Introduction
Reason for audit
Antipsychotic-related movement disorders are a common and well-recognised problem in patients treated with typical antipsychotics. These side effects can be physically disabling and subjectively distressing for patients, may be an important cause of poor medication adherence (Barnes & McPhillips, Reference Barnes and McPhillips1996) and are associated with poor quality of life (Browne et al. Reference Browne, Roe, Lane, Gervin, Morris, Kinsella, Larkin and Callaghan1996). Although they are less common with newer atypical drugs there is significant evidence that they still remain a problem for a considerable proportion of patients (Gervin & Barnes, Reference Gervin and Barnes2000). Point prevalence rates of rigidity as high as 17% with risperidone and 13% with clozapine have been demonstrated (Miller et al. Reference Miller, Mohr, Umbricht, Woerner, Fleischhacker and Lieberman1998) and there are reports of incidence rates of akathisia varying between 2.8% and 16% with olanzapine and 2–5% with quetiapine (Hirose, Reference Hirose2003). Tardive dyskinesia has been demonstrated to occur at an incidence rate of 0.74% with atypical antipsychotics and although rare may be more persistent once emerged when compared with typical drugs (Tenback et al. Reference Tenback, Vanharten, Slooff and Van Os2010).
The clinical guidelines for schizophrenia produced by the National Institute for Clinical Excellence (2009) and the American Psychiatric Association (APA) Practice Guidelines on treatment of schizophrenia (American Psychiatric Association, 2004) both highlight the importance of regularly assessing for the presence of EPSE.
However, rates of clinician under-recognition and under-diagnosis of these conditions are high (Weiden et al. Reference Weiden, Mann, Mattson and Frances1987; Hansen et al. Reference Hansen, Brown, Weigel and Casey1992) and medical records generally show poor levels of assessment and documentation of EPSE (Cortese et al. Reference Cortese, Jog, McAuley, Kotteda and Costa2004) This may be due to the lack of training, formal or informal, that clinicians receive in assessment of drug-related movement disorders, resulting in low clinician confidence levels, especially in non-consultant hospital doctors (NCHDs) (Kuruvilla et al. Reference Kuruvilla, Sedano-Ruiz and Ley2006).
Aim
To review current practice in our service regarding monitoring of EPSE in patients on antipsychotic treatment seen in the outpatient clinic.
Objectives
-
∙ To review records from patients seen in outpatient clinics (covering a catchment area of ~60 000) over a 2-week period and audit documentation regarding the presence or absence of EPSE.
-
∙ To present audit findings to relevant staff members.
-
∙ To organise a teaching session on the assessment of EPSE at the weekly local academic meeting and thus increase clinical confidence levels in assessment, diagnosis and management of these side effects.
-
∙ To re-audit following the teaching session and compare with initial results.
Guidelines/standards
The clinical guidelines for schizophrenia produced by the National Institute of Clinical Excellence state that in patients on antipsychotic treatment, side effects should be monitored and recorded regularly and systematically by the clinician throughout treatment, especially during titration periods.
The APA Practice guidelines on treatment of schizophrenia are more specific and state that patients receiving antipsychotic medication treatment on a sustained basis (for >4 weeks) should be evaluated at a minimum of every 3 months for signs of dyskinetic movements.
Method
Criteria to be measured
Patients on antipsychotic medication should be assessed for EPSE at every outpatient review and the presence or absence of EPSE should be clearly documented in patient records. EPSE include dystonia, parkinsonism, akathisia and tardive dyskinesia.
Standard/target
A target of 75% of patients on antipsychotic treatment should be monitored for EPSE on reviewing outpatient records for a particular 2-week period. This will take into account patients in whom it may not be possible to monitor EPSE at that time for any reason, for example, a patient who is very unwell or who refuses to cooperate with a physical exam.
Data collection
A list of patients (n=92) seen in outpatient clinics over a 2-week period was compiled and checked against the antipsychotic register to identify patients on antipsychotics (n=43).
The clinical record from each outpatient clinic was reviewed retrospectively, and data collected on whether or not EPSE had been assessed as well as on diagnosis and class of antipsychotic(s) prescribed.
The same data was collected for the re-audit over another 2-week interval. During this period 94 patients were seen in the outpatient clinic of whom 61 were on antipsychotic treatment.
Results of initial data collection
The outpatient record of 43 patients was analysed. The length of time from last outpatient review to current outpatient clinic ranged from 1 to 45 weeks, with an average of 10 weeks (s.d.=8).
Documentation regarding EPSE was present in 14% of patient records. Consultants documented presence or absence of EPSE in 19% of cases while non-consultant hospital doctors (NCHD) made no reference to these side effects in any case.
In the cases in which documentation was present, 66% documented an absence of EPSE while 33% described the presence of EPSE (Fig. 1).

Fig. 1 Frequency of outpatient records with EPSE documentation according to clinician group (Initial audit).
Of the total sample, 72% of patients were on atypical antipsychotics alone, 7% were taking only a typical antipsychotic and 21% were prescribed both classes of medication. Of those on only atypical medication, 19% were taking more than one atypical medication.
Regarding the impact of class of antipsychotics, EPSE were assessed in 10% of patients on atypical antipsychotics, 33% of those on typical antipsychotics and 22% of those on both (Fig. 2).

Fig. 2 Frequency of outpatient records with EPSE documentation according to class of antipsychotic medication (Initial audit).
Regarding the influence of diagnosis, EPSE were more likely to be checked for in patients with psychosis (19%) compared with those with bipolar affective disorder (BPAD) (9%) or depression (0%) (Fig. 3).

Fig. 3 Frequency of outpatient records with EPSE documentation according to patient diagnosis (Initial audit).
Action plan
Audit findings were presented at the weekly local academic meeting. This was followed by a teaching session on the assessment of EPSE. Teaching covered the assessment, diagnosis and management of dystonia, parkinsonism, akathisia and tardive dyskinesia. Copies of the relevant rating scales were distributed (Simpson Angus Scale, Barnes Akathisia Scale and Abnormal Involuntary Movements Scale).
Use of these scales, although useful for monitoring EPSE, is not feasible as a method of screening for EPSE in busy, time-pressured outpatient clinics where a clinician reviews each patient in ~15–20 minutes. Thus, we suggested the use of a brief 1-minute screening tool, which we had developed and introduced this to our colleagues.
We also placed a copy of this on the desks in the outpatient clinics to remind clinicians to check for EPSE (Fig. 4).

Fig. 4 One minute screening test for EPSE.
Results of re-audit
A total of 61 patient records were reviewed. The average length of time from last review to current outpatient review was 16 weeks (s.d.=12) with a range from 1 to 56 weeks.
The results of the re-audit revealed an overall level of documentation of EPSE of 42%. Consultants documented the presence of absence of EPSE in 37% while the level of documentation by NCHDs had risen to 75%.
Of the patients who had been assessed for EPSE, 92% of records reported EPSE as being absent while in 8% they were present (Fig. 5).
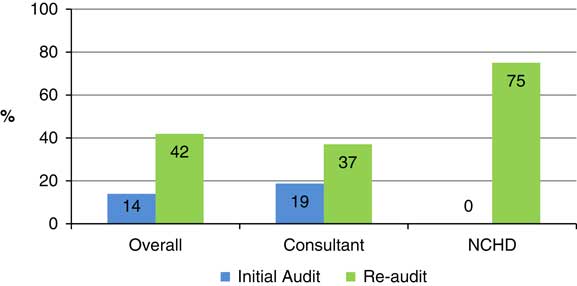
Fig. 5 Frequency of outpatient records with EPSE documentation according to clinician group (Initial audit cf. re-audit).
Similar to the initial results, the majority of patients (84%) were taking atypical medication, while only 5% were on typical medication and 11% were taking both. In those taking atypicals, 23% were on more than one drug.
The impact of antipsychotic class followed the same trend, with clinicians checking for EPSE more commonly in those taking typical antipsychotics (67%) than in those on atypicals (40%). Those patients being treated with both typical and atypical medication were checked for EPSE in 43% of cases (Fig. 6).

Fig. 6 Frequency of outpatient records with EPSE documentation according to class of antipsychotic medication (Initial audit cf. re-audit).
In relation to the impact of diagnosis in the re-audit, again clinicians were most likely to check for EPSE in patients with a diagnosis in psychosis (54%) but in contrast to the initial results, documentation on EPSE was present more often in those with depression (36%) than in those with BPAD (30%) (Fig. 7).

Fig. 7 Frequency of outpatient records with EPSE documentation according to diagnosis (Initial audit cf. re-audit).
Discussion
The results of our initial audit are consistent with existing data that suggests that medical records generally show poor levels of documentation regarding EPSE (Cortese et al. Reference Cortese, Jog, McAuley, Kotteda and Costa2004), that clinicians are poorly trained in their assessment (Kuruvilla et al. Reference Kuruvilla, Sedano-Ruiz and Ley2006) and that as a result, they frequently under-recognise and under-diagnose these important side effects (Weiden et al. Reference Weiden, Mann, Mattson and Frances1987; Hansen et al. Reference Hansen, Brown, Weigel and Casey1992). In our initial audit, there was documentation relating to EPSE in only 19% of patients reviewed by the consultant while there was no documentation in patients reviewed by NCHDs. This likely reflects a lack of training and poor confidence levels in trainees.
The APA guidelines state that dyskinetic movements should be evaluated in patients on sustained antipsychotic treatment at a minimum of every 3 months. In the initial audit and re-audit the average lengths of time between the current outpatient review and last outpatient clinic were 10 and 16 weeks, respectively, consistent with the presumption that these are stable patients attending for routine review. However, there were a small number of patients (<8%) who had been seen in the preceding 1–2 weeks. Presumably, these patients were attending frequently because they were unstable. It may still be reasonable to expect that EPSE should be assessed in this group of patients as if they were unstable there is a strong possibility their medication dose was being titrated. According to the NICE guidelines, side effects should be monitored and recorded, especially during titration periods.
We had expected that in the majority of cases in which documentation relating to EPSE existed, it would be because EPSE were present. However, this was not the case and in the majority of instances in both stages of the audit, documentation reported an absence of EPSE (66% and 92%, respectively). This suggests that in some patients, clinicians are screening for dyskinetic movements in the absence of obvious clinical signs or patients spontaneously reporting symptoms.
As expected, EPSE were assessed more often in patients on typical antipsychotics than in those prescribed atypical medications, a trend that was seen in both the initial audit and re-audit. This finding was not surprising given the much higher incidence of EPSE with typical antipsychotics. However, as detailed in the introduction, even with the newer atypical agents, movement disorders are seen in a significant proportion of patients (Gervin & Barnes, Reference Gervin and Barnes2000) and need to be actively screened for by clinicians. The APA guidelines make no distinction between the different classes of antipsychotic medication in terms of EPSE assessment.
A consistent finding between both initial audit and re-audit results was that clinicians were more likely to assess for EPSE in patients with a diagnosis of a psychotic disorder than in those with bipolar disorder or depression. It is known that a significant proportion of never-medicated patients with schizophrenia exhibit spontaneous dyskinesias (9%) and parkinsonian symptoms (17%) (Pappa et al. Reference Pappa and Dazzan2009) and perhaps this might account for the greater tendency to assess for EPSE in these patients. Interestingly, in a systematic review by Gao (Pappa et al. Reference Pappa and Dazzan2009) it was found that bipolar patients, especially in depression, were more vulnerable to having acute antipsychotic-induced movement disorders than those with schizophrenia.
Following the teaching session, rates of documentation of EPSE improved significantly, especially in the case of NCHDs (from 0% to 75%). This supports the theory that poor levels of assessment and documentation are related to lack of training and low confidence levels. Consultant rates also improved (from 29% to 41%) but the target criteria of 75% was not achieved.
Conclusion
In our practice, clinicians are generally poor to assess and record EPSE in patients on antipsychotic treatment, especially in those prescribed atypical medications. However, rates of assessment and documentation improved significantly following a teaching session on the topic, especially in NCHDs. It is likely that further training on the assessment, diagnosis and management of these important side effects would be beneficial in promoting best clinical practice in our service and improving the quality of patient care.









