Introduction
Schizophrenia is a chronic disorder, of variable clinical characteristics and outcome, reflected in the heterogeneous response to antipsychotic medication. Approximately 50–70% of patients with their first episode of schizophrenia (FES) will respond to the first antipsychotic medication prescribed, this figure falling to 20% for those who require a trial of a second (Agid et al., Reference Agid, Arenovich, Sajeev, Zipursky, Kapur, Foussias and Remington2011). Antipsychotic medication (excluding clozapine) has its greatest effect within the first 2 weeks and thereafter the improvements are more marginal (Agid et al., Reference Agid, Kapur, Arenovich and Zipursky2003). Despite the expansion in our therapeutic armamentarium over the past decades, up to one-third of patients do not respond to non-clozapine antipsychotics (Wimberley et al., Reference Wimberley, Støvring, Sørensen, Horsdal, MacCabe and Gasse2016; Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a) and are described as having treatment resistant schizophrenia (TRS).
Defining treatment resistance
The concept of TRS first appeared in the literature in the mid-1960s (Itil et al., Reference Itil, Keskiner and Fink1966), but definitions remained inconsistent, rendering the literature difficult to interpret. A recent systematic review of randomised controlled trials (RCTs) in TRS identified 42 studies; of these half did not define what they meant by treatment resistance and only two of the 42 studies used the same criteria (Howes et al., Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren, Birnbaum, Bloomfield, Bressan, Buchanan, Carpenter, Castle, Citrome, Daskalakis, Davidson, Drake, Dursun, Ebdrup, Elkis, Falkai, Fleischacker, Gadelha, Gaughran, Glenthoj, Graff-Guerrero, Hallak, Honer, Kennedy, Kinon, Lawrie, Lee, Leweke, MacCabe, McNabb, Meltzer, Moller, Nakajima, Pantelis, Reis Marques, Remington, Rossell, Russell, Siu, Suzuki, Sommer, Taylor, Thomas, Ucok, Umbricht, Walters, Kane and Correll2017). International consensus guidelines on treatment resistance (and response) in schizophrenia were therefore developed by the Treatment Response and Resistance in Psychosis (TRRIP) group (Howes et al., Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren, Birnbaum, Bloomfield, Bressan, Buchanan, Carpenter, Castle, Citrome, Daskalakis, Davidson, Drake, Dursun, Ebdrup, Elkis, Falkai, Fleischacker, Gadelha, Gaughran, Glenthoj, Graff-Guerrero, Hallak, Honer, Kennedy, Kinon, Lawrie, Lee, Leweke, MacCabe, McNabb, Meltzer, Moller, Nakajima, Pantelis, Reis Marques, Remington, Rossell, Russell, Siu, Suzuki, Sommer, Taylor, Thomas, Ucok, Umbricht, Walters, Kane and Correll2017) in an attempt to construct a unified definition of TRS. According to these guidelines, the following defines TRS: the presence of persistent significant symptoms in a person with a diagnosis of schizophrenia, who has not had a response to at least two antipsychotic trials of adequate dose, duration and adherence. In defining adequate treatment, the guidelines follow the recommendation of the National Institute of Clinical Excellence (NICE) and indicate that each antipsychotic treatment last for at least 6 weeks, with each drug administered at an ‘adequate’ therapeutic dosage (NICE., 2014), equivalent to the minimum effective dose/target dosage (or the midpoint of the target range as specified in the product summary characteristics) – or to a daily dose equivalent to 600 mg of chlorpromazine (Leucht et al., Reference Leucht, Samara, Heres, Patel, Furukawa, Cipriani, Geddes and Davis2015b; Leucht et al., Reference Leucht, Samara, Heres and Davis2016). In effect, this means a minimum duration of antipsychotic treatment of 12 weeks is required before treatment resistance can be diagnosed.
In recognition of the possibility that unrecognised treatment non-adherence may mimic TRS, the guidelines recommend that at least one treatment episode utilise a long-acting injection antipsychotic formulation (depot) for at least 4 months before diagnosing treatment resistance. Alternatively, the use of plasma antipsychotic concentrations can be informative. Although not routinely used in clinical practice, a growing range of second-generation antipsychotics have suggested therapeutic ranges (minimum target threshold: amisulpride 200 μg/l, aripiprazole 150 μg/l, olanzapine 20 μg/l, quetiapine 100 μg/l and risperidone 20 μg/l (total risperidone and 9-hydroxyrisperidone) (McCutcheon et al., Reference McCutcheon, Beck, Bloomfield, Marques, Rogdaki and Howes2015), though in Ireland, samples need to be processed at UK laboratories. A recent observational study of 99 people referred to a TRS service identified that 35% of antipsychotic plasma concentrations were sub-therapeutic, and of these, a third were undetectable (McCutcheon et al., Reference McCutcheon, Beck, D’Ambrosio, Donocik, Gobjila, Jauhar, Kaar, Pillinger, Reis Marques, Rogdaki and Howes2018).
Similarly, it is important not to conflate treatment non-adherence due to intolerability with treatment non-response and resistance.
Factors to consider in differentiating TRS from treatment non-response due to other causes are shown in Box 1.
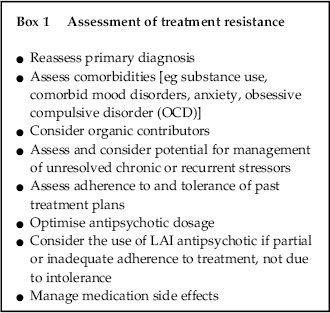
Box 1 Assessment of treatment resistance
Epidemiology of TRS
Schizophrenia has a relatively low incidence (approx. 15.2/100 000), and a lifetime prevalence of approximately 7/1000 (McGrath et al., Reference McGrath, Saha, Chant and Welham2008; Moreno-Kustner et al., Reference Moreno-Kustner, Martin and Pastor2018). TRS is highly disabling and affects approximately 20–30% of those diagnosed with schizophrenia (Wimberley et al., Reference Wimberley, Støvring, Sørensen, Horsdal, MacCabe and Gasse2016; Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a, Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017). In Ireland with a population of approximately 5 million, given that the lifetime risk for schizophrenia is 0.7%, there will be approximately 35–40 000 people with schizophrenia. A conservative estimate is that 20% (Bachmann et al., Reference Bachmann, Aagaard, Bernardo, Brandt, Cartabia, Clavenna, Coma Fuste, Furu, Garuoliene, Hoffmann, Hollingworth, Huybrechts, Kalverdijk, Kawakami, Kieler, Kinoshita, Lopez, Machado-Alba, Machado-Duque, Mahesri, Nishtala, Piovani, Reutfors, Saastamoinen, Sato, Schuiling-Veninga, Shyu, Siskind, Skurtveit, Verdoux, Wang, Zara Yahni, Zoega and Taylor2017) of those (i.e. 7000–8000) will meet the criteria for TRS. However, there is little contemporary epidemiological data on psychotic disorders in Ireland.
Recovery and outcome in schizophrenia
Antipsychotic treatment failure and intolerability come with a high clinical and economic cost (Kennedy et al., Reference Kennedy, Altar, Taylor, Degtiar and Hornberger2014). Our systematic review and meta-analysis of remission (defined as an improvement in symptoms ± a specified duration criteria (e.g. >6 months) for persistence of mild or absent symptoms) and recovery (defined as sustained improvement in both clinical and functioning domains ± a duration of sustained improvement for ≥1 year) in 5000 people with FES, found a recovery rate of 30% (95% CI=19.7–43.6., N=12 studies) at 5 years follow-up, with 56.0% (95% CI=47.5–64.1, N=25 studies) meeting criteria for remission at 7.5 years follow-up (Lally et al., Reference Lally, Ajnakina, Stubbs, Cullinane, Murphy, Gaughran and Murray2017a). Remission and recovery rates may be overestimated with shorter duration of follow-up, but our average length of follow-up was 5 and 7.5 years, respectively, and we did not identify that recovery rates decreased during periods of follow-up longer than 2 years.
This study highlighted a better long-term prognosis in FES, and a more positive outlook for people diagnosed with schizophrenia than previously suggested, given that a 2013 review of outcomes in FES and multi-episode schizophrenia estimated that only one in seven patients attain a functional recovery (Jaaskelainen et al., Reference Jaaskelainen, Juola, Hirvonen, McGrath, Saha, Isohanni, Veijola and Miettunen2013). Estimates of the prevalence of TRS derived from clinical samples should be interpreted with this in mind; the prevalence of TRS is likely to be overestimated in most studies as patients with early remission and recovery may not be included.
Although waiting until a second antipsychotic trial fails before defining a treatment resistant course of illness may seem arbitrary at first glance, this is supported by evidence indicating that the response rate drops precipitously after successive failed trials of medication. Approximately 70% of FEP patients remit on their first anti-psychotic, (Agid et al., Reference Agid, Arenovich, Sajeev, Zipursky, Kapur, Foussias and Remington2011) but after the second drug, the response rate drops to less than 5% (Kane et al., Reference Kane, Honigfeld, Singer and Meltzer1988). With early use of clozapine, a response of 60–70% can be achieved in TRS with improvement observed up to a year after initiation (Meltzer, Reference Meltzer1992). Findings from OPTiMiSE (‘Optimisation of Treatment and Management of Schizophrenia in Europe’), a large scale FES study investigating the benefits of antipsychotic switching in patients not achieving remission on first-line amisulpride, indicate that clozapine is effective in substantially reducing psychotic symptoms after 12 weeks of use, when introduced as a second- or third-line treatment (Kahn et al., Reference Kahn, Winter van Rossum, Leucht, McGuire, Lewis, Leboyer, Arango, Dazzan, Drake, Heres, Díaz-Caneja, Rujescu, Weiser, Galderisi, Glenthøj, Eijkemans, Fleischhacker, Kapur, Sommer, Kahn, Sommer, Winter-van Rossum, Somers, Ywema, Kapur, McGuire, Leboyer, Meyer-Lindenberg, Lewis, Leucht, Arango, Fleischhacker, Meijering, Petter, Van de Brug, Schotsman, Zwerver, Peuskens, De Hert, Thys, Hranov, Hranov, Libiger, Köhler, Mohr, Glenthoj, Broberg, Düring, Baandrup, Jamain, Heres, Rujescu, Giegling, Weiser, Bar Heim, Davidson, Galderisi, Bucci, Mucci, Rybakowski, Remlinger-Molenda, Gonen, Radu, Díaz-Marsá, Rodriguez, Palomo, Rodriguez-Jimenez, García-Portilla, Bernardo, Bobes, Vilares Oliveira, Berger, Wildt, Dazzan, Perez-Iglesias, Drake, Gregory, Wilson, Díaz-Caneja and Eijkemans2018).
Clinical management of TRS
Clozapine is the only evidence-based effective treatment for TRS, as reflected in international guidelines (Nielsen et al., Reference Nielsen, Young, Ifteni, Kishimoto, Xiang, Schulte, Correll and Taylor2016), with reported clinical response in 60–70% of patients (Meltzer, Reference Meltzer1992; Agid et al., Reference Agid, Arenovich, Sajeev, Zipursky, Kapur, Foussias and Remington2011) and meta-analyses identifying an overall response rate of 40–60% (Chakos et al., Reference Chakos, Lieberman, Hoffman, Bradford and Sheitman2001; Siskind et al., Reference Siskind, Siskind and Kisely2017).
In naturalistic settings compared to no antipsychotic treatment, clozapine is associated with decreased rehospitalisation (Nielsen et al., Reference Nielsen, Nielsen and Correll2012; Stroup et al., Reference Stroup, Gerhard, Crystal, Huang and Olfson2016; Kirwan et al., Reference Kirwan, O’Connor, Sharma and McDonald2017; Taipale et al., Reference Taipale, Mehtala, Tanskanen and Tiihonen2017) and reduced hospitalisation and risk of relapse (Tiihonen et al., Reference Tiihonen, Mittendorfer-Rutz, Majak, Mehtala, Hoti, Jedenius, Enkusson, Leval, Sermon, Tanskanen and Taipale2017). Its use is associated with reductions in comorbid substance use (Brunette et al., Reference Brunette, Drake, Xie, McHugo and Green2006), hostility and aggression (Krakowski et al., Reference Krakowski, Czobor, Citrome, Bark and Cooper2006; Frogley et al., Reference Frogley, Taylor, Dickens and Picchioni2012).
Clozapine use is also associated with lower all-cause mortality (Tiihonen et al., Reference Tiihonen, Lonnqvist, Wahlbeck, Klaukka, Niskanen, Tanskanen and Haukka2009; Hayes et al., Reference Hayes, Downs, Chang, Jackson, Shetty, Broadbent, Hotopf and Stewart2015), completed suicide (Meltzer et al., Reference Meltzer, Alphs, Green, Altamura, Anand, Bertoldi, Bourgeois, Chouinard, Islam, Kane, Krishnan, Lindenmayer and Potkin2003; Ringback Weitoft et al., Reference Ringback Weitoft, Berglund, Lindstrom, Nilsson, Salmi and Rosen2014) and self-harm (Ringback Weitoft et al., Reference Ringback Weitoft, Berglund, Lindstrom, Nilsson, Salmi and Rosen2014; Wimberley et al., Reference Wimberley, MacCabe, Laursen, Sorensen, Astrup, Horsdal, Gasse and Stovring2017). An important meta-analysis identified that those continuously treated with clozapine had lower all-cause mortality over a 7-year follow-up compared to those continuously treated with other antipsychotics (Vermeulen et al., Reference Vermeulen, van Rooijen, van de Kerkhof, Sutterland, Correll and de Haan2018). This allies to previous work showing that most major side effects with clozapine can be managed without a need for discontinuation (Nielsen et al., Reference Nielsen, Correll, Manu and Kane2013), and that in certain situations clozapine rechallenge can be successful (Manu et al., Reference Manu, Sarpal, Muir, Kane and Correll2012; Lally et al., Reference Lally, Malik, Krivoy, Whiskey, Taylor, Gaughran, Flanagan, Mijovic and MacCabe2017b, Lally et al., Reference Lally, Al Kalbani, Krivoy, Murphy, Gaughran and MacCabe2018), indicates that concerns regarding the detrimental effect of clozapine on longer term mortality compared to other antipsychotics may be overestimated.
When to use clozapine
In a longitudinal study of 246 people with FES, 34% met the criteria for treatment resistance over a 5 year follow-up period (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a), of whom 70%, 23% of the total study population, were treatment resistant from illness onset. This raises the possibility that TRS may be a distinctive and homogenous schizophrenia subgroup, in line with the biological differences seen between treatment resistant and treatment responsive schizophrenia (Demjaha et al., Reference Demjaha, Egerton, Murray, Kapur, Howes, Stone and McGuire2014).
The question of staging and early recognition of treatment resistance in people with schizophrenia is of utmost importance. Recent longitudinal data indicate that earlier use of clozapine and fewer pre-clozapine antipsychotic trials are associated with better treatment outcomes for people with TRS (Ucok et al., Reference Ucok, Cikrikcili, Karabulut, Salaj, Ozturk, Tabak and Durak2015). A retrospective analysis from Japan identified a critical time window of 2.8 years after illness onset, subsequent to which clozapine response was poorer (response rates of 82% v. 32%) (Yoshimura et al., Reference Yoshimura, Yada, So, Takaki and Yamada2017). Emerging evidence to suggest additional benefits with earlier use of clozapine exists (Agid et al., Reference Agid, Arenovich, Sajeev, Zipursky, Kapur, Foussias and Remington2011; Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a, Kahn et al., Reference Kahn, Winter van Rossum, Leucht, McGuire, Lewis, Leboyer, Arango, Dazzan, Drake, Heres, Díaz-Caneja, Rujescu, Weiser, Galderisi, Glenthøj, Eijkemans, Fleischhacker, Kapur, Sommer, Kahn, Sommer, Winter-van Rossum, Somers, Ywema, Kapur, McGuire, Leboyer, Meyer-Lindenberg, Lewis, Leucht, Arango, Fleischhacker, Meijering, Petter, Van de Brug, Schotsman, Zwerver, Peuskens, De Hert, Thys, Hranov, Hranov, Libiger, Köhler, Mohr, Glenthoj, Broberg, Düring, Baandrup, Jamain, Heres, Rujescu, Giegling, Weiser, Bar Heim, Davidson, Galderisi, Bucci, Mucci, Rybakowski, Remlinger-Molenda, Gonen, Radu, Díaz-Marsá, Rodriguez, Palomo, Rodriguez-Jimenez, García-Portilla, Bernardo, Bobes, Vilares Oliveira, Berger, Wildt, Dazzan, Perez-Iglesias, Drake, Gregory, Wilson, Díaz-Caneja and Eijkemans2018), much earlier than the 2.8 years critical time period identified.
We know that people with TRS experience delays of 4–5 years before starting clozapine (Howes et al., Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor2012). Each non-clozapine antipsychotic trial before clozapine is associated with a further 10% reduction in clozapine response rates (Nielsen et al., Reference Nielsen, Nielsen and Correll2012) while in women the functional Improvement achieved with clozapine decreases by 15% (HRR, 0.85; 95% CI, 0.72–1.00) for each year delay to initiation (Kohler-Forsberg et al., Reference Kohler-Forsberg, Horsdal, Legge, MacCabe and Gasse2017). Further, high-dose antipsychotic polypharmacy is used in 36.2–65% of patients before receiving clozapine (Taylor et al., Reference Taylor, Young and Paton2003; Howes et al., Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor2012; Ucok et al., Reference Ucok, Cikrikcili, Karabulut, Salaj, Ozturk, Tabak and Durak2015), which is not evidence-based practice, and increases the risk of adverse events. In Ireland, a retrospective analysis of 171 FEP cases who presented from 1995 to 1999, identified that 16% (n=28) commenced clozapine in the follow-up period, with a mean delay of 6.7 years and an average of 4.85 antipsychotic trials prior to clozapine use (Doyle et al., Reference Doyle, Behan, O’Keeffe, Masterson, Kinsella, Kelly, Sheridan, Keating, Hynes, Madigan, Lawlor and Clarke2017).
Clozapine underutilisation
Despite its superior and unique effectiveness in TRS, there is marked geographical variation in prescription of clozapine, which in most countries is prescribed to far fewer than the approximately 30% of patients who are likely to benefit from it. Clozapine prescription rates in people with schizophrenia vary from 2% to 5% (Stroup et al., Reference Stroup, Gerhard, Crystal, Huang and Olfson2014) in the United States to 20–30% in the UK, Finland and New Zealand (Downs & Zinkler, Reference Downs and Zinkler2007; Wheeler, Reference Wheeler2008; Tiihonen et al., Reference Tiihonen, Haukka, Taylor, Haddad, Patel and Korhonen2011). There are several possible reasons for deciding against starting clozapine. It is likely that the fear of side effects (by clinicians and patients alike) and the inconvenience of blood monitoring limit its uptake. Clinician unfamiliarity with the use of clozapine, complex pathways to qualify for clozapine use, clinician overestimation of the prevalence and severity of side effects and poor communication all contribute (Nielsen et al., Reference Nielsen, Dahm, Lublin and Taylor2010; Verdoux et al., Reference Verdoux, Quiles, Bachmann and Siskind2018).
Predicting TRS/clozapine responders
Our findings indicate that two distinct patterns of treatment resistance develop in patients, with the majority displaying treatment resistance from the onset, and a smaller subset of patients developing treatment resistance after periods of relapse (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a). While there is a large literature investigating the predictors of treatment response and remission from illness onset (Carbon & Correll, Reference Carbon and Correll2014), treatment resistance has only recently been examined longitudinally as an outcome measure in FEP (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a, Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes, Heslin, Reininghaus, Donoghue, Lomas, Charalambides, Onyejiaka, Fearon, Jones, Doody, Morgan, Dazzan and Murray2017).
An early age of onset (<20 years old) and male sex are consistent predictors for TRS (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a). Severity of psychotic symptoms at first contact for psychosis does not predict TRS, though those with TR from onset have more psychotic symptoms at first contact than those with emergent resistance (Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou, Mondelli, Reis Marques, Pariante, Dazzan, Shergil, Howes, David, MacCabe, Gaughran and Murray2016a). Greater impairment on the Global Assessment of Functioning (GAF) scale is associated with an higher risk of TRS within 2 years of first schizophrenia diagnosis (Horsdal et al., Reference Horsdal, Wimberley, Kohler-Forsberg, Baandrup and Gasse2017).
What if, at the early stages of antipsychotic treatment we could identify those patients likely to respond to clozapine – and those likely to have adverse effects? The available neuroimaging and genetic biomarkers cannot yet reliably guide the early use of clozapine (Lally et al., Reference Lally, Gaughran, Timms and Curran2016b, Samanaite et al., Reference Samanaite, Gillespie, Sendt, McQueen, MacCabe and Egerton2018). Of 379 investigated gene variants, only three (DRD3 Ser9Gly, HTR2A His452Tyr and C825T GNB3) have independently replicated significant findings in clozapine response prediction. Replicated putative central biomarkers of clozapine response include a lower ratio of the dopamine and serotonin metabolites, homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA) in the CSF. Higher prefrontal cortical volumes and increased prefrontal activity on imaging may predict clozapine response (Samanaite et al., Reference Samanaite, Gillespie, Sendt, McQueen, MacCabe and Egerton2018). Neuroimaging studies have indicated a potential role for glutamate in TRS cases compared to treatment responsive schizophrenia, with higher glutamate levels in the anterior cingulate cortex (Demjaha et al., Reference Demjaha, Egerton, Murray, Kapur, Howes, Stone and McGuire2014; Mouchlianitis et al., Reference Mouchlianitis, Bloomfield, Law, Beck, Selvaraj, Rasquinha, Waldman, Turkheimer, Egerton, Stone and Howes2016), and relatively normal dopamine functioning in TRS (Demjaha et al., Reference Demjaha, Murray, McGuire, Kapur and Howes2012), with increased levels of glutamatergic metabolites in the ACC in those with TRS compared to controls (Demjaha et al., Reference Demjaha, Egerton, Murray, Kapur, Howes, Stone and McGuire2014; Iwata et al., Reference Iwata, Nakajima, Plitman, Caravaggio, Kim, Shah, Mar, Chavez, De Luca, Mimura, Remington, Gerretsen and Graff-Guerrero2018).
Standardised definitions of TRS and treatment response will allow for the development of comparable, large, homogenous samples to prospectively assess links between genetic and neuroimaging data and clozapine response and tolerability. Such studies will need to account for factors such as concurrent medication use, tobacco smoking, clozapine dose and plasma concentrations. The current best available clinical marker of TR is the careful assessment of antipsychotic non-response with assured adherence and tolerability. Biomarker testing to improve the predictability of response to clozapine is the subject of a number of multicentre/international collaborations but the clinical utility of such an approach will depend on the emergence of an effective alternative to clozapine for people with TRS.
Clozapine non-responders
For the 30% of TRS patients who fail to respond to clozapine (Meltzer, Reference Meltzer1992; Lally et al., Reference Lally, Tully, Robertson, Stubbs, Gaughran and MacCabe2016c) and the 25% in whom clozapine is discontinued due to adverse events (Davis et al., Reference Davis, Fuller, Strauss, Konicki and Jaskiw2014; Mustafa et al., Reference Mustafa, Burke, Abukmeil, Scanlon and Cox2015), there is little to guide subsequent pharmacological strategies. If a patient was not to respond to clozapine after 3 months of therapeutic dosing (clozapine plasma concentrations 0.35–0.5 mg/l) (Schulte, Reference Schulte2003; Remington et al., Reference Remington, Agid, Foussias, Ferguson, McDonald and Powell2013), then the following steps would be considered (Box 2).
Box 2 Management of clozapine non-response

It is important to assess for and manage comorbid conditions. People with TRS have more comorbid alcohol (51%) and substance abuse (51%), than those with non-TRS (27–35% and 28–35%, respectively). While this can complicate the consistency of adherence to clozapine, an optimised trial of clozapine may give an individual with TRS, the best chance of successfully managing their comorbid substance use. Suicidal ideation is noted in 44% of people with TRS (Kennedy et al., Reference Kennedy, Altar, Taylor, Degtiar and Hornberger2014) and it is important to be aware of the possibility of a comorbid mood disorder. In clozapine-treated patients, OCD rates of 47% have been identified (Fernandez-Egea et al., Reference Fernandez-Egea, Worbe, Bernardo and Robbins2018), 3-fold higher than in non-TRS (Swets et al., Reference Swets, Dekker, van Emmerik-van Oortmerssen, Smid, Smit, de Haan and Schoevers2014), with some authors believing OCD to be released by clozapine use (Schirmbeck & Zink, Reference Schirmbeck and Zink2012).
Clozapine augmentation strategies
The practice of augmentation with a second antipsychotic varies, occurring in 11.7–72% of clozapine-treated patients (Wheeler, Reference Wheeler2008; Pai & Vella, Reference Pai and Vella2012; Ucok et al., Reference Ucok, Cikrikcili, Karabulut, Salaj, Ozturk, Tabak and Durak2015). A recent systematic review and meta-analysis of 46 studies reported improvement in total psychotic symptoms with augmentation with aripiprazole, fluoxetine and sodium valproate, although the quality of included studies was noted to be poor (Siskind et al., Reference Siskind, Lee, Ravindran, Zhang, Ma, Motamarri and Kisely2018). Single studies supporting the efficacy of paroxetine, duloxetine and lithium carbonate in reducing total psychotic symptoms compared to placebo were identified (Siskind et al., Reference Siskind, Lee, Ravindran, Zhang, Ma, Motamarri and Kisely2018). Leucht et al. (Reference Leucht, Helfer, Dold, Kissling and McGrath2015a) examined the augmentation with lithium in general schizophrenia and noted that the response was limited to those with an identified affective component to their illness (Leucht et al., Reference Leucht, Helfer, Dold, Kissling and McGrath2015a). Clinical recommendations, including those in the Maudsley Guidelines (Taylor et al., Reference Taylor, Barnes and Young2018), emphasise the importance of recognising and treating co-occurring mood symptomatology. Overall, caution is required and other meta-analyses have identified that clozapine augmentation with a second medication, including a second antipsychotic, an antidepressant, lamotrigine, topiramate or glycine was not superior to placebo in improving psychopathology (Sommer et al., Reference Sommer, Begemann, Temmerman and Leucht2012; Correll et al., Reference Correll, Rubio, Inczedy-Farkas, Birnbaum, Kane and Leucht2017), while sodium valproate is highly teratogenic.
An earlier meta-analyses of 14 RCTs of antipsychotic augmentation concluded that clozapine augmentation with a second antipsychotic was modestly superior to placebo and well tolerated (Taylor et al., Reference Taylor, Smith, Gee and Nielsen2012). However, the most recent meta-analysis (Galling et al., Reference Galling, Roldan, Hagi, Rietschel, Walyzada, Zheng, Cao, Xiang, Zink, Kane, Nielsen, Leucht and Correll2017) focused solely on antipsychotic augmentation after a non-response, rather than concurrent initiation and augmentation trials and provided no evidence for enhanced efficacy of antipsychotic augmentation in high‐quality studies. Some evidence for improvement in negative symptoms with aripiprazole augmentation was seen.
Guidelines for clozapine augmentation from 15 years ago would have favoured a trial of amisulpride, based largely on anecdotal evidence and pharmacodynamic properties of the compound, which may synergistically augment clozapine. However, to date there is no trial evidence to support this or indeed alternative antipsychotic augmentation strategies. A recent RCT of clozapine augmentation failed to find an effect of amisulpride compared to placebo in reducing psychotic symptoms, although recruitment was underpowered (Barnes et al., Reference Barnes, Leeson, Paton, Marston, Davies, Whittaker, Osborn, Kumar, Keown, Zafar, Iqbal, Singh, Fridrich, Fitzgerald, Bagalkote, Haddad, Husni and Amos2017) and amisulpride may merit further investigation in larger studies. An earlier single sulpiride trial showed efficacy as an augmentation agent in improving total, positive and negative symptoms (Shiloh et al., Reference Shiloh, Zemishlany, Aizenberg, Radwan, Schwartz, Dorfman-Etrog, Modai, Khaikin and Weizman1997).
Siskind’s recent meta-analysis identified that aripiprazole showed effects in reducing total psychotic symptoms, but the effect was lost when poor-quality studies were removed (Siskind et al., Reference Siskind, Lee, Ravindran, Zhang, Ma, Motamarri and Kisely2018). The two high-quality placebo-controlled trials of aripiprazole augmentation show divergent results, with evidence for benefits for negative symptoms in one trial (Chang et al., Reference Chang, Ahn, Park, Lee, Kim, Kang and Kim2008) and positive symptoms in a later trial (Muscatello et al., Reference Muscatello, Bruno, Pandolfo, Mico, Scimeca, Di Nardo, Santoro, Spina and Zoccali2011). Aripiprazole has, however, shown efficacy in relation to weight loss when combined with clozapine (mean difference (95% CI) of −1.36 kg (−2.35 to −0.36) (n=3 studies; p=0.008) (Srisurapanont et al., Reference Srisurapanont, Suttajit, Maneeton and Maneeton2015) and is used in low doses to improve the tolerance of clozapine.
Various non-antipsychotic agents, such as antiepileptics/mood stabilisers (lamotrigine, topiramate, sodium valproate, lithium carbonate,), antidepressants (citalopram, fluoxetine, fluvoxamine, mirtazapine), glutamatergic agents (CX 516, D-cycloserine, D-serine, glycine, sarcosine), allopurinol, memantine, telmisartan and tetrabenazine have been trialled as clozapine augmentation (Elkis & Buckley, Reference Elkis and Buckley2016; Siskind et al., Reference Siskind, Lee, Ravindran, Zhang, Ma, Motamarri and Kisely2018). Among these, sodium valproate (6 RCTs, n=430) has shown efficacy in reducing total psychopathology and positive symptoms compared to clozapine monotherapy. Prescribing of valproate is, however, a problem in women of childbearing age, given its teratogenicity. Similar findings were reported for topiramate (5 RCTs, n=270), but it is associated with a high rate of discontinuation (Zheng et al., Reference Zheng, Xiang, Yang, Xiang and de Leon2017). Lamotrigine has shown some evidence of efficacy, but this effect is lost in meta-analyses when outlier studies are removed (Sommer et al., Reference Sommer, Begemann, Temmerman and Leucht2012; Zheng et al., Reference Zheng, Xiang, Yang, Xiang and de Leon2017).
The divergent findings from clozapine augmentation trials mean that the evidence base does not allow for assured recommendations, or for the development of treatment algorithms for clozapine non- or suboptimal response. Limitations to studies include the variable definitions of clozapine resistance and the dose and short duration of use of the antipsychotic augmentation agents. Current evidence suggests that augmentation agents may need to be used for longer than the standard 6-week antipsychotic monotherapy trial to enhance effectiveness (Correll et al., Reference Correll, Rummel-Kluge, Corves, Kane and Leucht2009). It remains the case that augmentation interventions are used as individual patient trials and if no symptomatic improvement is seen then the medication should be stopped, to minimise the risk of adverse effects.
Electroconvulsive therapy
An intriguing finding is the relatively high-response rate in clozapine non-responders to augmentation with electroconvulsive therapy (ECT) in open trials (Petrides et al., Reference Petrides, Malur, Braga, Bailine, Schooler, Malhotra, Kane, Sanghani, Goldberg, John and Mendelowitz2015). A 2005 Cochrane Review of ECT for schizophrenia noted that in treatment resistant psychosis, the recommended number of ECT treatments was 12–20, higher than in affective disorders (Tharyan & Adams, Reference Tharyan and Adams2005). In our recent meta-analysis, we identified a 66% response to clozapine augmentation with ECT, with an average of 11 treatments used (Lally et al., Reference Lally, Tully, Robertson, Stubbs, Gaughran and MacCabe2016c). To date, it is not possible to identify specific clinical factors that may predict response to ECT augmentation of clozapine. Further, the use of ECT to augment clozapine is far from standard clinical practice in the UK or Ireland, with the usual course of treatment being to augment with other medications, or the addition of psychotherapy.
A note of caution is raised from a recent small single-blind sham-controlled trial that investigated the efficacy of augmenting clozapine with 12 sessions of ECT (n=13) or Sham ECT (n=12) in clozapine resistant schizophrenia (Melzer-Ribeiro et al., Reference Melzer-Ribeiro, Rigonatti, Kayo, Avrichir, Ribeiro, Santos, Fortes and Elkis2017). This pilot study did not identify a significant difference in PANSS total, positive and negative scores between the groups, with only one ECT-treated patient having the 40% or more reduction in PANSS scores seen in the Petrides trial, one with a 30% or more reduction and only 2 with a 20% or more reduction, The authors note the small sample size and suggest a marked placebo (Sham ECT) response likely impacted on the pilot study findings (Melzer-Ribeiro et al., Reference Melzer-Ribeiro, Rigonatti, Kayo, Avrichir, Ribeiro, Santos, Fortes and Elkis2017).
Clozapine augmentation with cognitive behavioural therapy
Cognitive behavioural therapy (CBT) is widely used in patients with schizophrenia, especially in the treatment of positive symptoms such as delusions and hallucinations and in the management of associated emotional distress. A meta-analysis of 12 RCTs of CBT use in medication resistant psychosis showed significant improvement in positive psychotic symptoms compared to controls, supporting the use of CBT as an adjunctive treatment in TRS (Burns et al., Reference Burns, Erickson and Brenner2014). Two small unrandomised RCTs assessed the efficacy of CBT in clozapine non-responders, with benefits seen for total psychotic and general psychopathology symptoms compared to a befriending control intervention (total n=21) (Barretto et al., Reference Barretto, Kayo, Avrichir, Sa, Camargo, Napolitano, Nery, Pinto, Bannwart, Scemes, Di Sarno and Elkis2009) and improvements in positive symptoms compared to supportive therapy (total n=37) (Antonio Pinto et al., Reference Antonio Pinto, Rosa, Domenico and Luigi1999). The recent Focusing On Clozapine Unresponsive Symptoms (FOCUS) randomised clinical trial is the largest and most rigorous trial of CBT for clozapine resistant psychosis and failed to identify any significant differences in the primary outcome of Positive and Negative Syndrome Scale (PANSS) total score at 21 months (mean difference −0·89, 95% CI −3·32 to 1·55; p=0·48), between those treated with CBT and treatment as usual (Morrison et al., Reference Morrison, Pyle, Gumley, Schwannauer, Turkington, MacLennan, Norrie, Hudson, Bowe, French, Byrne, Syrett, Dudley, McLeod, Griffiths, Barnes, Davies, Kingdon, Aydinlar, Courtley, Douglas-Bailey, Graves, Holden, Hutton, Hutton, Irving, Jackson, Lebert, Mander, McCartney, Munro-Clark, Murphy, Spanswick, Steele, Tip and Tully2018). This is an important null study finding and fails to support widespread use of CBT for clozapine augmentation in clozapine resistant schizophrenia and other psychotic disorders. These study findings need to be considered alongside the overall small effect size for total symptom improvement in non-TRS, and lack of significant benefit for positive symptoms identified in meta-analysis of RCTs of CBT use (Jauhar et al., Reference Jauhar, McKenna, Radua, Fung, Salvador and Laws2014).
An important consideration is for carer support and family interventions for those with TRS. Family interventions incorporate psychotherapeutic interventions focused on psychoeducation, facilitating communication and supporting families in developing coping skills and identifying appropriate support services. Family interventions have shown reductions in relapse and rehospitalisation rates, and improved medication adherence in psychotic disorders, along with reduced expressed emotion in families (Pharoah et al., Reference Pharoah, Mari, Rathbone and Wong2010). It is important to note that the vast majority of family intervention studies have not focused on TRS, highlighting an unmet need in research of family interventions in this patient population and for practice implementation.
Affective symptoms
Comorbid mood disorders are often missed in treatment resistance but are important to bear in mind, and if comorbid depression is present, whether it is historically in a unipolar or bipolar context. Meta-analytic data exist to support antidepressant augmentation of first generation antipsychotics in non-TRS patients with predominant negative symptoms. The strongest evidence is for augmentation with selective serotonin reuptake inhibitors, although there is low-level evidence for the use of augmentation with mirtazapine with improvements on positive symptom severity (Galling et al., Reference Galling, Vernon, Pagsberg, Wadhwa, Grudnikoff, Seidman, Tsoy-Podosenin, Poyurovsky, Kane and Correll2018).
In a meta-analysis of non-TR schizophrenia cases, a significant risk difference was found in favour of antidepressant treatment, with a number needed to treat of 5 (95% CI 4–9), but the effect did not persist after sensitivity analysis (Gregory et al., Reference Gregory, Mallikarjun and Upthegrove2017). It is worth noting that the bulk of the agents showing effectiveness in clozapine augmentation in the Siskind et al. (Reference Siskind, Lee, Ravindran, Zhang, Ma, Motamarri and Kisely2018) meta-analysis were antidepressants or mood stabilisers, although the presence or absence of affective disorder was not included as a variable in the analysis.
Negative symptoms
To date, no pharmacological strategies have demonstrated consistently replicable effects on primary negative symptoms. However, there is scope for better outcomes, particularly in negative symptoms secondary to depression, positive psychotic symptoms or motor side effects, which may be more amenable to treatment, and for which clozapine treatment may have advantages.
Clozapine refusal
Patients sometimes refuse clozapine due to dislike of phlebotomy or needle phobia. Possible strategies may include the use of the smallest calibre needles, the application of EMLA cream prior to phlebotomy and consideration for the use of psychological interventions based on exposure techniques where appropriate.
An alternative strategy is the use of finger prick capillary blood sampling. This could be considered if all attempts to perform venous sampling fail. A single puncture site on the palmar surface of the distal phalanx of the 3rd or 4th digit is used, with the first drop of blood discarded before collecting a volume of approximately 125–250 ul (approximately 4–5 drops of blood). Prior discussion with the local haematology laboratory is essential to ensure that granulocyte counts can be reliably measured from a capillary sample as this is not standard and confirmation with the clozapine regulatory body is needed (e.g. ZTAS or CPMS).
Intramuscular (IM) clozapine is an unlicensed product that has been used as a short-term intervention to potentially enable the initiation of clozapine in those who are refusing oral administration. It is started with a view to establishing regular oral clozapine as soon as possible, and clozapine tablets are offered to the patient as an alternative before each injection. Current formulations of clozapine IM are 25 mg/ml and each ampoule contains 5ml (125 mg). The maximum single IM dose is 100 mg, administered in the gluteal muscle, which restricts the potential for dose escalation.
In an Israeli retrospective analysis of the use of parenteral clozapine in 59 clozapine-treated patients who became noncompliant, 27% (n=16) were switched to oral clozapine within 3 days and a further 71% (n=42) by 7 days. One patient continued with IM clozapine for 8 days. There were no adverse events reported, though patients were already established on clozapine for ‘a few weeks’ prior to the use of parenteral clozapine (Lokshin et al., Reference Lokshin, Lerner, Miodownik, Dobrusin and Belmaker1999). Seventeen patients with TRS were identified for treatment with IM clozapine in a Dutch cohort (Schulte et al., Reference Schulte, Stienen, Bogers, Cohen, van Dijk, Lionarons, Sanders and Heck2007), of whom 10 started IM injections, while 7 chose oral clozapine in preference. The duration of IM treatment was 1–4 days for four patients (40%), 7–11 days for three patients (30%) and 1–3 months for three patients (30%). The maximum daily dosage of IM clozapine, given in one or two injections, was 12.5–25 mg for four patients, and 50 mg, 150 mg, 200 mg, 225 mg, 300 mg and 500 mg for six patients, respectively (the mg/ml dose used was not provided). Clozapine was discontinued in two patients, one who developed leucopenia and another who developed impaired liver function. A further patient continued IM treatment for 90 days without any evidence that they would switch to oral clozapine, necessitating the ending of the IM regimen (Schulte et al., Reference Schulte, Stienen, Bogers, Cohen, van Dijk, Lionarons, Sanders and Heck2007).
Alternatives to clozapine in TRS
As clozapine may not be suitable for some patients, for example due to intolerability, adverse events or if they are deemed to be non-rechallengeable, alternative treatments for TRS have been tried. The best evidence is for the use of high-dose olanzapine with some trial data (olanzapine mean dose 35 mg (Meltzer et al., Reference Meltzer, Bobo, Roy, Jayathilake, Chen, Ertugrul, Anil Yagcioglu and Small2008); mean olanzapine dose of 20.5 mg and 67% treated with 25 mg/day) (Tollefson et al., Reference Tollefson, Birkett, Kiesler and Wood2001) providing support for equivalent reductions in psychotic symptoms and relapses in comparison to clozapine. Of note, while Meltzer et al. (Reference Meltzer, Bobo, Roy, Jayathilake, Chen, Ertugrul, Anil Yagcioglu and Small2008) found an equivalent reduction in PANSS score on high-dose olanzapine, those randomised to clozapine had better function and fewer emergent cardiometabolic risk factors. Other trials found high-dose olanzapine to be inferior to clozapine in adults (mean olanzapine dose 50 mg/day) (Conley et al., Reference Conley, Kelly, Richardson, Tamminga and Carpenter2003) and adolescents (mean olanzapine dose 26.2 mg/day) (Kumra et al., Reference Kumra, Kranzler, Gerbino-Rosen, Kester, DeThomas, Kafantaris, Correll and Kane2008).
Meta-analysis of antipsychotic augmentation with the selective oestrogen receptor modulator (SERM) raloxifene in non-TR schizophrenia suggests that it is useful in improving symptoms compared to placebo (de Boer et al., Reference de Boer, Prikken, Lei, Begemann and Sommer2018). In a RCT of 56 post-menopausal women with TRS, raloxifene at 120 mg/day was associated with a greater reduction in PANSS total score relative to placebo (β=−6.37; 95% CI, −11.64 to −1.10; p=0.02) and an increased probability of clinical response (hazard ratio, 5.79; 95% CI, 1.46–22.97; p=0.01) (Kulkarni et al., Reference Kulkarni, Gavrilidis, Gwini, Worsley, Grigg, Warren, Gurvich, Gilbert, Berk and Davis2016). Raloxifene was well tolerated and offers potential for its use in this difficult to treat patient cohort and follows on previous trials from the same centre showing an effect of adjunctive oestradiol 200 mcg in symptom improvement, particularly positive symptoms (Kulkarni et al., Reference Kulkarni, Gavrilidis, Wang, Worsley, Fitzgerald, Gurvich, Van Rheenen, Berk and Burger2015). However, other studies have failed to find a benefit for adjunctive raloxifene in improving cognitive symptoms in non-TR schizophrenia (Kulkarni et al., Reference Kulkarni, Gavrilidis, Gwini, Worsley, Grigg, Warren, Gurvich, Gilbert, Berk and Davis2016; Weiser et al., Reference Weiser, Levi, Burshtein, Hagin, Matei, Podea, Micluţia, Tiugan, Pacala, Grecu, Noy, Zamora and Davis2017), or in improving symptom severity (Weiser et al., Reference Weiser, Levi, Burshtein, Hagin, Matei, Podea, Micluţia, Tiugan, Pacala, Grecu, Noy, Zamora and Davis2017).
Key areas for clinical and academic focus to optimise the management of TRS are outlined in Box 3.
Box 3 The management of TRS, clinical and research priorities

Conclusion
The evidence highlights clozapine as the cornerstone of the pharmacological management of TRS. Clozapine is a uniquely effective medication with over half of those treated responding and with additional benefits in reducing suicide, aggression, violence, alcohol and substance abuse, psychiatric rehospitalisation and all-cause mortality. Despite there being no comparable alternatives, clozapine remains underutilised and initiation is delayed. Increasing evidence suggests that it should be used earlier in the course of illness, with better longer term outcomes associated with earlier use.
Despite being available for over 25 years, the incorporation of clozapine initiation into routine practice needs more work. Although non-clozapine antipsychotics confer little or no benefit for a third of all people with schizophrenia, TRS remains a poor relation in the academic community, with a comparative paucity of studies on its epidemiology, genetic, molecular and neuroimaging characteristics, and the response to pharmacotherapeutic/psychotherapeutic interventions.
With no current credible therapeutic alternatives, it is worth considerable investment in clinical services and academic structures to optimise our use and understanding of clozapine and of strategies, which may help when clozapine fails or is not, tolerated. It is important to maintain an awareness of the high rates of comorbidity in TRS, acknowledging that addressing these may considerably improve function or quality of life in someone for whom antipsychotics are having little effect. Novel psychotherapeutic approaches, such as Avatar Therapy may also hold potential. As TRS research is now moving more into the personalised sphere, this opens the possibility of identifying effective interventions for subgroups of people with TRS. In the meantime, collaborations between clinicians, academics, service users, families, service-planners and industry are needed to scan the horizon for future developments in the prevention and management of TRS.
Conflict of interest
FG declares a potential conflict of interest, although not in relation to this work.
Financial Support
FG has received support or honoraria for CME, advisory work and lectures from Lundbeck, Otsuka, and Sunovion, collaborated on research funded by an NHS Innovations/Janssen-Cilag award and has a family member with professional links to Lilly and GSK, including shares. FG is in part, funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research & Care Funding Scheme and the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The other author has no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; there are no other relationships or activities that could appear to have influenced the submitted work.
Ethical Standards
The authors (JL and FG) assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committee on human experimentation with the Helsinki Declaration of 1975, as revised in 2008.


