INTRODUCTION
The development of highly productive, short-stemmed wheat cultivars that are resistant to lodging has remained one of the main breeding objectives in most countries for many years. The introduction of the cultivars Norin 10, Saitama 27 and Akakomughi semi-dwarfing (Rht) genes in the first half of the 20th century led to significant worldwide increases in wheat yields (Gale & Youssefian Reference Gale, Youssefian and Russell1985). The major Rht genes are classified into two groups according to their response to exogenous gibberellic acid (GA): GA-insensitive and GA-responsive (Gale & Gregory Reference Gale and Gregory1977). The Norin 10-derived GA-insensitive alleles Rht-B1b (previously termed Rht1) and Rht-D1b (Rht2), located on chromosomes 4B and 4D, respectively, cause a reduction in plant height of c. 15% and can increase yield by up to 20% (Worland & Law Reference Worland and Law1986). Their distribution is, however, restricted to geographic areas that are not subjected to heat stress during ear emergence, as this has been demonstrated to reduce plant fertility (Worland & Law Reference Worland and Law1986). Poor seedling emergence from depth because of shorter coleoptiles and lower adaptation, especially to dry environments, are other known problems connected with the presence of the Rht-B1b and Rht-D1b alleles (Trethowan et al. Reference Trethowan, Singh, Huerta-Espino, Crossa and van Ginkel2001). Landjeva et al. (Reference Landjeva, Korzun, Stoimenova, Truberg, Ganeva and Börner2008) showed that seedling growth was significantly affected by the allelic status at the Rht loci, background genes and the water potential. In Southern Europe, it appeared advantageous to incorporate the Saitama 27-derived Rht-B1d allele, characterized by a weaker GA-insensitivity, less reduction in height and lower temperature sensitivity (Worland & Petrovic Reference Worland and Petrovic1988; Ganeva et al. Reference Ganeva, Korzun, Landjeva, Tsenov and Atanasova2005).
The GA-responsive Rht genes, Rht8 and Rht9, were introduced into Italian germplasm from the Japanese cultivar Akakomughi, and the 192-bp allele of the microsatellite marker, WMS 261, on chromosome 2D, was initially reported to be diagnostic for Rht8 (Korzun et al. Reference Korzun, Röder, Ganal, Worland and Law1998). However, it was recently reported by Ellis et al. (Reference Ellis, Bonnett and Rebetzke2007) that the 192-bp allele at this locus is not always diagnostic for the height-reducing gene Rht8, stating that the presence of the Xgwm261 192-bp allele is only indicative of Rht8 in wheat cultivars that have inherited this allele from Akakomughi or a Strampelli wheat ancestor. This allele now prevails in Southern and South-Eastern European genotypes and in Southern Ukraine, implying an adaptive significance of the 192-bp allele for these climatic conditions, evidently due to the beneficial effects on increased fertility (Chebotar et al. Reference Chebotar, Korzun and Sivolap2001; Worland et al. Reference Worland, Sayers and Korzun2001). The Xgwm261 192-bp allele was also found to be prevalent in Chinese wheat cultivars (Liu et al. Reference Liu, Liu, Zhang, Wang, Sun, Guo and Zhang2005). The Xgwm261 locus is highly polymorphic with three main allelic variants, characteristic of cultivars Ciano 67 (165-bp), Cappelle-Desprez (174-bp) and Mara (192-bp). According to Korzun et al. (Reference Korzun, Röder, Ganal, Worland and Law1998), it was possible to attribute a 70–80 mm reduction in plant height to the Xgwm261 192-bp allele compared to the Xgwm261 174-bp allele. A height reduction of c. 30 mm was detected between the Xgwm261 174-bp allele and the Xgwm261 165-bp allele. Screening of over 800 wheat cultivars from 20 countries (Worland et al. Reference Worland, Sayers and Korzun2001) showed a high proportion (0·9) of these three alleles. Ten novel alleles at the Xgwm261 locus were also detected, the most frequent being the 197-bp allele, present in 51 cultivars coming mainly from Austria, Holland and Switzerland. Four novel alleles (180, 198, 200 and 204-bp) were detected in the US and New Zealand wheat cultivars by Ahmad & Sorrells (Reference Ahmad and Sorrells2002).
Marker locus Xgwm261 is in tight linkage on chromosome 2D with the semi-dwarfing gene Rht8 and also with the photoperiod insensitive Ppd-D1a gene, which enabled simultaneous breeding for short stem and earliness connected with the transfer of a Rht8/Ppd-D1a linkage block (Pestsova & Röder Reference Pestsova and Röder2002). Ppd-D1a is extremely important in increasing the adaptability and yield in cultivars from Southern Europe. A selective advantage for the preservation of the linkage between Ppd-D1a and Rht8 has been observed in Italian, Yugoslavian and Russian breeding programmes. In west-European cultivars and CIMMYT wheats, the absence of an adaptive advantage for the linkage block containing Ppd-D1a/Rht8 led to the breakage of this linkage (Worland et al. Reference Worland, Korzun, Röder, Ganal and Law1998). The effects of the Ppd-D1a allele on yield potential appeared to be largely neutral in the conditions of the UK, but Ppd-D1a was associated with increased early biomass production and a more efficient production of grain dry matter (improved harvest index (HI)) (Foulkes et al. Reference Foulkes, Sylvester-Bradley, Worland and Snape2004). Addisu et al. (Reference Addisu, Snape, Simmonds and Gooding2010) recently demonstrated that the presence of Ppd-D1a in west-European regions may be also beneficial for early resource capture, which is highly desirable especially under organic agriculture (Wolfe et al. Reference Wolfe, Baresel, Desclaux, Goldringer, Hoad, Kovacs, Lőschenberger, Miedaner, Ǿstergård and Lammerts van Bueren2008).
It was clearly shown by Reynolds & Borlaug (Reference Reynolds and Borlaug2006) that crop improvement in general must embrace the problems associated with a highly heterogeneous and unpredictable environment, and it is highly desirable to achieve synergies between genetic improvement and innovative crop management practices. The best evidence of wide adaptation in variable environments was given by CIMMYT's shuttle breeding strategy, which resulted in the development of some cultivars (e.g. Siete Cerros, Anza, Sonalika and Seri 82) that were grown on several million ha (Braun et al. Reference Braun, Rajaram, van Ginkel and Tigerstedt1997). However, it is obvious that widely adapted genotypes are the best performers in only a narrow range of so-called mega-environments and usually not including those with severe stresses (Ceccarelli Reference Ceccarelli1989). Therefore, the identification of the range of target environments in which the genotypes are likely to perform well after the application of certain farming practices will be a key objective (Wolfe et al. Reference Wolfe, Baresel, Desclaux, Goldringer, Hoad, Kovacs, Lőschenberger, Miedaner, Ǿstergård and Lammerts van Bueren2008). It can be expected that a high degree of buffering capacity is related to an appropriate plant growth type (i.e. the presence of certain Rht and Ppd alleles). An insight into the breeding history, reflecting the changes of breeding goals and requirements of agricultural practice (Phillips & Wolfe Reference Phillips and Wolfe2005; Peltonen-Sainio et al. Reference Peltonen-Sainio, Muurinen, Rajala and Jauhiainen2008; Wolfe et al. Reference Wolfe, Baresel, Desclaux, Goldringer, Hoad, Kovacs, Lőschenberger, Miedaner, Ǿstergård and Lammerts van Bueren2008; Sener et al. Reference Sener, Arslan, Soysal and Erayman2009; Acreche & Slafer Reference Acreche and Slafer2009; Šíp et al. Reference Šíp, Růžek, Chrpová, Vavera and Kusá2009) could be helpful to find plant types suitable for certain growing conditions. Mohammadi et al. (Reference Mohammadi, Amri, Haghparast, Sadeghzadeh, Armion and Ahmadi2009) stated that a careful definition, with the participation of farmers, of target environments will ensure that the cultivars developed are adapted to the target conditions. Farmers and breeders are now faced with the effects of climatic changes, needs for organic production, lowering of inputs in agriculture and wider applications of reduced tillage systems, while keeping the productivity at the highest possible level. This study follows the dynamics of the distribution of alleles affecting plant height and photoperiodic response in different European winter wheat cultivars that were registered in Middle Europe (especially the Czech and Slovak Republics) over the last 30 years in order to test the hypothesis that regional allelic combinations exist, and to find the appropriate combinations of alleles to use in certain growing regions and current agricultural systems.
MATERIALS AND METHODS
Plant material, data sources and description of growing conditions
This study focuses on 85 winter wheat cultivars that were registered in the Czech Republic during the period 1981–2009, and were still registered up to 1 January 2010. Their detailed characteristics are available on the website of the Central Institute for Supervising and Testing in Agriculture (CISTA), Brno, Czech Republic (http://www.ukzuz.cz./Folders/2220-1-Plant+Varieties.aspx). The collection of winter wheat cultivars grown in Slovakia included 40 cultivars registered by the Slovak Central Controlling and Testing Institute in Agriculture (CCTIA) since 1976. All cultivars were the highest yielding on the date of registration. The Czech collection includes cultivars bred in eight European countries (31 in the Czech Republic, 31 in Germany, eight in Slovakia, eight in the Netherlands, three in France, two in Austria, one in Denmark and one in the UK). The Slovak collection contained only cultivars of Slovak origin, because they have dominated cultivation up to the present time. Breeders’ S1 seed (Pre-Basic) was used for the analyses of cultivars registered in the Czech and Slovak Republics.
Despite being close geographically, the main wheat-growing areas of the Czech Republic and the Slovak Republic represent different and variable environmental conditions in the middle of Europe. Thus, a different germplasm has been exploited in wheat breeding in these regions. A characteristic feature of Czech wheat breeding and cultivation is the wide use of germplasm from the UK and Western Europe, and previously, to a large extent also from the Ukraine and Russia, as well as the registration of cultivars that come from these regions. In Slovakia, Eastern and South European, germplasm has a higher importance, allowing for an earlier genotype.
The Czech Republic lies in a temperate climatic zone with pleasantly mild summers. The temperature difference between summers and winters is relatively high due to its landlocked geographical position. Alternation between hard and relatively mild winters is quite typical for this region. The climate in Slovakia can be considered similar, but the tendency to a continental type of climate is more marked. The main difference concerns the relatively low proportion of agricultural land situated in maize-growing regions in the Czech Republic (0·07), while it is higher in Slovakia (0·37). The proportions of agricultural land used for cereal, sugar beet, potato and upland fodder crops in the growing regions of the Czech Republic are 0·41, 0·24, 0·18 and 0·10, respectively. The growing of cereals in Slovakia is highly concentrated in the maize and sugar beet regions, occupying 0·59 of the agricultural land in total. The remaining 0·41 of land is situated in the potato and upland regions, where cereal growing is very limited.
Brief characteristics of the main regions used for growing wheat
The maize-growing region typically is at altitudes up to 250 m asl, has an annual average precipitation of 500–600 mm, an annual mean air temperature of 9–10°C and 0·30–0·50 of the year lacks rainfall. The characteristics of the sugar beet growing region are altitude: 250–350 m asl, annual average precipitation: 500–650 mm, annual mean air temperature: 8–9°C, proportion of the year that lacks rainfall: 0·10–0·60. Those of the cereal growing region (Czech Republic) are altitude: 300–600 m asl, annual average precipitation: 550–700 mm, annual mean air temperature: 5–8·5°C, proportion of the year that lacks rainfall: 0·05–0·40 and those of the potato growing region are altitude: 400–650 m asl, annual average precipitation: 550–900 mm, annual mean air temperature: 5–8°C and proportion of the year that lacks rainfall: 0·05–0·30.
The proportion of agricultural land used for growing wheat fluctuates according to the season, but it is, in general, higher in the sugar beet-, maize- and cereal-growing regions (0·29–0·36) than in the potato-growing region (0·23–0·25). Yield potential of the registered wheat cultivars, estimated from the Official Trials of CISTA and CCTIA (2005–09) was 9·32 t/ha, and was highest in the sugar beet region (yield was 10·7% higher than average), whereas it was 3–5% below average in the remaining growing regions.
Characterization of Rht and Ppd genotypes
Responses to exogenously applied GA were determined using the method of Gale & Gregory (Reference Gale and Gregory1977). To distinguish between GA-responsive and GA-insensitive genotypes, the method consisted of a 14-day treatment with 50 ppm GA3. Measurements of shoot length were taken 10 and 14 days after GA treatment and in control (untreated) plants. A genotype was considered to be GA-sensitive when the increase in shoot length between the GA-treated and control plants was statistically significant.
Genomic DNA was extracted from individual plants using commercial DNeasy Plant Mini Kits (Qiagen) following manufacturer's instructions. For detection of both the Rht-B1 and Rht-D1 genes (except for the Rht–D1a allele), located on wheat chromosomes 4B and 4D, respectively, polymerase chain reaction (PCR) conditions and primers were as published by Ellis et al. (Reference Ellis, Spielmeyer, Gale, Rebetzke and Richards2002). To detect the Rht-D1a allele, reaction conditions were: initial denaturation at 94°C for 3 min, followed by 45 cycles (94°C for 1 min, 58°C for 1 min and 72°C for 1 min) and finished at 72°C for 10 min. The PCR products were separated on agarose gels and visualized using ethidium bromide.
Probable allelic variation associated with the Rht8 locus on chromosome 2D was assessed using the microsatellite marker WMS 261 (Korzun et al. Reference Korzun, Röder, Ganal, Worland and Law1998) with slight changes in reaction mixture and conditions. The reaction mixture (total volume of 15 μl) contained: 1·5 μl of 2 mM Deoxyribonucleoside triphosphate (dNTP), 1·5 μl of both 2 μM primers, 1·5 μl of buffer, 0·07 of Taq polymerase and 5 μl of template DNA (20 μg. μl-1). PCR conditions were: 94°C 1 min (94°C for 1 min, 60°C 1 min, 72°C 1 min)×30 cycles and final step at 72°C for 4 min.
PCR products were then separated on 5% polyacrylamide gel (1 litre of stock solution contained 125 ml of 40% acrylamide/bis-acrylamide solution [19:1 ratio], Sigma Aldrich product no. A9926) and visualized by silver staining (Bassam et al. Reference Bassam, Caetano-Anollés and Gresshoff1991). The Xgwm261 allele size was determined by juxtaposition with control cultivars Bezostaya 1 for the 192-bp allele, Mironovskaya 808 for the 174-bp allele, Kosutka for the 165-bp allele. Varieties carrying alleles larger than 192-bp were screened using the ABI PRISM® 310 Genetic Analyser.
Allelic variants at the Ppd-D1 locus on chromosome 2D were also examined. Primers and PCR conditions were used as described in detail by Beales et al. (Reference Beales, Turner, Griffiths, Snape and Laurie2007). A 288-bp product was indicative of the presence of the photoperiod-insensitive Ppd-D1a allele and a 414-bp product of the presence of the photoperiod-sensitive Ppd-D1b allele.
Pedigree analyses
Pedigrees of the registered cultivars were examined using the European Wheat Database (EWDB) held at the Crop Research Institute, Prague (http://www.ecpgr.cgiar.org/databases/crops/wheat.htm, verified 17 August 2010). The data on the presence of the Rht and Ppd alleles examined in different cultivars included in the pedigree diagrams were mainly obtained from the literature (Worland et al. Reference Worland, Korzun, Röder, Ganal and Law1998; Chebotar et al. Reference Chebotar, Korzun and Sivolap2001; Ganeva et al. Reference Ganeva, Korzun, Landjeva, Tsenov and Atanasova2005). The cultivars that still required examination were analysed in the Crop Research Institute, Prague and the Research Institute of Plant Production Piešťany, if the seed was available in the Gene Bank Departments of these institutes.
RESULTS
Gibberellin tests and molecular genotyping for GA-insensitive Rht genes
The results of the gibberellin tests corresponded with the molecular analyses. Screening showed that 54 out of the 85 cultivars registered in the Czech Republic (0·64) were GA responsive and 31 (0·37) were GA insensitive. Among the cultivars registered in the Slovak Republic, the proportions were very similar (0·65 of GA-sensitive cultivars and 0·35 of GA-insensitive cultivars). However, as demonstrated in Table 1, the proportion of Rht-B1b/d and Rht-D1b genotypes differed greatly between the two countries. While in Slovakia only the presence of Rht-B1b or d was detected by the diagnostic molecular marker, in the Czech Republic, the presence of Rht-D1b was dominant (0·84 of GA-insensitive cultivars). From pedigree analyses, the predominant allele at Rht-B1 should be Rht-B1d, because 12 out of 16 GA-insensitive winter wheat cultivars (cvars Agra, Astella, Barbara, Baletka, Danubia, Istra, Regia, Solara, Solaris, Solida, Velta and Venistar) have the cultivar Produttore (descendant of Saitama 27) in their pedigree. In the cultivar Viador, the source of the Rht genes is not clear from pedigree analysis. In Ilona and Iris, the source of the Rht-B1b allele could be Siete Cerros. The widely grown Czech cultivar Rheia, which is high yielding particularly in the maize-growing region, has in its pedigree the cultivar Vlada, in which the phenotyping results showed a significantly longer shoot length than in Siete Cerros, which may indicate the presence of weaker insensitivity characteristic of Rht-B1d.
Table 1. Distribution of winter wheat cultivars grown in the Czech and Slovak Republics according to their reaction to GA (I: presence of genes Rht-B1b or d and Rht-D1b), alleles at the Xgwm261 locus and alleles at the Ppd-D1 locus
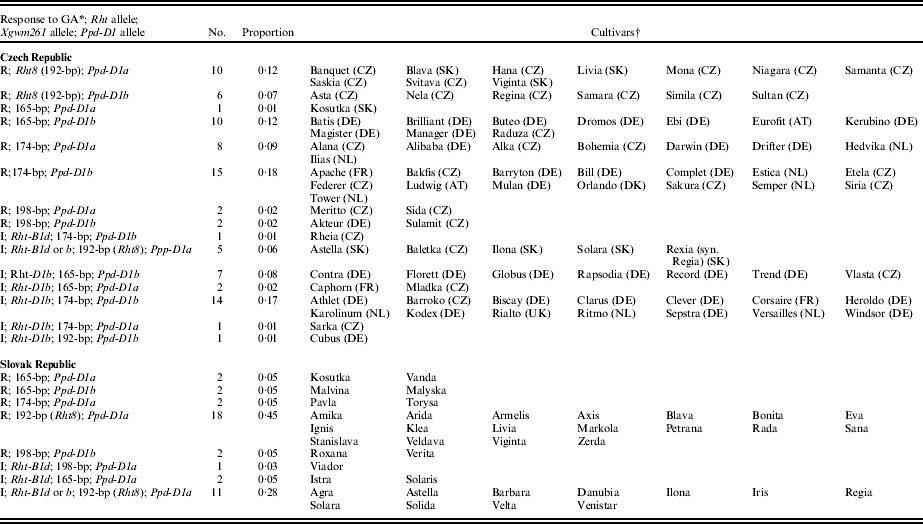
* R, responsive to GA; I, insensitive to GA.
† In brackets – country of origin based on EVIGEZ database (http://genbank.vurv.cz/genetic/resources/); in Slovak Republic all cultivars SK.
It is clear from Table 2 that Rht-D1b cultivars appeared in Czech agriculture at a higher frequency after 1995, and for the whole period 1981–2009, the presence of Rht-B1d or b was the exception. In Slovakia, this allele eventually dominated. The occurrence of Rht-B1d or b genotypes has dropped from being common during the period 1976–94 to a low presence currently.
Table 2. Effect of registration year on the presence of alleles at the Xgwm261 locus, GA-insensitive alleles Rht-B1b or d and Rht-D1b and alleles at the Ppd-D1 locus in the winter wheat cultivars grown in the Czech and Slovak Republics

* S=sensitive to GA.
Distribution of allelic variants at Xgwm261 and Ppd-D1
Among the cultivars examined at the Xgwm261 molecular marker locus associated with Rht8 height allelic variants, four different alleles were detected in both countries: 165, 174, 192 and 198-bp. However, the distribution of these alleles was different in the neighbouring countries. As shown in Tables 1 and 2, the 174-bp allele was the most frequent from 1995 in the Czech Republic, detected in 39 out of 85 cultivars (0·46). In total, 20 cultivars (0·24) were found to carry the 165-bp allele, 22 cultivars (0·26) the 192-bp allele and only four cultivars (0·05) the 198-bp allele. Relative to the year of registration, the proportion of 165-bp allele increased from 0·07 to 0·38. Before 1995, 10 (0·67) cultivars carried the Xgwm261 192-bp allele that could be associated with the Rht8 dwarfing gene, but only three cultivars (0·12) were detected as carrying this allele in the registration period 2005–9. The Xgwm261 192-bp allele was common in cultivars registered in the Slovak Republic (detected in 30 out of 40 cultivars, 0·75) and its occurrence was independent of the year of registration. The remaining alleles occurred at a lower proportion, which ranged from 0·13 (165-bp allele), 0·08 (198-bp allele) to 0·05 (174-bp allele).
In the Slovak Republic, 36 out of 40 cultivars (0·90) were found to carry the allele Ppd-D1a for insensitivity to photoperiod (Table 1). The linkage between the alleles 192-bp (indicative of Rht8) and Ppd-D1a has not been broken in any cultivar. The proportion of Ppd-D1a in cultivars registered in the Czech Republic was 0·34 (in 29 out of 85 cultivars), and 15 out of 29 cultivars (0·52) have this allele associated with the 192-bp allele.
The most frequent allelic combinations among GA responsive genotypes were Xgwm261 174-bp/Ppd-D1b (15 cultivars), 165-bp/Ppd-D1b (10 cultivars) and Xgwm261 192-bp/Ppd-D1a (10, mostly older, cultivars) in the Czech Republic. The GA-insensitive gene Rht-D1b is often combined with alleles 174-bp and Ppd-D1b (14 cultivars, Table 1). In the Slovak Republic, the following allelic combinations were the most frequent: Xgwm261 192-bp /Ppd-D1a (18 cultivars) and Rht-B1b or d/Xgwm261 192-bp (Rht8)/Ppd-D1a (12 cultivars).
Sources of different allelic variants at the Xgwm261, Rht and Ppd-D1 loci in cultivars adapted to target environments
The classification of winter wheat cultivars grown in Middle Europe by the presence of the GA-insensitive dwarfing genes (Rht-B1b, Rht-B1d and Rht D1b) and alleles at the Xgwm261 and Ppd-D1 loci is given in Table 1. Successful cultivars were traced back to find probable sources of Xgwm261 192-bp, 174-bp and 165-bp alleles on 2D and Rht genes on chromosomes 4B and 4D (Figs 1–4).

Fig. 1. Pedigrees of Czech winter wheat cultivars carrying Xgwm261 192-bp allele (lower case, underlined). Bold letters: allele 174-bp. Capital letters: not tested. Dashed lines connecting the cultivars denote probable gene flow from well-known gene sources.

Fig. 2. Pedigrees of Slovak winter wheat cultivars carrying Xgwm261 192-bp allele (lower case, underlined). Bold letters, lower case: allele 174-bp. Italics: GA-insensitive (Rht-B1b or d). Capital letters: not tested. Dashed lines connecting the cultivars denote probable gene flow from well-known gene sources.

Fig. 3. Pedigrees of Czech winter wheat cultivars carrying Xgwm261 174-bp allele (bold lower case). Italics: GA-insensitive (Rht-D1d). Capital letters, underlined: 165-bp allele. Capital letters: not tested. Dashed lines connecting the cultivars denote probable gene flow from well-known gene sources.

Fig. 4. Pedigrees of Czech and adapted foreign winter wheat cultivars carrying Xgwm261 165-bp allele (capitals, underlined). Italics: GA-insensitive (Rht-D1d). Lower case, bold: 174-bp allele. Capital letters: not tested. Dashed lines connecting the cultivars denote probable gene flow from well-known gene sources.
It is obvious from Fig. 1 that the main ‘domestic’ sources of the Xgwm261 192-bp allele, which may be diagnostic for Rht8, were cvars Hana, Viginta and Slavia, registered in the Czech Republic in 1985, 1984 and 1976, respectively. All these cultivars occupied large areas and were widely exploited in the Czech and Slovak wheat-breeding programmes. The older Russian cultivars (especially Mironovskaya 808) evidently contributed the high levels of winter-hardiness and bread-making quality in these cultivars. The Ukrainian cultivars (Bezostaya 1 and Ilichevka) were evidently donors of the 192-bp Rht8-associated allele for all these cultivars (directly for cvars Viginta and Slavia and probably through the Serbian line NS-984-1 for Hana). It is generally known that the cultivar Hana, which also possesses Ppd-D1a, was widely and successfully used as a donor of improved bread-making quality and earliness. Figure 2 also shows that the second important transfer of the Akakomughi Rht8 gene (see pedigree of the Slovak cultivar Ilona) could also have happened through the Mexican cultivar Siete Cerros, in which, however, the origin of the Xgwm261 192-bp allele could not be ascertained by pedigree analysis. The alternative route for this allele to Ilona can be via the Russian cultivar Kavkaz (derived from Bezostaya 1). It is evident from Table 1 that in the Czech cultivars Asta, Nela, Samara, Simila and Sultan, the 192-bp (Rht8)/Ppd-D1a linkage block was broken, as in the current GA-insensitive German cultivar Cubus, and the famous older cultivar Regina, which was widely grown particularly in the potato growing region (the source of Rht8 gene in cvars Sultan, Cubus and Regina) was not found. As shown in Table 2, the presence of the Xgwm261 192-bp (Rht8) in modern cultivars grown in the Czech Republic is very rare (since 2005, it is only found in cvars Simila, Baletka and Sultan), but it is still frequent in Slovakia, where the 192-bp (Rht8)/Ppd-D1a linkage has not yet been broken. In Slovak cultivars, the 192-bp (Rht8) genotype was often combined with Rht-B1d or Rht-B1b (possible pathways can be found in Fig. 2). The probable donor of the Rht-B1d allele from cvar Saitama 27 to modern Slovak cultivars could be the line SO 985, derived from the cross between the cultivars Nebojska and Produttore. The presence of Norin 10 dwarfing genes has not been detected in any Rht8 cultivar bred in the Czech Republic.
As seen in Tables 2 and 3, the 174-bp allele of the Xgwm261 locus became highly prevalent in the Czech wheat-growing regions after 1995, and Ppd-D1b at the expense of Ppd-D1a. This can be connected to the introduction of UK and West European high-yielding cultivars that also often carry Rht-D1b. As seen in Fig. 3, cvar Capelle–Desprez probably was the source of the 174-bp allele for the modern Czech cultivars Sakura and Bohemia, through cvar Maris Huntsman and subsequently cvars Virtue, Mercia and Estica. Cultivar Maris Huntsman also can be considered as the probable source of the 174-bp allele for the successful cultivars of foreign origin such as Darwin, Drifter, Complet, Biscay, Mulan and Ritmo (see http://genbank.vurv.cz/ewdb, verified 17 August 2010). The cultivars Heines VII, Clement and Apollo could be sources of the 174-bp allele for the very high-yielding modern cultivar Etela (bred by the Limagrain Central Europe Cereals, Ltd, Plant Select, Hrubčice, CZ). The proportion of Rht-D1b and Rht-D1a cultivars carrying the 174-bp and Ppd-D1b alleles was almost identical (0·17 and 0·18). However, most of the registered GA-insensitive cultivars are foreign, mainly west European cultivars. The short, early and lodging-resistant Czech cultivar Sarka appears to be highly adapted to these climatic conditions and carries Rht-D1b (from the British cultivar Avalon) in combination with Ppd-D1a. Although sources of Rht-D1b (British cultivars Virtue and Mercia) are involved in the pedigrees of modern Czech cultivars, the selection for greater plant height, earliness, high grain quality and winter hardiness probably resulted in the retention of non-Rht-D1b genotypes. To obtain the necessary earliness, the presence of Ppd-D1a was evidently beneficial in nine cultivars, and among them Alana, Bohemia and the Dutch cultivar Ilias were widely exploited in agricultural practice.
Table 3. Distribution of Rht and Ppd alleles among commercial winter wheats in the Czech and Slovak Republics; area of seed multiplication in 2007
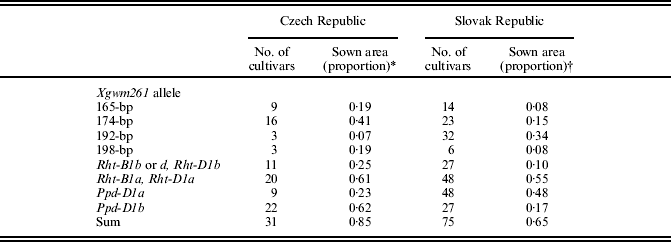
* Included only registered cultivars with sown area >0·01.
† 0·35 of area is occupied by Slovak and foreign cultivars in which the allelic composition was not yet identified.
It is obvious from Fig. 4 that the key source of the Xgwm261 165-bp allele appears to be the Canadian spring wheat cultivar Marquis, released in 1911 and widely exploited by spring wheat breeders in the USA and Canada. The route for the spread of the 165-bp allele to and around Europe was evidently through the North American cultivars Thatcher, Marquillo and probably also Reward (parent of the Slovak landrace Nebojska). The presence of this allele can be traced back to Marquis and alternatively also to the Italian cultivar Mentana, selected by Strampelli (Strampelli Reference Strampelli1932). From cvar Mentana this allele was transferred to the Brazilian cultivar Frontana and to semi-dwarf CIMMYT cultivars. However, no clear route for the 165-bp allele from the well-known Mexican sources such as cvar Ciano 67 was not found in any 165-bp genotype examined; however, the UK cultivars Rapsodia and Caphorn have Ciano 67 in their pedigree. There are no data available on the presence of this allele in the Mexican cultivar Yaktana 64, derived from Frontana, which is a parent of the old awned Slovak cultivar Kosutka, widely grown particularly in warm, dry regions. Among the GA-responsive cultivars, the German cultivars Ebi and Batis were widely exploited in agriculture. Probable donors of the 165-bp allele in this genotype group were the German cultivars Urban and Diplomat that have cvar Marquis in their pedigrees. Another important branch is GA-insensitive cultivars, in which sources of Rht-D1b were the British cultivars Maris Marksman, Norman, Haven, Brimstone, PBIS 95-82 and CWW 4442. Many of these cultivars were also successful in agriculture, mainly the Czech cultivar Mladka that showed broad adaptability (very high yields in all regions in non-stressed conditions of the years 2000, 2001 and 2002), the German cultivars Rapsodia, Florett and Globus, and the Czech cultivar Vlasta suitable for growing in the potato region. The combination of the ‘Mexican’ 165-bp allele with Ppd-D1a occurred in three cultivars and the numbers of Rht-D1a and Rht-D1b genotypes carrying Ppd-1Db were 10 and 7, respectively (Table 1).
DISCUSSION
These results suggest that selection and adaptation to particular environments in Middle Europe are reflected by the presence of particular allelic combinations of Rht and flowering time loci. In this adaptation spectrum, there are common and also distinct features relating to the breeding history and the evolution of foreign cultivars particularly in two neighbouring countries, the Czech Republic and Slovakia. These correspond to the climatic diversity of the middle European regions (the predominant wheat growing area and main breeding centre is situated in the maize region in Slovakia, while in the Czech Republic it is found in cereal, sugar beet and maize regions), and to a certain degree with specific historical developments. The breeding objectives did not differ very much, but the significance of the ‘border position’ of the Czech Republic between the continental and maritime types of climate evidently played an important role. The breeders soon became aware of the usefulness of combining properties of west and east European germplasm in order to obtain broader adaptation and good bread-making quality (Šíp et al. Reference Šíp, Amler, Bobková and Škorpík1991). Pedigree analyses (Figs 1–4) also show that in the Czech Republic the cultivated modern winter wheat cultivars are derivatives of geographically diverse germplasm, and also that the allelic combinations are more variable than in the cultivars grown in the Slovak Republic. The genetic diversity studies of Martynov et al. (Reference Martynov, Dobrotvorskaya, Stehno and Dotlačil1997) showed that the majority of Czech and Slovak cultivars released in the period 1954–94 were related because they had common ancestors (35 of 62 cultivars analysed were found to be descendants of Canadian cultivars Marquis or Garnet). Martynov et al. (Reference Martynov, Dobrotvorskaya, Stehno and Dotlačil1997) also documented that in the two decades before 1994, the ratio of ‘local germplasm’ in the pedigrees of ‘modern’ cultivars has decreased substantially, and that the Ukrainian cultivars Mironovskaya 808 and Bezostaya 1 had the largest contribution (0·80). Cultivars containing Xgwm261 192-bp (Rht8) and Ppd-D1a came to dominate in both countries (in Slovakia often in combination with Rht-B1d or Rht-B1b). However, while these genotypes are still highly prevalent in Slovakia, a decline of cultivars containing Xgwm261 192-bp (Rht8) and Ppd-D1a has occurred since 1995 in the Czech Republic (Table 3), and, moreover, selection performed in relatively colder districts has led to genotypes that break the 192-bp (Rht8)/Ppd-D1a linkage block, unlike in Slovakia. However, it should be noted that the breakage of this linkage need not necessarily concern the association between Rht8 and Ppd-D1a, because, as documented by Ellis et al. (Reference Ellis, Bonnett and Rebetzke2007), we cannot preclude a lack of association of the 192-bp allele with Rht8. The wide use of foreign winter wheat cultivars, especially from the UK, Germany, France and the Netherlands, has contributed to an increase in genetic diversity in winter wheat cultivars grown in the Czech Republic, as documented by Stehno et al. (Reference Stehno, Dotlačil, Faberová, Martynov and Dobrotvorskaya2003). Recently, the frequent registration of foreign cultivars is also a characteristic of the Slovak Republic system. Among winter wheats registered in Slovakia in 2007, 0·09 of the cultivars are of Czech or west European origin (registered also in the Czech Republic), although not yet cultivated on a large area. Efforts to broaden the genetic base and increase the availability of different growth types are now characteristics of both countries.
In Slovakia, there was a very low frequency of the Xgwm261 174-bp allele, the most frequent in the Czech Republic. While the routes of the Xgwm261 192-bp and 174-bp alleles are more easily identifiable, an inspection into the sources of the third important allele, 165-bp, is more complicated and often not clear. These investigations indicate that the main, obvious, donors of this allele were the Canadian cultivar Marquis and the Brazilian cultivar Frontana (derived from the Strampelli cultivar Mentana), mainly via the German cultivars Urban and Kronjuwel (derived from the US cultivar Thatcher). No clear paths, however, lead to the Mexican spring wheat cultivars, except for the Slovak cultivar Iris, coming from the cross between cvars Siete Cerros and Kavkaz. The GA-insensitive dwarfing gene Rht-D1b was not introduced to the UK and the other western European cultivars from CIMMYT germplasm, but via Chile. The possible bridge could be the US cultivar Vogel-8058. Alternatively, as documented by Šíp et al. (Reference Šíp, Škorpík and Chrpová1995), the CIMMYT cultivars were widely exploited in Czech breeding programmes for the development of spring wheat cultivars carrying Rht-B1b gene. In winter wheat, there is little evidence of wide exploitation of the Mexican sources of either Norin 10 genes or the 165-bp allele of the Xgwm261 locus.
There is a striking difference between these two countries in the use of GA-insensitive Rht genes located on chromosomes 4B and 4D. Only two cultivars bred in the Czech Republic (Rheia and Baletka) appear to carry the Rht-B1d Saitama 27 allele, and both cultivars were high yielding, particularly in the maize-growing region (Bobková et al. Reference Bobková, Šíp and Škorpík2002; Laml & Pánek Reference Laml and Pánek2008). In the warmer conditions of neighbouring Slovakia, the Rht-D1b allele was not detected and the majority of GA-insensitive genotypes probably carry Rht-B1d. These findings support the usefulness of exploiting the Rht-B1d allele in the conditions of Southern Europe, often in combination with Rht8 and Ppd-D1a (Worland & Petrovic Reference Worland and Petrovic1988; Worland et al. Reference Worland, Sayers and Korzun2001). It has been stated that the Rht-D1b genotypes do not perform well in warmer areas, which is consistent with these and other results (Šíp et al. Reference Šíp, Chrpová, Žofajová, Pánková, Užík and Snape2010). The effects of the Norin 10 dwarfing genes can be considered rather similar (Gale & Youssefian, Reference Gale, Youssefian and Russell1985) and dependent on genetic background. Ellis et al. (Reference Ellis, Spielmeyer, Gale, Rebetzke and Richards2002) found that the Rht-D1b allele accounted for a larger proportion of phenotypic variance in plant height than Rht-B1b. The high preference for the Rht-D1b allele in the conditions of the Czech Republic (western Europe) can be explained by the germplasm used for crossing and by the better conditions for the desirable selection of ‘tall-dwarfs’ (Šíp et al. Reference Šíp, Amler, Bobková, Škorpík, Miller and Koebner1988).
Undoubtedly, the most important indication of the value of a cultivar is its area grown. It is generally known that not only yielding ability but also grain quality and resistance to the important biotic and abiotic stresses are decisive for cultivar registration and spread. Table 3 shows that 0·85 of the wheat acreage in the Czech Republic is occupied by cultivars with an identified composition of alleles, a relatively higher acreage is now sown to wheat cultivars possessing the alleles 174-bp (0·41) and Ppd-D1b (0·62), and that are responsive to GA (0·61). A low proportion of the sown area at present is occupied by the carriers of the 192-bp allele (0·07). It is interesting that three out of four cultivars carrying the rare 198-bp allele (which cannot be separated accurately from the central European 197-bp allele identified by Worland et al. Reference Worland, Sayers and Korzun2001) are now on 0·19 of the area (the German cultivar Akteur on 0·11 and the Czech cultivars Meritto and Sulamit on the rest). This allele can be considered as height promoting, because the average plant height of these cultivars was 0·99 m, while it was 0·92, 0·91 and 0·91 m for the alleles 174, 165 and 192-bp, respectively (Šíp et al. Reference Šíp, Chrpová, Žofajová, Pánková, Užík and Snape2010). On the other hand, in Slovakia, (Rht8)192-bp and Ppd-D1a cultivars are still widely grown (on more than half the area), but at present a relatively small area is sown to GA-insensitive genotypes (0·10). GA responsiveness and the presence of (Rht8)192-bp and Ppd-D1a are characteristics of the most widespread modern Slovak cultivars Armelis, Axis and Bonita, and probably also Alacris, coming from the cross between Hana and Zerda.
A decline in the utilization of Rht-B1b and Rht-B1d in winter wheat breeding, as well as the absence of Rht-D1b in Slovak cultivars and a relatively low frequency of this GA-insensitive gene in modern cultivars of Czech origin, can, to a certain extent, be ascribed to problems with grain quality and winter hardiness (Šíp et al. Reference Šíp, Chrpová, Žofajová, Pánková, Užík and Snape2010) that probably still exist. It has been demonstrated with spring wheat Rht isolines (Chapman et al. Reference Chapman, Mathews, Trethowan and Singh2007) that over a large sample of environments tall isolines may yield the same or better than short lines, because in recent genetic backgrounds the tall isolines are shorter than their predecessors as a consequence of direct or indirect selection for several minor genes for reduced height (Trethowan et al. Reference Trethowan, Singh, Huerta-Espino, Crossa and van Ginkel2001). In winter wheat, the requirements for obtaining good bread-making quality and a high winter-hardiness level in combination with high and stable grain yield can probably be achieved better with GA responsiveness than GA insensitivity. This is because the GA-insensitive dwarfing genes Rht-B1b and Rht-D1b are environmentally sensitive (Worland et al. Reference Worland, Sayers and Korzun2001), and there may be also problems with plant establishment, due to a shortening of coleoptile length, and relatively lower initial growth performance (Rebetzke et al. Reference Rebetzke, Bruce and Kirkegaard2005; Richards et al. Reference Richards, Watt and Rebetzke2007; Tambussi et al. Reference Tambussi, Bort and Araus2007). A preference for selecting GA-sensitive genotypes can be explained by desirable positive effects on cell size (greater leaf area development (LAD)) and high early vigour, opening new avenues for increasing water use efficiency (Botwright et al. Reference Botwright, Rebetzke, Condon and Richards2005; Tambussi et al. Reference Tambussi, Bort and Araus2007). According to recent findings (Holzapfel et al. Reference Holzapfel, Voss, Miedaner, Korzun, Häberle, Schweizer, Mohler, Zimmermann and Hartl2008), a decline in the utilization of Rht-D1b can also be attributable to greater Fusarium head blight susceptibility.
Figure 5 shows the changes in average plant height and ear emergence in the four time periods. It is evident that there was a substantial decline in plant height around 2000 then, more recently, a rapid increase. In comparison with older cultivars, the currently used cultivars are later in heading, although some earlier types are still present. Preferential selection for taller and later flowering cultivars could be justified by better establishment under variable sowing conditions, a deeper rooting system, and a longer period for grain yield formation (higher LAD), resulting in better yielding ability and adaptability to different conditions. The comparison of Ppd-D1a and Ppd-D1b NILs by Foulkes et al. (Reference Foulkes, Sylvester-Bradley, Worland and Snape2004) showed that the later flowering Ppd-D1b genotypes appeared to be able to extract more soil water during the season and largely maintain water uptake during grain filling under late drought compared to Ppd-D1a genotypes. It is assumed that the extended period to flowering may favour the development of a larger, more extensive, root system. This property is improved by Ppd-D1b, and could be more advantageous than early flowering and drought escape, a strategy adopted in southern European countries. However, the preference for Ppd-D1a is characteristic of a very successful breeding programme at Úhřetice, which is generally oriented towards the development of relatively taller cultivars than the other programmes in the Czech and Slovak Republics. The widespread use of the cultivars Alana, Alka, Mladka, Meritto and Bohemia, carrying Ppd-D1a mostly in combination with the Xgwm261 allele 174-bp, shows that earlier cultivars can be advantageous for these conditions, provided plants are taller and their root system is highly developed. A widely grown tall (1·10 m) and early bread wheat cultivar Bohemia (Bobková & Hromádko Reference Bobková and Hromádko2008) can indicate a possible future preference for Ppd-D1a and alleles contributing to greater plant height, provided the culm is stiff and lodging resistance is also achievable. Satisfactory stem strength characteristics are, therefore, highly desirable. Addisu et al. (Reference Addisu, Snape, Simmonds and Gooding2010) found that the presence of Ppd-D1a was beneficial for early resource capture (early season growth), which is highly desirable under organic growing systems. Reynolds et al. (Reference Reynolds, Calderini, Condon and Vargas2007) demonstrated a recent move from increased dry matter partitioning to grain towards increases of above-ground biomass by improving radiation use efficiency. Improving the balance between the source and sink is, according to those authors, currently the most promising approach for increasing yield, biomass and radiation use efficiency.

Fig. 5. Changes in mean plant height and ear emergence in four periods of cultivar release (1: 1981–94; 2: 1995–99; 3: 2000–04; 4: 2005–09).
It can be implied from the recent wide exploitation of foreign, especially German, cultivars that broader adaptation and high yield potential are the most desirable properties. Šíp et al. (Reference Šíp, Chrpová, Žofajová, Pánková, Užík and Snape2010) demonstrated that broader adaptability to different growing conditions in a genotype can be created by combining Xgwm261 alleles 174, 165 or 198-bp with Ppd-D1b. Indeed, obtaining broader adaptation in a genotype is undoubtedly becoming more and more important in all European regions owing to the relatively greater variation in weather conditions between years rather than between regions. However, there is danger that these genotypes will not be able to exploit certain growing conditions most effectively. Therefore, as stated by Reynolds & Borlaug (Reference Reynolds and Borlaug2006), a more complete knowledge of target environments is strategically useful in terms of determining which new traits and genes may be useful and the appropriate allocation of resources. Finding an optimum plant type (ideotype) is undoubtedly the most important starting point and orientation to certain allelic combinations can be considered as more strategically promising than seeking improvement (historical patterns) in single yield components (Sener et al. Reference Sener, Arslan, Soysal and Erayman2009).
In conclusion, the environments under study, in which different European wheat cultivars were exploited, can be considered as representative of the dominant wheat growing areas in Europe, and it can be expected that the results obtained will have broader applicability, especially for appropriate use of certain Rht and Ppd allelic combinations and modelling the optimum plant type for certain growing conditions. Monitoring of evolutionary changes during a 30-year period helped to identify the progressive decline and increase in frequency of certain alleles and to identify allelic combinations that may be indicative of improved adaptability to different growing conditions. According to these results, a steady decline in utilization of the GA-insensitive semi-dwarfing genes and a preference for the Xgwm261 alleles different from 192-bp can be expected in the future. The presence of the allele 192-bp (Rht8) can be recommended only in warmer (maize) growing regions. Improved properties (broader adaptation, suitability for organic and low-input agriculture) can be seen in the combination of the Ppd-D1a allele with height neutral or height-promoting Xgwm261 alleles. However, genetic studies of lodging resistance in these plant types are highly desirable. Broad adaptation under variable growing conditions of Central Europe is more likely achievable with the most frequent combination of Ppd-D1b and 174-bp Xgwm261 allele. Useful sources of these alleles can be found in pedigree schemes that are presented in this paper. In general, this study can help in optimizing the breeding strategy and detect regions where certain cultivar types can perform well, because it is undoubtedly highly desirable to find the best use for the different arrays of germplasm produced in international breeding programmes.
This research was supported by the Ministry of Agriculture of the Czech Republic, project No. 0002700604.










