dias, Lopez, Silva, Pardo, Silva Filho, Vitti, Kebreb and France2009).
Statistical analysis
Experimental measurements of Ca and P were analysed as a completely random design using a general linear models procedure. The data were taken from 18 animals, six for each treatment (dicalcium phosphate ingestion). Means were compared using the Tukey test, declaring significant differences between means when P<0·05. Polynomial orthogonal contrasts were performed using the GLM procedure (SAS 1999). Probability values for the linear response (orthogonal polynomial contrast) are reported. The correlations between the parameters expressed as Pearson product–moment correlation coefficients were determined using PROC CORR of SAS, whereas the linear regression equations between the two parameters were obtained using PROC REG.
RESULTS
DM intake was similar for both diets supplemented with dicalcium phosphate (1·12 and 1·13 kg/day for diets providing 12·5 and 25 g of dicalcium phosphate/day, respectively), whereas DM intake was reduced by 7% (P<0·05) with the unsupplemented diet (1·04 kg/day).
Calcium
There were significant (P<0·05) differences among treatments in total and endogenous Ca excreted in faeces and Ca truly absorbed from the diet, whereas Ca excreted in urine was similar for all treatments (P>0·05) (Table 2). Endogenous faecal Ca was used to calculate true Ca availability coefficients, which were 0·54, 0·41 and 0·38 for 0, 12·5 and 25 g of dicalcium phosphate/day, respectively (P<0·05).
Table 2. Calcium intake (mg/kg BW/day), excretion (mg/kg BW/day), retention (mg/kg BW/day) and Ca in bone (mg Ca/g bone)
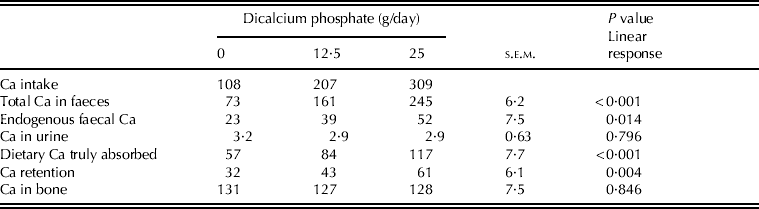
Calcium retention increased linearly with dicalcium phosphate intake (P<0·05), the greatest retention corresponding to the highest intake of dicalcium phosphate. The concentration of Ca in bone was similar for all treatments (P>0·05).
Phosphorus
Daily ingestion of dicalcium phosphate had a significant (P<0·05) linear effect on total and endogenous P excreted in faeces and P truly absorbed from the diet, whereas urinary P was similar for all treatments (P>0·05) (Table 3). True P absorption coefficients were 0·66, 0·62 and 0·64 for the diets with 0, 12·5 and 25 g of dicalcium phosphate/day, respectively (P>0·05).
Table 3. Phosphorus intake (mg/kg BW/day), excretion (mg/kg BW/day), retention (mg/kg BW/day) and P in bone (mg P/g bone)
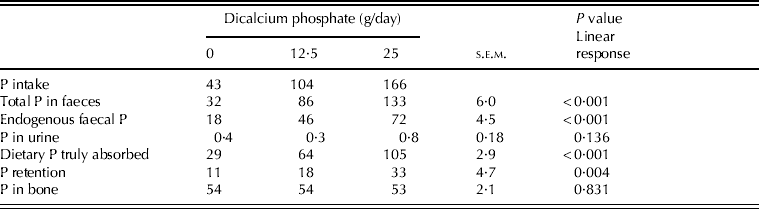
Retained P (mg/kg BW/day) was different among treatments (P<0·05) (Table 3) and increased linearly with dicalcium phosphate intake (P<0·05). However, the proportion of P retained related to P intake was not different among treatments: 0·24, 0·17 and 0·20 for 0, 12·5 and 25 g of dicalcium phosphate/day (P>0·05).
Phosphorus concentration in the rumen (g P/l) was lowest for the control and similar for the other treatments (Table 4). Calculated P flow into the rumen (S r) increased linearly with ingested P, whereas P turnover time (T) decreased linearly with ingested P (Table 4).
Table 4. Phosphorus concentration in rumen, endogenous P flow to rumen and P turnover time in the rumen
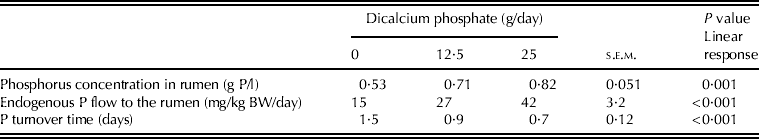
Endogenous P excreted in faeces (mg/kg BW/day), was related to endogenous P flow entering the rumen (S r, mg/kg BW/day) as shown by the linear equation:
Relationships between Ca and P
Endogenous Ca excreted in faeces was poorly related to endogenous P in faeces (both in mg/kg BW/day):
 $$\eqalign{{\rm Endogenous}\;{\rm P}\;{\rm in\ faeces} =\ & 0 {\cdot} {\rm 6}0\;{\rm (}{\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 251)} \cr & \hskip-6pt \times {\rm endogenous}\;{\rm Ca}\;{\rm in}\;{\rm faeces} \cr & \hskip-5pt + {\rm 22}\;{\rm (}{\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;{\rm 1}0 {\cdot} {\rm 8)} \cr & (n = {\rm 18};\;R^{\rm 2} = 0 {\cdot} {\rm 26}; \cr & P = 0 {\cdot} 0{\rm 3}0)} $$
$$\eqalign{{\rm Endogenous}\;{\rm P}\;{\rm in\ faeces} =\ & 0 {\cdot} {\rm 6}0\;{\rm (}{\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 251)} \cr & \hskip-6pt \times {\rm endogenous}\;{\rm Ca}\;{\rm in}\;{\rm faeces} \cr & \hskip-5pt + {\rm 22}\;{\rm (}{\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;{\rm 1}0 {\cdot} {\rm 8)} \cr & (n = {\rm 18};\;R^{\rm 2} = 0 {\cdot} {\rm 26}; \cr & P = 0 {\cdot} 0{\rm 3}0)} $$Dietary P truly absorbed (mg/kg BW/day) was related to Ca truly absorbed (mg/kg BW/day):
 $$\eqalign{{\rm P \ truly}\;{\rm absorbed} = \ & 0 {\cdot} {\rm 85}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 167}) \cr & \times {\rm Ca}\;{\rm truly}\;{\rm absorbed} \cr & -{\rm 6} {\cdot} {\rm 7}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;{\rm 15} {\cdot} {\rm 2}) \cr & \hskip 1pt (n = {\rm 18},\;R^{\rm 2} = 0 {\cdot} {\rm 62};\;P \lt 0 {\cdot} 00{\rm 1})} $$
$$\eqalign{{\rm P \ truly}\;{\rm absorbed} = \ & 0 {\cdot} {\rm 85}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 167}) \cr & \times {\rm Ca}\;{\rm truly}\;{\rm absorbed} \cr & -{\rm 6} {\cdot} {\rm 7}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;{\rm 15} {\cdot} {\rm 2}) \cr & \hskip 1pt (n = {\rm 18},\;R^{\rm 2} = 0 {\cdot} {\rm 62};\;P \lt 0 {\cdot} 00{\rm 1})} $$However, when Ca and P true absorption were expressed as a proportion of the corresponding intakes, the relationship between the true absorption coefficients of both minerals was not significant:
 $$\eqalign{{\rm P \ true}\;\,{\rm absorption} = \ & 0 {\cdot} 0{\rm 81}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 149}) \cr & \hskip-3pt \times {\rm Ca}\;{\rm true}\;{\rm absorption} \cr & \hskip-3pt -0 {\cdot} {\rm 6}0{\rm 3}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} 0{\rm 68}) \cr & \hskip-1pt (n = {\rm 18},\;R^{\rm 2} = 0 {\cdot} 0{\rm 18};\;P \gt 0 {\cdot} {\rm 05})} $$
$$\eqalign{{\rm P \ true}\;\,{\rm absorption} = \ & 0 {\cdot} 0{\rm 81}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 149}) \cr & \hskip-3pt \times {\rm Ca}\;{\rm true}\;{\rm absorption} \cr & \hskip-3pt -0 {\cdot} {\rm 6}0{\rm 3}\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} 0{\rm 68}) \cr & \hskip-1pt (n = {\rm 18},\;R^{\rm 2} = 0 {\cdot} 0{\rm 18};\;P \gt 0 {\cdot} {\rm 05})} $$Calcium to P ratio was similar in bone (P>0·05): 2·40, 2·41 and 2·39 for diets providing 0, 12·5 and 25 g of dicalcium phosphate/day, respectively and both minerals were linearly correlated:
 $$\eqalign{{\rm Ca(mg}/{\rm g}\;{\rm bone)} = \ & {\rm 2} {\cdot} 0\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 49}) \times {\rm P(mg}/{\rm g}\;{\rm bone)} \cr & \hskip 1pt -{\rm 24}\;{\rm (}{\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;{\rm 26} {\cdot} {\rm 4}0{\rm )} \cr & \hskip 3pt {\rm (}n = {\rm 18};\;R^{\rm 2} = 0 {\cdot} {\rm 68};\;P \lt 0 {\cdot} 00{\rm 1)}.} $$
$$\eqalign{{\rm Ca(mg}/{\rm g}\;{\rm bone)} = \ & {\rm 2} {\cdot} 0\;({\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;0 {\cdot} {\rm 49}) \times {\rm P(mg}/{\rm g}\;{\rm bone)} \cr & \hskip 1pt -{\rm 24}\;{\rm (}{\scale75%{\rm {S}}}.{\scale75%{\rm {E}}}.\;{\rm 26} {\cdot} {\rm 4}0{\rm )} \cr & \hskip 3pt {\rm (}n = {\rm 18};\;R^{\rm 2} = 0 {\cdot} {\rm 68};\;P \lt 0 {\cdot} 00{\rm 1)}.} $$DISCUSSION
Calcium and P were provided in the diet at levels considered deficient for the diet without dicalcium phosphate supplementation and above Ca and P requirements for 8-month-old sheep (late maturing) for diets supplemented with 12·5 and 25 g of dicalcium phosphate/day (as, according to NRC (2007), Ca and P requirements for these animals would be 130 and 98 mg/kg BW/day, respectively). Mineral shortage may explain the reduced feed intake in the unsupplemented group, as deficiency of P might result in appetite loss (NRC 2007).
Calcium
Dietary Ca was increased by supplementation with dicalcium phosphate. True absorption of Ca as a proportion of Ca intake was greatest for the control group, where dicalcium phosphate was not provided. Miller (Reference Miller1975) and Martz et al. (Reference Martz, Belo, Weiss and Belyea1999) also reported that true absorption of Ca decreased as dicalcium phosphate intake increased. Roque et al. (Reference Roque, Dias, Vitti, da Silva Bueno, da Cunha, dos Santos and Bueno2007) reported a coefficient of 0·40 for true Ca absorption of dicalcium phosphate offered to sheep fed diets according to NRC (1985) recommendations for growing lambs. Their value is in close agreement with that observed in the current study for the group receiving either 12·5 g of dicalcium phosphate/day, which provided the same amount of Ca as in Roque et al. (Reference Roque, Dias, Vitti, da Silva Bueno, da Cunha, dos Santos and Bueno2007) or 25 g of dicalcium phosphate/day, which provided a higher amount of dietary Ca. However, this value (0·40) is well below the value of Ca absorption of 0·68 suggested by NRC (2007) for growing lambs. According to Chrisp et al. (Reference Chrisp, Sykes and Grace1989a), the maximum or ‘potential’ availability should be assessed in animals in which intake is lower than the requirement, which would explain the highest Ca availability in the unsupplemented diet.
Young et al. (Reference Young, Lofgreen and Luick1966) did not observe a correlation between endogenous faecal Ca and absorption of Ca. However, in their study Ca intake did not affect endogenous Ca excretion in faeces, as observed in the present study (Table 2). Braithwaite (Reference Braithwaite1982) reported a significant linear relationship between endogenous faecal loss of Ca and feed intake, whereas endogenous Ca excretion was unaltered by Ca intake. Conversely, Chrisp et al. (Reference Chrisp, Sykes and Grace1989b) showed a positive relationship between Ca intake and endogenous faecal Ca in growing and lactating sheep. Chrisp et al. (Reference Chrisp, Sykes and Grace1989b) concluded that simple relationships between endogenous Ca excretion and either feed or Ca intake do not account for the complexity of the processes occurring in the gut. Therefore, along with a greater loss of endogenous Ca associated with increased feed intake, there is a variable faecal endogenous loss of Ca, which is dependent on the net Ca requirement in relation to Ca intake or the amount of Ca available for absorption in the alimentary tract (Chrisp et al. Reference Chrisp, Sykes and Grace1989b). According to those authors, when the demand for Ca is high and the pool of absorbable Ca is small, a greater proportion of endogenous Ca secreted is re-absorbed resulting in lower excretion of endogenous Ca in faeces. This assumption offers a reasonable explanation for the lowest endogenous faecal Ca excretion in the unsupplemented control group. Likewise, Martz et al. (Reference Martz, Belo, Weiss and Belyea1999) observed low endogenous faecal excretion of Ca in ruminants fed small amounts of feed with low intakes of Ca. Endogenous faecal Ca values for the supplemented lambs were within the range (35–50 mg/kg BW/day) found by Chrisp et al. (Reference Chrisp, Sykes and Grace1989b) for young sheep fed 1·5–2 times the estimated maintenance energy requirement.
Concentration of Ca in bone was not affected by increasing intakes of dicalcium phosphate. The short experimental period could explain the lack of response regarding Ca concentration in bone with increasing ingestion of Ca and there are studies showing that Ca ingestion may not affect the concentration of Ca in bone. Braithwaite & Riazuddin (Reference Braithwaite and Riazuddin1971) observed no changes in the rapidly and slowly exchangeable bone Ca pool in young and mature sheep fed variable amounts of Ca, while Belonje & van den Berg (Reference Belonje and Van Den Berg1983) observed no significant change in bone Ca concentration related to Ca intake in sheep fed different amounts of Ca and P for 98 days. In the current study, other bone measurements such as bone ash and bone DM content were not significantly different among treatments (data not shown).
Phosphorus
Dicalcium phosphate is a common dietary P supplement used in animal diets. Lofgreen (Reference Lofgreen1960) reported 0·50 as the true availability of this source of dietary P, noticeably lower than the average values observed in the present study, which ranged from 0·62 to 0·66. The different true P absorption coefficients are probably related to the category of animal used for study, as growing animals are able to absorb nutrients more efficiently than mature animals (Braithwaite & Riazuddin Reference Braithwaite and Riazuddin1971). In a meta-analysis study, Bravo et al. (Reference Bravo, Sauvant, Bogaert and Meschy2003) found true P absorption coefficients of 0·60 and 0·70 for diets containing large amounts of hay and silage, respectively. The database used included results from studies in sheep, cattle and goats in different physiological and productive states (growing, lactating, gestating, etc.), and with diets covering a wide range of P contents (from 2·0 to 6·4 g of P/kg DM). According to Bravo et al. (Reference Bravo, Sauvant, Bogaert and Meschy2003), the efficiency of P absorption decreases when concentration of P in the digestive tract is high, due to the combination of saturated active absorption and passive diffusion. It seems that in the current study the difference between P intakes was not enough to affect true P absorption (P>0·05).
Endogenous P loss in the control diet (unsupplemented) was close to value of 20 mg/kg BW/day used by NRC (1985) as the total endogenous losses for growing sheep. Loss of metabolic P is expected even in animals fed an inadequate P diet (Vitti et al. Reference Vitti, Kebreab, Lopes, Abdalla, de Carvalhos, de Resende, Crompton and France2000). Endogenous P loss in all treatments represented approximately 0.55 of total faecal P, which is noticeably lower than the 0·85 value suggested by NRC (2007) as the proportion of endogenous faecal P present in total P excreted in faeces. However, the values found in the present paper are in line with the values reported by Lofgreen (Reference Lofgreen1960) who reported that endogenous faecal P accounted for 0·56 of total P excreted in faeces of mature wethers. As a result of the significant contribution of endogenous P to total faecal excretion, true P availability was 0·66, 0·62 and 0·64, whereas apparent availability was 0·25, 0·18 and 0·21 for dicalcium phosphate intakes of 0, 12·5 and 25 g/day.
Endogenous P excreted in faeces was related to endogenous P flow entering the rumen (S r) (r=0·86) and both increased linearly with increasing dicalcium phosphate intake. In agreement, Dias et al. (Reference Dias, Lopez, Silva, Pardo, Silva Filho, Vitti, Kebreb and France2009) found that P intake in sheep supplemented with mono-ammonium phosphate affected positively endogenous P flow to the rumen and inversely P turnover, as observed in the current study (Table 4). The flows of endogenous P entering the rumen were smaller than those for endogenous P excreted in faeces, indicating a contribution of endogenous P from the lower gastrointestinal tract.
Endogenous P in faeces and P intake were inversely related to P turnover (r=−0·68 and r=−0·78, respectively). According to Young et al. (Reference Young, Lofgreen and Luick1966), a greater metabolic faecal P excretion associated with increased P intake suggests an accelerated rate of P secretion to the gastrointestinal tract and thus a shorter turnover time of P in the digestive compartments.
Phosphorus intake affected linearly P concentration in the rumen (Table 4). Secretion of P through saliva also contributes to P in the rumen (Bannink et al. Reference Bannink, Šebek, Dijkstra, Vitti and Kebreab2010), as shown earlier by the flow of endogenous P entering the rumen. Salivary P is important not only as a buffer against volatile fatty acids but also as a means to control the amount of P secreted into the gut (Tomas Reference Tomas1974a,Reference Tomasb). In contrast, very small amounts of Ca are returned to the rumen via saliva secretion (Grace et al. Reference Grace, Carr and Reid1985), explaining why for Ca the endogenous loss accounts for a smaller proportion of total faecal excretion than for P.
Concentration of P in bone was not affected by dicalcium phosphate intake (Table 3). Accordingly, Wan Zahari et al. (Reference Wan Zahari, Thompson, Scott and Buchan1990) did not observe differences in P concentration in the bone of sheep fed different amounts of this mineral. Conversely, other studies showed a correlation between P intake and P content in bone (Williams et al. Reference Williams, Lawrence, McDowell, Wilkinson, Ferguson and Warnick1991; Esser et al. Reference Esser, Hoffman, Coblentz, Orth and Weigel2009). The lack of effect of dietary P on P content in bone could be related to the short timeframe of the experiment.
Calcium and phosphorus interactions
Calcium retention was weakly related to P retention (r=0·30). Conversely, Braithwaite (Reference Braithwaite1984) found a strong correlation between the retention of these minerals using data from several studies with sheep. However, Ca truly absorbed from the diet was related to dietary P truly absorbed (r=0·81). This relationship can be explained by the fact that as the intake of both minerals is increased, the amount of each mineral truly absorbed is also increased. Indeed, when true absorption coefficients (g truly absorbed/g ingested) were correlated, the relationship was not significant (r=0·14, P=0·593). The absorption of these minerals is uncoupled to allow the absorption of each element to be adjusted to differences in physiological demand (Pfeffer et al. Reference Pfeffer, Beede, Valk, Pfeffer and Hristov2005). The metabolism of Ca and P are closely related and the excess or deficit of one mineral may affect the utilization of the other (Veum Reference Veum, Kebreab and Vitti2010). However, in ruminants the Ca to P ratio only affects the absorption of these minerals if the diet is inadequate in Ca and P (Veum Reference Veum, Kebreab and Vitti2010) and optimal performance in ruminants can be attained with dietary Ca to P ratios ranging from 1·5 : 1 to 2·5 : 1 (NRC 2007). The Ca : P ratios of the three diets used in the current experiment fell within this range and thus it can be expected that the Ca : P ratio did not affect the absorption of either mineral. Chapuis-Lardy et al. (Reference Chapuis-Lardy, Fiorini, Toth and Dou2004) suggested that P supplementation by adding monocalcium or dicalcium phosphate to ruminant diets increased faecal excretion of P and Ca. Roque et al. (Reference Roque, Dias, Vitti, da Silva Bueno, da Cunha, dos Santos and Bueno2007), assessing the availability of different sources of Ca to growing sheep, found that Ca from dicalcium phosphate was less available than Ca from limestone, probably due to the link between Ca and P in the former source. The lack of mineral supplementation in the control treatment associated with slightly greater Ca and P availability for this treatment suggests that the Ca and P present in an organic form together with the low level of Ca and P in this diet may have favoured utilization of these minerals. Rumen microbes and their enzymes are able to digest organic forms of these minerals leading to their efficient absorption from the gastrointestinal tract (Bravo et al. Reference Bravo, Sauvant, Bogaert and Meschy2003; Kincaid & Rodehutscord Reference Kincaid, Rodehutscord, Pfeffer and Hristov2005; Bannink et al. Reference Bannink, Šebek, Dijkstra, Vitti and Kebreab2010).
Endogenous Ca in faeces was poorly correlated with endogenous P excreted in faeces (r=0·48). This weak relationship would be a result of the different roles of endogenous Ca and endogenous P in the metabolism of these elements.
The Ca : P ratio in bone was similar for all treatments with a mean value of 2·4. This ratio is greater than the stoichiometric value of hydroxyapatite (2·16), which is the main mineral form in which Ca and P are accreted in bone. This difference suggests that sheep were mineralizing the bone matrix with Ca present in carbonate form. Taylor et al. (Reference Taylor, Knowlton, Mcgilliard, Swecker, Ferguson, Wu and Hanigan2009) observed a dietary Ca effect on bone Ca but not on bone P and suggested that bone was mineralized with Ca in carbonate form.
Although supplementation with dicalcium phosphate is recommended under some circumstances, especially for diets deficient in Ca and P, this nutritional strategy needs to be implemented with caution in order to avoid increased feeding costs and unnecessary waste of these minerals, which would consequently cause a potential environmental problem.
Funding was provided, in part, by the Canada Research Chairs and NSERC Discovery programs. JF and SL gratefully acknowledge receipt of collaborative grant no. SAB2010-0151 from the Spanish ‘Ministerio de Educación’ under the programme ‘Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+i 2008-2011 – subprograma EXTESP-EDU’ to undertake this work.






