Introduction
Because of public health concerns over the use of conventional antibiotics as growth promoters in livestock, the use of non-antibiotic chemical substances has come into force (Yang et al., Reference Yang, Iji and Choct2009). Nutritional rather than pharmacological approaches are required to enhance the production and natural defence mechanisms of livestock. Organic acids have been suggested as potential alternatives to antibiotic growth promoters (Van Immerseel et al., Reference Van Immerseel, Fievez, de Buck, Pasmans, Martel, Haesebrouck and Ducatelle2004), with higher bactericidal activity (Leeson et al., Reference Leeson, Namkung, Antongiovanni and Lee2005). In poultry production, organic acids such as formic, benzoic and propionic acids have been used mainly to sanitize feed with bacterial infections (Thompson and Hinton, Reference Thompson and Hinton1997). However, later it was proven that the use of organic acids in feed reduces pathogen colonization of the intestinal wall and production of toxic components, thus preventing damage to epithelial cells (Langhout, Reference Langhout2000). The use of organic acids also improves the digestibility of proteins, and they serve as substrates in intermediary metabolism (Kirchgessner and Roth, Reference Kirchgessner and Roth1988). The butyrate derived from fermentation of non-starch polysaccharides in the gut has a significant role in the normal development of intestinal epithelium, with improved gut health (Brouns et al., Reference Brouns, Kettlitz and Arrigoni2002). It has been reported to have a significant influence on gene expression and protein synthesis in the body and thus has a direct bearing on mucosal cell proliferation, maturation and differentiation. Butyric acid (BA) is considered a major modulator of epithelial cell activity, which reflects significant changes in the gut microflora of rats (Sharma et al., Reference Sharma, Schumacher, Ronaasen and Coates1995). Several studies have demonstrated that supplementation of broiler diets with organic acids increased growth performance, and reduced disease and management problems (Ao et al., Reference Ao, Kocher and Choct2012). However, a comprehensive study is needed to ascertain the positive effects of BA supplementation in broiler chicken at the gross as well as molecular level. Thus, the objectives of the current study were based on the hypothesis that the use of BA as a substitute for antibiotic growth promoter improves the performance, immunity, gut health and serum biochemistry of broiler chicken.
Materials and methods
Birds, treatments and feeding management
Two hundred and forty 1-day-old chicks from the same hatch were procured from the hatchery of Central Avian Research Institute and distributed randomly into four treatment groups, each having six replicates with ten chicks in each (four treatments × six replicates). Following a completely randomized design, four experimental diets were formulated viz. control, with no additive, bacitracin methylene di-salicylate (BMD) at 20 mg bacitracin/kg diet (BMD-supplemented), 3 g BA/kg diet and 4 g BA/kg diet. The BA was purchased from Shree Ram Enterprises, Mumbai, India as sodium butyrate with >98% assay and BMD with 44% bacitracin activity was purchased from Alpharma Animal Health Division, New Jersey, USA. The ingredients and nutrient composition of the basal diets provided to the birds in both starter (0–21 days) and finisher (21–42 days) phases is given in Table 1 and birds were allowed to eat and drink ad libitum. The light/dark regime started at 24 h light on day 1 followed by a decrease of 1 h per day until reaching 18 h light, which was continued until the 42nd day. The birds were reared in specially designed electrically heated battery brooders under uniform management conditions, with one replicate (ten birds) per 0.76 × 0.76 × 0.46 m3 cabin.
Table 1. Ingredients and composition of basal diet
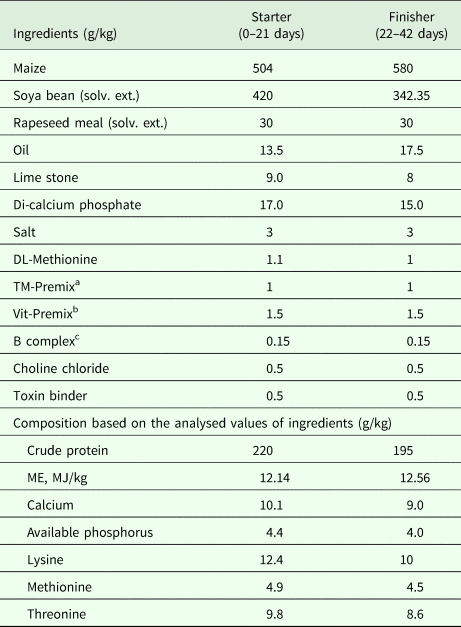
ME, metabolizable energy.
a Trace mineral (TM) premix supplied: Mg, 300; Mn, 55; I, 0.4; Fe, 56; Zn, 30 and Cu, 4 mg kg-1 diet.
b Vitamin (Vit) premix supplied: vitamin A, 8250 IU; vitamin D3, 1200 ICU; vitamin K, 1 mg; vitamin E, 40 IU.
c B complex: vitamin B1, 2 mg; vitamin B2, 4 mg; vitamin B12, 10 µg; niacin, 60 mg; pantothenic acid, 10 mg; choline, 500 mg kg-1 diet.
Growth performance
The weekly body weight and daily FI of birds at 08:00 h were recorded to arrive at overall BWG, FI and feed conversion ratio (FCR). Birds were monitored daily to record mortality, if any.
Gene expression
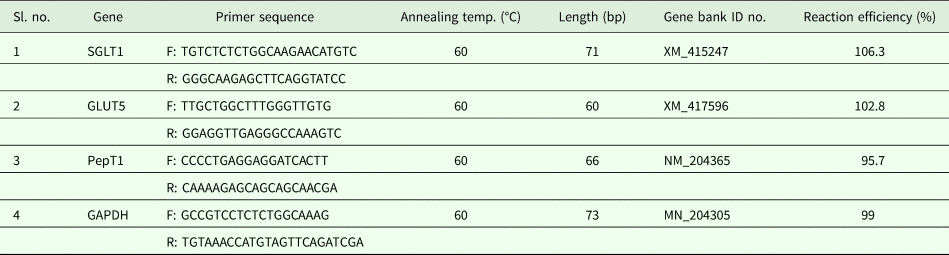
Six birds from each dietary treatment were taken at 7 days and 21 days post-hatching for gene expression analysis. The birds were slaughtered after stunning in an electrical water bath by severing the trachea and both carotid arteries and samples of the jejunum from each were collected aseptically in RNAlater. The tissue samples were homogenized using an automated Kinematica polytron PT 10/35 GT Homogenizer (Thermo Fisher Scientific, India) and total RNA extracted using the Trizol method (Chomczynski, Reference Chomczynski1993) followed by cDNA synthesis immediately by using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, India) as per the manufacturer's instructions. The purity and concentration of total RNA was analysed using a nanodrop spectrophotometer (Nano Drop 1000, Thermo-Scientific, Singapore), considering the optical density values at ratios of 260 and 280 nm. The purity was further verified electrophoretically by ethidium bromide staining. Any RNA showing contamination with DNA was incubated with RNase free DNase (Biogene, Cambridge, MA, USA) at 37 °C (at 1 unit per 1 µl). The DNase was subsequently inactivated by incubation at 65 °C for 10 min. The relative gene expression of nutrient transporters such as PepT1, SGLT1 and GLUT5 was quantified with respect to the housekeeping gene GAPDH using real-time polymerase chain reaction (PCR) (qPCR) detection (IQ5 Multicolor Real-time PCR Detection System, Bio-Rad Laboratories Inc., Hercules, CA, USA). All reactions were performed in nuclease-free eight tube-strips with optically clear flat caps (Axygen Scientific, Inc., Corning, NY, USA). The forward and reverse primers used in the study are given in Table 2 (Mott et al., Reference Mott, Siegel, Webb and Wong2008).
Table 2. Primer sequences used in the study
Immunological studies
The immune response of birds to different dietary treatments was measured in terms of antibody titre against sheep red blood cells (RBC) (humoral immunity) and foot web index (cell-mediated immunity) at the age of 28 days. For humoral immunity, three birds from each replicate (18 birds/treatment) were injected intravenously via the jugular vein with a 1% suspension of sheep RBC (SRBC). Blood samples were collected from the SRBC-injected birds at 0 and 6 days post-inoculation. All the samples were incubated at 37 °C for 1 h to aid clotting and retraction then centrifuged at 15 000 g for 5 min for collection of sera. All micro-titre plates (U-bottomed) were rinsed with phosphate-buffered saline (PBS; pH 7.6) and dried before the haemagglutination antibody (HA) titre was estimated by a micro-haemagglutination method using two-fold serial dilutions of sera.
For cell-mediated immunity, three birds from each replicate (18 birds/treatment) were injected with 0.2 ml of the protein form of the mitogen phytohaemagglutinin-P (PHA-P), a lectin extract from the red kidney bean (1 mg/ml PBS). This was injected intra-dermally into the left foot web and sterile PBS (0.2 ml) was injected in the right foot web to serve as a control. Measurements were made at 0 and 24 h post-injection, to measure the cell-mediated immune response against PHA-P mitogen.
Carcase characteristic traits
At the end of the experimental period 12 birds from each treatment group (two birds/replicate) were selected randomly and slaughtered after a 12 h fast with ad lib drinking water, for evaluation of carcase characteristics and organ weights.
Histomorphometry of jejunum
The histomorphometry of jejunum was performed by measuring the villi height and width under a high-resolution microscope with micrometry and photographic attachments. The birds used in jejunum sample collection for the gene expression study were also used to collect jejunum samples for histomorphometry at 7 days post-hatching and for histomorphometry at 42 days post-hatching (six birds in each treatment group). The jejunum samples were subjected to microtomy and staining with haematoxylin and eosin to prepare tissue sections for microscopic examination. Villus height was measured from the top of the villus to the top of the lamina propria and villus width was taken as an average of proximal, middle and distal width. A total of 15 measurements were taken per bird.
Serum biochemistry
At the end of the feeding trial, blood samples from the birds slaughtered for the study of carcase characteristics were collected in sterile glass test tubes without anticoagulant. Test tubes containing the blood were kept in a slanted position at room temperature for 30 min to facilitate separation of serum. Serum was harvested by centrifugation at 3000 rpm for 10 min, decanted into plastic vials and stored at −20 °C for further processing. The serum was used for (a) estimation of enzymes such as alkaline phosphatase (ALP; Kind and King, Reference Kind and King1954), AST and alanine aminotransferase (ALT; Reitman and Frankel, Reference Reitman and Frankel1957), (b) kidney function tests such as serum creatinine (Reitman and Frankel, Reference Reitman and Frankel1957) and uric acid by the modified phosphotungstate method, (c) serum mineral estimation such as calcium (Baginski et al., Reference Baginski, Marie, Clark and Zak1973) and phosphorus (Morin and Prox, Reference Morin and Prox1973), and (d) serum total cholesterol (Wybenga et al., Reference Wybenga, Pileggi, Dirstine and di Giorgio1970), total protein (Vatzidis, Reference Vatzidis1977) and albumin (Gustafsson, Reference Gustafsson1978) estimation using Cogent diagnostics kits (SPAN Diagnostics, Gujarat, India).
Statistical analysis
Data obtained in the experiment were analysed using SPSS version 20.0, following standard procedures, by one-way analysis of variance. The post-hoc analysis for comparing group means was performed using Duncan's multiple range test with significance level set at P < 0.05 and expression analysis of nutrient transporter genes was carried out using REST 2009 software (https://www.gene-quantification.de/rest-2009.html). Linear and quadratic polynomial contrasts were performed to study the effect of BA levels. Replicate was used as an experimental unit for the study of growth performance, immune response, carcase characteristic traits and serum biochemistry of chicken, whereas the individual bird was used as an experimental unit for gene expression and histomorphometry study.
Results
Growth performance
The results of growth performance analysis reveal that birds fed the control diet had significantly (P = 0.023) lower BWG compared to the birds fed BMD or BA, which did not differ statistically from each other (Table 3). FI of birds was significantly (P = 0.041) higher in the BMD-supplemented group compared to the control, whereas that of birds fed 3 or 4 g BA/kg diet did not differ significantly from either control or BMD-supplemented groups. The FCR of birds fed 3 or 4 g BA/kg diet was significantly (P = 0.018) better compared to the control or BMD-supplemented birds, which were statistically similar to each other. Only one bird died from each of the control, BMD and 4 g BA/kg diet groups during the whole experimental period, indicating no dietary effect on the mortality pattern of birds.
Table 3. Effect of dietary BA supplementation on production performance (n = 60) and immunity (n = 18) of broiler chicken

BMD, bacitracin methylene di-salicylate; BA, butyric acid; FCR, feed conversion ratio; HA, haemagglutination; CMI, cell mediate immunity; s.e.m., standard error of mean.
Gene expression
The results of gene expression analysis for the PepT1, the SGLT1 and the GLUT5 in the jejunum of broiler chicken at 7 and 21 days post-hatching are presented in Table 4. At 7 days post-hatching, significant up-regulation of GLUT5 (P = 0.025), SGLT1 (P = 0.018) and PepT1 (P = 0.032) was observed in birds supplemented with BMD or BA compared to the control group, whereas BMD- and BA-supplemented groups did not differ significantly from each other. At 21 days post-hatching, significant up-regulation of GLUT5 (P = 0.029) and PepT1 (P = 0.045) was observed in birds supplemented with BMD or BA compared to the control group. However, significant (P = 0.012) up-regulation was observed for SGLT1 expression in birds supplemented with 3 or 4 g BA/kg diet compared to the control or BMD-supplemented groups, which were statistically similar to each other.
Table 4. Effect of BA supplementation on the fold expression of nutrient transporter genes in jejunum of broiler chicken (n = 6)
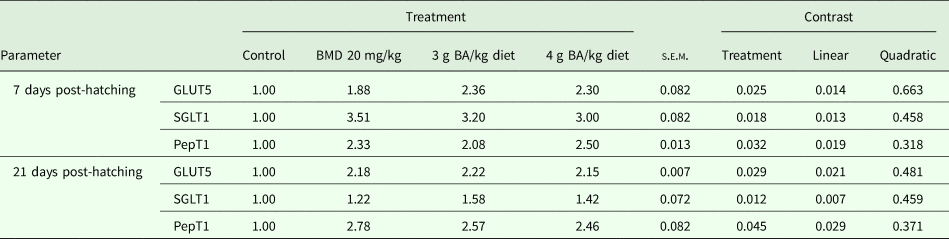
BMD, bacitracin methylene di-salicylate; BA, butyric acid; s.e.m., standard error of mean; GLUT5, glucose transporter 5; SGLT1, sodium-dependent glucose transporter 1; PepT1, peptide transporter.
Immune response
The immune response of birds under different dietary treatments is given in Table 3. The HA titre of birds in the control group was significantly (P = 0.015) lower followed by the BMD and 3 g BA-supplemented birds, which were statistically similar to each other. The highest titre was observed in birds supplemented with 4 g BA/kg diet. The cell-mediated immune response of birds was significantly (P = 0.015) higher in the 4 g BA-supplemented group, followed by the 3 g BA-supplemented group, which was statistically different compared to the control and BMD-supplemented groups, which were statistically similar to each other.
Carcase characteristics
The effects of dietary supplementation with BA on carcase characteristics are presented in Table 5. The results reveal that none of the carcase traits were influenced by dietary supplementation of BA in the diets of broiler chicken with respect to the control or BMD supplementation.
Table 5. Effects of BA supplementation on carcase traits and relative organ weight (g/kg live weight) of broiler chicken (n = 12)

BMD, bacitracin methylene di-salicylate; BA, butyric acid; s.e.m., standard error of mean.
Histomorphometry of the jejunum
The effect of BA supplementation on the histomorphometric measurements of the jejunum at the end of the experimental trial is shown in Table 6. The results revealed no significant difference in villus height and width 7 days post-hatching among different treatment groups. However, at 42 days post-hatching, villus height was significantly (P = 0.039) higher in birds supplemented with 4 g BA/kg diet compared to the control and 3 g BA-supplemented groups, whereas the BMD-supplemented group did not differ significantly from either of the BA-supplemented groups. Similarly, villus width was significantly (P = 0.042) higher in birds fed 4 g BA/kg diet compared to other treatment groups, which did not differ significantly from each other.
Table 6. Effects of BA supplementation on histomorphometry of jejunum of broiler chicken (n = 6)
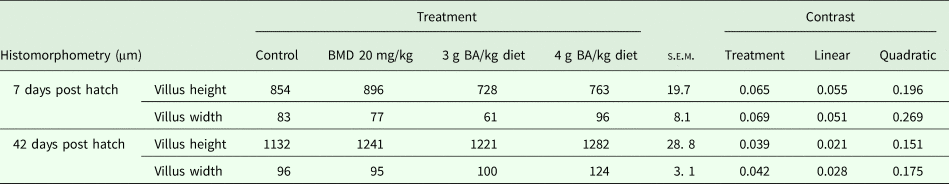
BMD, bacitracin methylene di-salicylate; BA, butyric acid; s.e.m., standard error of mean.
Serum biochemistry
The serum biochemistry of broiler chicken as affected by dietary supplementation of BA is given in Table 7. The serum total protein and creatinine levels were significantly (P = 0.024) higher in birds supplemented with 3 or 4 g BA/kg diet compared to the control or BMD-supplemented groups, which did not differ significantly from each other. The serum albumin was significantly (P = 0.015) higher in the 4 g BA-supplemented group, followed by the 3 g BA-supplemented group, which was statistically different from the control and BMD-supplemented groups, which were statistically similar to each other. Serum uric acid level was significantly (P = 0.045) higher in the control group followed by the BMD group and lower in both of the BA-supplemented groups, which did not differ significantly from each other. Among the estimated serum enzymes, significantly (P = 0.017) lower values of AST were observed in the control group followed by the statistically similar BMD-supplemented group, while higher values were observed in both of the BA-supplemented groups, which did not differ significantly from each other. On the other hand, no significant differences were observed in serum ALT and ALP due to BA supplementation in broiler chicken diets. In the case of serum minerals, significantly lower phosphorus (P = 0.046) and calcium (P = 0.025) levels were observed in the control group, followed by the statistically similar BMD-supplemented group, compared to either of the BA-supplemented groups, which did not differ significantly from each other. The serum cholesterol levels were significantly (P = 0.037) lower in the 4 g BA-supplemented group followed by the 3 g BA-supplemented compared to the control and BMD-supplemented groups, which were statistically similar to each other.
Table 7. Effects of BA supplementation on serum biochemistry of broiler chicken (n = 12)

BMD, bacitracin methylene di-salicylate; BA, butyric acid; s.e.m., standard error of mean; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase.
Discussion
Butyrate, mediating its effects via regulation of gene expression and protein synthesis, reduces apoptosis and enhances proliferation, differentiation and maturation of normal enterocytes, which may be the reason for enhanced body building and accumulation of proteins in muscles (Sengupta et al., Reference Sengupta, Muir and Gibson2006). Similarly, supplementation with antibiotics as growth promoters improves digestibility of protein, reduces ammonia and favours production of biogenic amines (Dierick et al., Reference Dierick, Vervaeke, Decuypere and Henderickx1986). On similar lines, the results of the current study clearly indicate that BA and BMD supplementation resulted in higher BWG compared to the control diet. Also, BMD supplementation increased the FI of birds, whereas BA supplementation had no effect. This may be the reason that BA supplementation improved feed efficiency and why no effect of BMD supplementation was observed on FCR of birds, since both BWG and FI increased simultaneously. Supplementation with BA causes a reduction in pH (Boling et al., Reference Boling, Douglas, Snow, Parsons and Baker2000), which improves absorption of nutrients and also causes the exclusion of harmful microbial load. It has been reported that the use of micro-encapsulated butyrate in young chicken decreases the caecum colonization of salmonella (Van Immerseel et al., Reference Van Immerseel, Fievez, de Buck, Pasmans, Martel, Haesebrouck and Ducatelle2004).
The observations of the current study are supported by the results of Panda et al. (Reference Panda, Rama Rao, Raju and Shyam Sunder2009) and Sikandar et al. (Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017). Further, Panda et al. (Reference Panda, Rama Rao, Raju and Shyam Sunder2009) reported that with respect to antibiotics, 4 g BA/kg diet in the broiler chicken was equally effective at maintaining BWG of birds but was superior in terms of feed efficiency. The organic acids in the gut have the ability to diffuse into enterocyte cytoplasm in un-dissociated forms due to its higher pH, which hastens pancreatic and bile secretion (Harada et al., Reference Harada, Hiroko, Kobayashi and Tsuchita1988). The presence in enterocytes of the receptor that responds to the dissociated proton causes an increase in secretin release. The improved performance of broiler chickens can be attributed to this increase of gut secretions in response to BA supplementation in the diet. Further, the modification of gut microstructure (Le Gall et al., Reference Le Gall, Gallois, Sève, Louveau, Holst, Oswald, Lallès and Guilloteau2009; Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010) in response to dietary supplementation of organic acids can contribute to better nutrient absorption and in turn better growth performance of animals and birds. To our knowledge there is no literature available pertaining to the effect of BA supplementation on the mortality pattern of broiler chickens.
The introduction of unusual feed ingredients or supplements in the ration of birds and animals causes adaptive conditioning of gene expression. The jejunum is the main seat of absorption in birds; therefore, the expression of nutrient transporters, responsible for dietary nutrient assimilation, influences overall nutritional status, growth and development. The current study revealed that the expression of nutrient transporter genes was up-regulated due to BMD or BA supplementation. However, at 21 days post-hatching expression of SGLT1 in the BMD-supplemented group was down-regulated with respect to the BA-supplemented groups. The major route for glucose assimilation in enterocytes is the SGLT1 transporter (Wright and Turk, Reference Wright and Turk2004). This may be why BA supplementation outperformed BMD supplementation in terms of feed efficiency in the current study, thus making the importance of SGLT1 explicit in broiler growth performance. The absorption of di- and tri-peptides occurs via proton-coupled PepT1 (Gilbert et al., Reference Gilbert, Wong and Webb2008) which is dependent on a pH gradient as well as a negative intracellular membrane potential (Adibi, Reference Adibi1997). Peptide transport by PepT1 is most efficient in an acidic environment (Steel et al., Reference Steel, Nussberger, Romero, Boron, Boyd and Hediger1997), which is provided by BA supplementation in broiler diets. However, the literature pertaining to the role of BA supplementation in the nutrient transporter gene expression of broiler chicken is not available.
BA acts as an immune modulator in chickens (Ahsan et al., Reference Ahsan, Cengiz, Raza, Kuter, Chacher, Iqbal, Umar and Çakir2016). Sodium butyrate supplementation in chickens (Sunkara et al., Reference Sunkara, Achanta, Schreiber, Bommineni, Dai, Jiang, Lamont, Lillehoj, Beker, Teeter and Zhang2011) has been reported to boost the production and secretion of immunoglobulins. In the current study, supplementation of 4 g BA/kg diet yielded better humoral and cell-mediated immune responses compared to the control, BMD and 3 g BA-supplemented groups. These results are supported by the observations of Sikandar et al. (Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017). Also, the thymus-dependent immunogens, sheep RBCs, have been shown to yield higher antibody titres in sodium butyrate-supplemented birds at 35 days of age (Sikandar et al., Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017). Similarly, Lohakare et al. (Reference Lohakare, Ryu, Hahn, Lee and Chae2005) reported higher Infectious Bursal disease titres post-vaccination in a group supplemented with ascorbic acid (2 g/kg diet); they argued that there is a possibility of enhanced differentiation of lymphoid organs due to ascorbic acid supplementation by increasing the activity of the hexose monophosphate pathway, thus increasing the circulating antibody. It has been reported that BA supplementation lowers the colonization and faecal shedding of salmonella (Van Immerseel et al., Reference Van Immerseel, Fievez, de Buck, Pasmans, Martel, Haesebrouck and Ducatelle2004), which indicates that butyrate may have a role in modulation of B and T cell function in response to antigenic exposure (Ahsan et al., Reference Ahsan, Cengiz, Raza, Kuter, Chacher, Iqbal, Umar and Çakir2016). BA boosts the immunity of chickens by the induction of host defence peptides (Sunkara et al., Reference Sunkara, Achanta, Schreiber, Bommineni, Dai, Jiang, Lamont, Lillehoj, Beker, Teeter and Zhang2011) and the regulation of immune cells by BA has been reported by Zhou et al. (Reference Zhou, Packialakshmi, Makkar, Dridi and Rath2014).
In the current study, BA supplementation did not show any significant effect on carcase traits of the birds with respect to the control and BMD-supplemented groups. These results are corroborated by the findings of Adil et al. (Reference Adil, Banday, Bhat, Mir and Rehman2010), who also reported no significant effect of organic acid supplementation on various carcase traits. However, in contrast to the current findings, Panda et al. (Reference Panda, Rama Rao, Raju and Shyam Sunder2009) reported that birds receiving diets supplemented with 2 g BA/kg diet yielded higher dressed weight compared to the control or antibiotic-supplemented birds. Similarly, Leeson et al. (Reference Leeson, Namkung, Antongiovanni and Lee2005) observed that carcase yield and breast meat yield increased in birds fed 2 g BA/kg diet. These differences may be attributed to differences in the genetics of experimental birds used, along with possible differences in other environmental conditions.
The surface area of villi determines the absorption activity of the intestines and the jejunum in poultry represents the area with the highest absorption activity. The histomorphological modulation of the small intestine is held to have a relationship with the production performance of animals. In the current study, BMD or BA supplementation revealed no significant effect on the histomorphometry of jejunum at 7 days post-hatching. This may be because 7 days' duration of BMD or BA supplementation is too short to show significant effects. However, at 42 days of age, supplementation of 4 g BA/kg diet increased the villus length and width, and thus its surface area, significantly. Similar results were reported by Sikandar et al. (Reference Sikandar, Zaneb, Younus, Masood, Aslam, Khattak, Ashraf, Yousaf and Rehman2017) and Adil et al. (Reference Adil, Banday, Bhat, Mir and Rehman2010). This increase in villus length, width and in turn surface area could be partly responsible for better weight gain and feed efficiency (Ashraf et al., Reference Ashraf, Zaneb, Yousaf, Ijaz, Sohail, Muti, Usman, Ijaz and Rehman2013) along with the contribution of up-regulated gene expression of nutrient transporters discussed earlier. Since the diets formulated were iso-nitrogenous and iso-caloric, the apparent enhancement in growth performance of the 4 g BA/kg diet supplemented birds was assumed to be a result of the mucosal architectural modulations in birds.
The results of the current study indicate a significant increase in serum protein, albumin, creatinine, AST, phosphorus and calcium due to BA supplementation with respect to the control and BMD supplementation. However, the reverse trend was observed in serum uric acid and cholesterol, where BA supplementation decreased both and BMD supplementation decreased only uric acid levels. Similar to the current results, Ali et al. (Reference Ali, Seddiek and Khater2014) observed significant increases in serum total protein, albumin and globulin at 4 g BA/kg diet glyceride supplementation in broiler chicken rations in normal and Eimeria maxima challenged-birds. In the same context, the current results were similar to Helal et al. (Reference Helal, Youssef, Moursi, Khalil and Abdel-Daim2015) who reported a significant increase in serum total protein; meanwhile, a significant decrease in serum albumin was observed in BA-supplemented broiler chicken compared to antibiotic-supplemented and control birds. However, in contrast to the present results, Hedayati et al. (Reference Hedayati, Manafi, Khalaji, Yari, Esapour, Nazari and Mohebi2015) observed significant declines in total protein due to the addition of acidifiers in the basal diet of chicken. Adil et al. (Reference Adil, Banday, Bhat, Mir and Rehman2010) also observed no significant effect on serum ALT, whereas, in contrast to the observation of the current study, they also reported no effect on AST levels due to the supplementation of organic acids in the broiler ration. Abdel Fattah et al. (Reference Abdel-Fattah, El-Sanhoury, El-Mednay and Abdel-Azeem2008) argued that dietary supplementation of organic acids could be done up to the level of 3 g/kg in the diet of broiler chickens without causing any adverse effect on kidney and liver functions.
Similar to the current study, significantly higher values of serum calcium and phosphorus have been observed by various researchers (Adil et al., Reference Adil, Banday, Bhat, Mir and Rehman2010; Kamal and Ragaa, Reference Kamal and Ragaa2014). The higher levels can be attributed to the fact that acidification of feed increases the absorption of cationic minerals in the intestine by decreasing the pH of digesta, which in turn inhibits phytic acid from the formation of cationic chelates (Boling et al., Reference Boling, Douglas, Snow, Parsons and Baker2000). In line with the results of the current study, reduced levels of serum total cholesterol and low-density lipoprotein cholesterol have been reported due to BA supplementation (Kamal and Ragaa, Reference Kamal and Ragaa2014). The acidification of feed helps improve gut health by facilitating the growth of beneficial bacteria such as lactobacillus. The lactobacillus possesses high hydrolytic activity against bile salts, which hastens their deconjugation (Surono, Reference Surono2003). The deconjugation process makes bile acids less able to be absorbed in the intestine and excretion of cholesterol and its fraction in faeces more likely (Klaver and Van der Meer, Reference Klaver and van der Meer1993), thus reducing the cholesterol accretion in the body. However, in contrast to the results of the current study Adil et al. (Reference Adil, Banday, Bhat, Mir and Rehman2010) observed no significant effect of BA supplementation on serum cholesterol levels.
Conclusion
In the current study it was revealed that BA supplementation in broiler chicken diets has positive effects on the growth performance, expression of nutrient transporter genes, immunity, intestinal histomorphometry and serum biochemistry of broiler chickens. Based on the results it was concluded that 4 g/kg diet BA supplementation in feed is optimum for desirable broiler production.
Author ORCIDs
N. A. Mir, 0000-0003-0929-0713.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical standards
All the experimental procedures carried out on the birds were approved by the Animal Ethics Committee of Indian Veterinary Research Institute, Izatnagar.
Conflict of interest
The authors declare no conflict of interest.









