Many introductions of invasive plants have been the direct result of human cultivation, from agriculture and horticulture. More than half of the naturalized (Mack and Erneberg Reference Mack and Erneberg2002) and invasive plants (Lehan et al. Reference Lehan, Murphy, Thorburn and Bradley2013) in the United States were deliberately introduced, and 85% of invasive woody plants were first introduced as ornamentals (Reichard and Hamilton Reference Reichard and Hamilton1997). There has been an increasing emphasis on using native plants as ornamentals, in part because horticulturalists recognize the problem of invasive plants (Peters et al. Reference Peters, Meyer and Anderson2006). Consumers are encouraged to use native plants in horticulture by government agencies, universities, environmental organizations, and for-profit vendors (Burghardt et al. Reference Burghardt, Tallamy and Shriver2009; Tallamy Reference Tallamy2007). Additionally, some state and local governments have prohibited the sale and use of plants deemed to be invasive or noxious. Nonetheless, there has been some resistance from horticulturalists to remove invasive plants from their inventories. Peters et al. (Reference Peters, Meyer and Anderson2006) note that characteristics that make plants suitable for mass production in horticulture (rapid reproduction, hardiness) are also associated with their potential to become invasive. Additionally, consumer demand for familiar horticultural products and a lack of effective communication about what species are considered problematic can increase the likelihood that invasive species persist in horticultural catalogs.
Box 1 Management Implications
Native species alternatives are often touted as replacements for invasive species with long histories in horticulture. In the case of the native American bittersweet and introduced invasive oriental bittersweet in the eastern United States, the two species are difficult to distinguish when plants are immature. In a survey of plants sold by vendors across the Midwest, by using genetic markers, we found that most products marketed as American bittersweet or Celastrus scandens were actually mislabeled “oriental bittersweet.” These mislabeled plants were less expensive than true American bittersweet. Parties intending to purchase and propagate American bittersweet may be contributing to the spread of the invasive. Special care should be taken to properly identify species when propagating bittersweet plants, especially when material is obtained from horticultural vendors. However, identification is not straightforward in the absence of flowers or fruit. As increased effort is put into preventing the spread of oriental bittersweet through statutory and other measures, the cryptic sale of oriental bittersweet through the horticultural industry should be considered an obstacle to attempts to curtail the invasive vine and to the conservation and restoration of American bittersweet populations.
Celastrus orbiculatus (oriental bittersweet, Celastraceae) is a highly invasive ornamental woody vine (or liana) introduced to the eastern United States (Leicht-Young and Pavlovic Reference Leicht-Young and Pavlovic2015). The species is widely recognized as a threat to native ecosystems because of its rapid growth, which crowds out native vegetation, negatively affects forestry operations, and can alter natural successional trajectories (Fike and Niering Reference Fike and Niering1999; Leicht-Young et al. Reference Leicht-Young, Silander and Latimer2007b). The species has been listed as a prohibited or restricted plant across much of its introduced range (e.g., Vermont, North Carolina, Minnesota). Celastrus scandens (American bittersweet, or American staff vine) is a congener native to the region that C. orbiculatus has invaded in North America. Celastrus scandens is also a woody vine and is widely marketed as an ornamental alternative to C. orbiculatus.
There is great potential for mislabeling the two species, with plants marketed as C. scandens and American bittersweet actually being C. orbiculatus or hybrids of the two species. Mislabeling may occur unintentionally, as it is difficult to distinguish the Celastrus species in the absence of reproductive structures (Leicht-Young et al. Reference Leicht-Young, Pavlovic, Grundel and Frohnapple2007a), and plants purchased from vendors are usually small individuals that have not begun to flower. Additionally, seeds collected from pistillate C. scandens may be sired by C. orbiculatus, and hybrids of the two species have been found in the wild (Zaya et al. Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015). There may also be an incentive for deliberate mislabeling, because C. orbiculatus grows more rapidly than C. scandens (Leicht-Young et al. Reference Leicht-Young, Silander and Latimer2007b), thus increasing yields while decreasing investment of time and resources, and C. orbiculatus has a long history in horticulture (Del Tredici Reference Del Tredici2014).
We used molecular markers to determine the species identity of commercially available plants marketed as C. scandens or American bittersweet. Our goal was to determine whether C. orbiculatus or hybrids were sold in place of C. scandens. If the ultimate source of marketed plants is seed collected from wild plants, it is possible that a large proportion of individuals are hybrids. Alternatively, C. orbiculatus may be substituted, intentionally or not, because the two species are difficult to distinguish morphologically in the absence of reproductive structures. Human commerce is among the most important dispersal agents of introduced species, and understanding commerce’s role in the continuing spread of C. orbiculatus is essential in any large-scale attempt to control its invasion and negative effects on natural communities, and on C. scandens in particular.
Materials and Methods
Study Species
Celastrus scandens L. (Celastraceae) is the only member of the genus native to North America (Hou Reference Hou1955). It is a woody vine, usually found in open habitat ranging from full sun to forest edges or gaps. Its range extends from southern Quebec to South Dakota, south to western Texas through Georgia (USDA–Natural Resources Conservation Service [USDA-NRCS] 2017). The native range of C. orbiculatus Thunb. is in Korea, Japan, and China (Hou Reference Hou1955), where it is one of approximately 25 species in the genus (Leicht-Young and Pavlovic Reference Leicht-Young and Pavlovic2015). It is found in thickets and lowland slopes, but can thrive in shaded habitat (e.g., forest understory) that would likely exclude C. scandens (Pavlovic and Leicht-Young Reference Pavlovic and Leicht-Young2011). Both species are usually dioecious, although rare individuals and populations displaying other breeding systems are known.
Celastrus orbiculatus was introduced as an ornamental vine to the eastern United States in 1874 (Del Tredici Reference Del Tredici2014). By the middle of the twentieth century, it was widely recognized as a pest species rapidly spreading in the eastern United States (Patterson Reference Patterson1974; EDDMapS 2017; USDA-NRCS 2017). Celastrus orbiculatus is a strong competitor that crowds out other vegetation and can be economically costly to forestry and alter natural succession (Fike and Niering Reference Fike and Niering1999; Leicht-Young et al. Reference Leicht-Young, Silander and Latimer2007b). There is strong evidence that C. orbiculatus interferes with successful reproduction in C. scandens through asymmetric pollen flow and hybridization (Zaya Reference Zaya2013), and declines in C. scandens have been observed in regions where invasion by C. orbiculatus is oldest and most extreme (Dreyer et al. Reference Dreyer, Baird and Fickler1987; Leicht Reference Leicht2005; RI Bertin, personal communication). As a result, C. scandens has been listed as a threatened, endangered, or extirpated species in multiple states.
Sampling
We purchased plants marketed as American bittersweet or C. scandens from 11 vendors in the midwestern United States (in Indiana, Illinois, Missouri, and Nebraska; Figure 1) in summer and fall of 2009. Purchases were made in person from six vendors, and the other five purchases were made via the Internet or by telephone. We sought vendors that targeted a retail audience, though it is possible that some may have also served as wholesalers, growers, or at other levels in the horticultural industry supply chain (Drew et al. Reference Drew, Anderson and Andow2010). Telephone or Internet orders were shipped as bare-root samples that we later potted. In-person purchases were potted plants. Approximately half of the samples acquired through in-person purchases were larger than shipped samples, though some in-person purchases were similar in size to shipped plants. Sampling locations ranged across the invasion front of C. orbiculatus (EDDMapS 2017). In total, 34 individuals were genetically tested, representing six named cultivars and plants not labeled with a cultivar name (Table 1).

Figure 1 Distribution of Celastrus vendors in the midwestern United States. Circles represent vendors that were visited in person; squares represent vendors that shipped the product. Red points represent vendors that exclusively sold C. scandens; yellow points represent vendors that exclusively sold C. orbiculatus; and the single orange point represents a vendor that delivered both species. The dashed line represents the approximate western edge of the C. orbiculatus invasion front (from EDDMapS 2017).
Table 1 Sources and genetic identities for Celastrus samples.Footnote a

a Two vendors sold multiple cultivars, and one vendor sold both species.
In addition to the purchased samples, we included three other types of control samples for genetic and statistical analysis. We used plants collected from the wild for which species identity was determined using reproductive morphology and verified genetically in a previous study as genetic benchmarks for C. scandens and C. orbiculatus (Zaya et al. Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015). In total, we used 182 C. scandens individuals from 15 populations in 9 states (Illinois, Indiana, Massachusetts, Michigan, Minnesota, North Carolina, Ohio, South Dakota, Wisconsin) and 180 C. orbiculatus individuals from 15 populations in 9 states (Connecticut, Illinois, Indiana, Massachusetts, Michigan, North Carolina, New Jersey, Tennessee, Virginia) as benchmarks. Data for these samples are not presented in detail here, but are summarized in Zaya et al. (Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015) and available through this paper's dataset (see Acknowledgments). Additionally, we included 16 hybrids produced through hand cross-pollination conducted at the Indiana Dunes National Lakeshore, Porter County, IN (Zaya et al. Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015). Resulting seeds were collected, put through cold stratification, and germinated according to the protocol outlined by Young and Young (Reference Young and Young1992). We soaked seeds for 24 h, sowed them in an equal mix of potting soil and sand, watered the mixture, placed them in a bag, and kept them refrigerated at 4 C for 90 d. After 90 d, we germinated the seeds in a greenhouse.
Genetic Analysis
Genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Germantown, MD). For 32 samples, DNA was extracted from 20 to 25 mg of ground leaf material following the manufacturer’s protocol. Two samples from the same supplier (Vendor H in Table 1) were not viable upon delivery and did not have leaf material available. For those two samples, we used 50 to 70 mg of scraped wood shavings for DNA extraction. We used a modified protocol developed by Rachmayanti et al. (Reference Rachmayanti, Leinemann, Gailing and Finkeldey2009), which included addition of polyvinylpyrolidone to the lysis buffer to help with DNA extraction from wood tissue. We successfully extracted DNA from wood shavings that came from the thickest part of the dead stem, near the base of the plant. The five nuclear microsatellite loci described by Zaya et al. (Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015) were used to genotype each individual. These five loci have been shown to distinguish the two species and their hybrids. Nuclear microsatellites are especially useful for the objectives of this study, because they are highly variable, making it possible to distinguish closely related species, and because they are codominantly inherited (one allele transmitted from each parent), which allows for accurate identification of hybrid individuals. Fragment sizes of PCR products were analyzed with the ABI 3730 DNA Analyzer, using a LIZ500 ladder (Applied Biosystems). All microsatellite genotypes were scored by analyzing the raw data using Applied Biosystems GeneMapper software v. 3.7.
Statistical Analysis
Species assignments were evaluated using the program Structure v. 2.3 (Falush et al. Reference Falush, Stephens and Pritchard2003). No a priori information on species identity was included in the analysis. Structure implements a Bayesian clustering approach and Markov chain Monte Carlo simulations to estimate the proportion of each individual’s genome originating from each inferred population. We used the admixture model, assuming correlated allele frequencies, and set the number of clusters, K, equal to 2. These settings have been shown to successfully discriminate these two species and their hybrids (Zaya et al. Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015). Identical genotypes were collapsed into a single record for the analysis. We conducted three independent runs, each with 250,000 iterations after an initial burn-in of 50,000 iterations. Individuals were classified to one of the two species groups using the proportion of ancestry, q, from each run. We set the threshold for classifying an individual in one of the two species categories as 0.9 (Manel et al. Reference Manel, Berthier and Luikart2002), so that if the maximum q was less than 0.9, a species was classified as a hybrid. All horticultural samples had a value of q greater than the threshold, and all individuals were assigned to the same group in each run. As each run gave the same result for every sample (test and control), we present the mean q values.
We used the nonparametric Mann-Whitney-Wilcoxon rank-sum test to compare prices of products identified as C. orbiculatus and C. scandens. The test was implemented in R v. 3.3.2 (R Core Team 2016).
Results and Discussion
All five primer pairs amplified polymorphic loci in all our test samples and the controls. Among the 34 horticultural samples tested, we identified 22 unique genotypes. For these unique genotypes, the mean number of alleles per locus was 10.2, and the mean observed heterozygosity was 0.72 (Table 2). Both the mean number of alleles and mean observed heterozygosity were greater in test samples that we identified as C. orbiculatus compared with those identified as C. scandens (Table 2). In control samples and other wild populations, large differences between species with respect to genetic diversity were only observed for one locus (CEOR7003; Zaya et al. Reference Zaya, Leicht-Young, Pavlovic, Feldheim and Ashley2015).
Table 2 Microsatellite genotypes for horticultural Celastrus samples.Footnote a
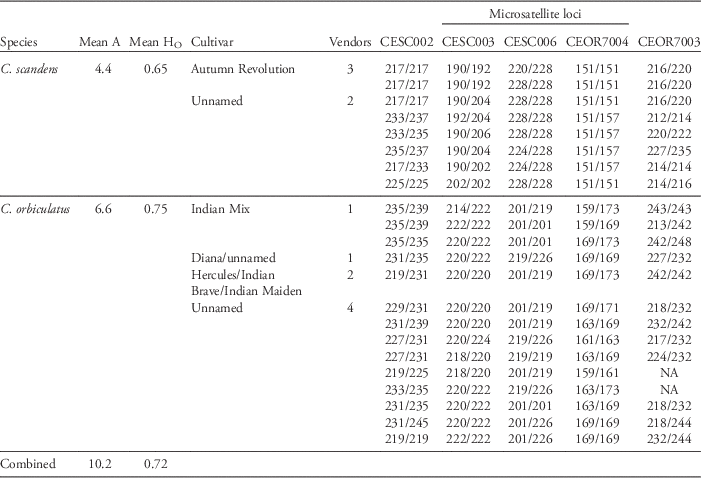
a Individuals are diploid, with two alleles per locus. The numbers under each locus heading represent the length of the allele in base pairs, and alleles are separated by a slash. Mean number of alleles (Mean A) and observed heterozygosity (Mean HO) were calculated across all five loci and across all cultivars, after excluding repeated genotypes. Genotypes were determined for 18 C. orbiculatus individuals and 16 C. scandens individuals. Vendors refers to the number of vendors, and NA indicates missing data. Note that the allele sizes used here depend on the usage of the method described by Schuelke (Reference Schuelke2000), and the same fluorescent label for each locus. The following Applied Biosystems standard dyes were used: NED, CESC006; VIC, CEOR7004 and CEOR7003; PET, CESC002 and CESC003.
Genetic tests indicated that 18 of 34 (53%) of the purchased samples clustered with C. orbiculatus. The other 16 samples all clustered with C. scandens (Table 1). None of the samples clustered with hybrids. All of the Structure assignments were highly supported by the inferred proportion ancestry, q (Figure 2). Every sample had a maximum q greater than 0.96, and all but one maximum q value was greater than 0.99. Two of the samples we purchased showed signs of reproductive structures, both carrying fruits in terminal panicles typical of C. scandens. Structure correctly classified both samples as C. scandens.

Figure 2 Bayesian clustering results from Structure with two inferred clusters (K=2) for Celastrus individuals. Results for each individual are represented in a single column. The colors in each column show the proportion of the individual’s genome assigned to the two clusters. “Test samples” (N=34) are plants purchased for this study. The other three categories were control plants of known genetic identity (N=378). For clarity, only 16 randomly selected control samples in each category are shown.
Four of the 11 vendors sold only C. scandens. Six vendors sold only C. orbiculatus. The last vendor, located in Nebraska and at the C. orbiculatus invasion front, sold both species (Figure 1). The mislabeled samples came under five cultivar names, some of which are racially insensitive: ‘Diana,’ ‘Hercules,’ ‘Indian Brave,’ ‘Indian Maiden,’ and ‘Indian Mix’ (Table 1). We found multiple samples with the same genotype due to asexual propagation. The identical genotypes included samples from the westernmost vendor in Nebraska and the easternmost vendor in southeastern Indiana. The Indian Maiden, Indian Brave, and Hercules cultivars had identical genotypes, as did Diana and an unnamed sample (Table 2). The only named cultivar that was genetically determined to be C. scandens was ‘Autumn Revolution,’ also known as C. scandens ‘Bailumn’ (Bailey Reference Bailey2009). Autumn Revolution was purchased from three of the five vendors that sold C. scandens. Six vendors sold plants that were not labeled with a cultivar name, and in four of those cases, genetic tests classified the samples as C. orbiculatus. The multilocus genotypes of the horticultural samples are provided as a reference to parties interested in testing commercial products of unknown species identity (Table 2).
Four of the six vendors that were visited in person exclusively sold C. scandens. All of the online or phone order shipments included C. orbiculatus, a group that includes the vendor that sent both species (Figure 1). The price of true C. scandens was more than twice the price of C. orbiculatus (Figure 3), a significant difference (Mann-Whitney-Wilcoxon rank-sum test: W=31, P<0.04). Interestingly, the vendor that sold both species charged more for C. scandens (US$19.95) than mislabeled C. orbiculatus (US$13.95).

Figure 3 Prices of correctly labeled C. scandens (n=5) and mislabeled C. orbiculatus (n=7). Heavy solid lines represent the median, while dashed lines represent the mean.
In testing the genetic identity of plants marketed as the native C. scandens, we found that the majority of vendors sampled in the midwestern United States were selling a mislabeled introduced species, C. orbiculatus. Mislabeled C. orbiculatus is available on both sides of the invasion front and can easily be shipped to any state in the contiguous United States. Some vendors also ship internationally, which could exacerbate the C. orbiculatus invasion in Canada and even in distant regions like New Zealand (Williams and Timmins Reference Williams and Timmins2003). None of the purchased samples were C. scandens × C. orbiculatus hybrids. We sampled near the invasion front, and it may be that the observed patterns would change farther east, where C. scandens has greatly declined, or farther west, where C. orbiculatus is not known to occur. Mislabeling of Celastrus is problematic, because the phenomenon promotes the spread of an invasive species inhibits the success of a native species, and consumers pay for a product that they did not choose—an aggressively growing plant that can be a nuisance.
A useful clue as to the accuracy of product labeling might be the mode of purchase. Four of six in-person purchases were accurately labeled. Every vendor that shipped a product provided us with C. orbiculatus, though one of those vendors sold both species. All of the shipped products were initially found through Internet searches, though some were ordered over the telephone. Shifts in distribution patterns and marketing strategies, prerequisites for widespread Internet purchasing, may accelerate the spread of incorrectly labeled Celastrus and invasive species in general (Drew et al. Reference Drew, Anderson and Andow2010). Additionally, mislabeled products can be shipped to areas where the sale and propagation of C. orbiculatus is illegal, such as the city of Chicago, IL—where all of the products included in this study were shipped. Although the municipal code restricting the sale of C. orbiculatus in Chicago may have influenced the accuracy of labels in the region where we made our in-person purchases, we do not believe this was the case, for three reasons. First, only two vendors were in the jurisdiction covered by the law at the time, and one of them sold the mislabeled product. Second, expertise is not available at the municipal level to discriminate the two Celastrus species, thus a mislabeled plant is likely to go unnoticed. Finally, the city has limited resources for enforcement of the law and is slow or unable to respond to reports of illegal sales, even for C. orbiculatus plants that are not mislabeled as “American bittersweet” (DNZ, personal observation).
The significant difference in price between mislabeled and correctly labeled plants may suggest that C. orbiculatus is easier to obtain or it can be propagated more efficiently. Celastrus orbiculatus has a long history in North American horticulture and has become more common than C. scandens in many regions of the United States. Ecological and physiological studies have found C. orbiculatus to be a stronger competitor than C. scandens, exhibiting more rapid growth (Leicht-Young et al. Reference Leicht-Young, Latimer and Silander2011), tolerance of a larger range of conditions (Leicht-Young et al. Reference Leicht-Young, Silander and Latimer2007b) and herbivory (Ashton and Lerdau Reference Ashton and Lerdau2008), and greater reproductive output (Dreyer et al. Reference Dreyer, Baird and Fickler1987; Zaya Reference Zaya2013). However, at this point, we can only speculate about the connection between species biology and product price. Because most Internet purchases were C. orbiculatus and most in-person purchases were C. scandens, myriad confounding factors (e.g., plant size, economic advantages for different types of vendors) may be influencing the price difference. Anecdotally, the case of the vendor that sold both species is revealing, as mislabeled plants cost 30% less. Whatever the cause, the lower price creates an incentive to purchase mislabeled C. orbiculatus.
Properly labeled C. scandens appears to be difficult to obtain, even when purchasing from vendors that claim to sell American bittersweet. Only one of the named cultivars turned out to be C. scandens, that one being Autumn Revolution, or C. scandens ‘Bailumn,’ patented by Bailey Nurseries Inc. (St Paul, MN; Bailey Reference Bailey2009). The availability of Autumn Revolution has increased recently, which means a properly labeled C. scandens can be more easily obtained. However, overreliance on this cultivar may be troublesome. Autumn Revolution has the potential to become naturalized, as individuals readily set germinable seed that can be widely dispersed by birds. Plants grow vigorously and set larger seeds at a greater rate than typical C. scandens (Bailey Reference Bailey2009; DNZ, personal observation). Also, the breeding system of Autumn Revolution is atypical in that all individuals have hermaphroditic flowers, while C. scandens is almost always dioecious (Bailey Reference Bailey2009). Eight of the nine Autumn Revolution samples that we tested, from three different vendors, were genetically identical (Table 2). The spread of the cultivar into the wild may threaten native C. scandens by decreasing genetic diversity of wild populations. Decreased genetic diversity may have several negative consequences, but in cultivated plants in particular, it may lead to increased susceptibility to disease (Zhu et al. Reference Zhu, Chen, Fan, Wang, Li, Chen, Fan, Yang, Hu, Leung, Mew, Teng, Wang and Mundt2000). The potential for intraspecific crossing, long-distance pollen dispersal, and introgression between horticultural plantings and wild conspecifics has been demonstrated and may threaten the genetic integrity of C. scandens. Johnson and Galloway (Reference Johnson and Galloway2008) provided evidence that individuals from natural Lobelia cardinalis L. (cardinalflower) populations were pollinated by horticultural L. cardinalis up to 1 km away, while Whelan et al. (Reference Whelan, Roberts, England and Ayre2006) found the potential for introgression of unusual morphological characteristics from garden populations of Grevillea macleayana (McGill) Olde & Marriott (Jervis Bay grevillea) into wild populations. It is currently challenging for conscientious midwestern U.S. consumers to find an alternative to Autumn Revolution. Only one vendor sold only C. scandens plants that were not Autumn Revolution. That vendor, located in Monee, IL, sold unnamed plants.
The rate of mislabeling found in this study (64% of vendors, and 53% of samples tested) is large compared with previous studies that used molecular markers to survey commercial plant products (Zaya and Ashley Reference Zaya and Ashley2012). For example, several studies have tested the accuracy of labels on herbal medication and have reported mislabeling of 8% to 60% of the products tested (Del Serrone et al. Reference Del Serrone, Attorri, Gallinella, Gallo, Federici and Palazzino2006; Fan et al. Reference Fan, Zhu, Chen, Yang, Cai and Komatsu2009; Feng et al. Reference Feng, Liu and He2010; LeRoy et al. Reference LeRoy, Potter, Woo, Heber and Hirsch2002; Lin et al. Reference Lin, Chen and Lin2008; Manissorn et al. Reference Manissorn, Sukrong, Ruangrungsi and Mizukami2010; Mihalov et al. Reference Mihalov, Marderosian and Pierce2000; Srirama et al. Reference Srirama, Senthilkumar, Sreejayan, Ravikanth, Gurumurthy, Shivanna, Sanjappa, Ganeshaiah and Shaanker2010; Vongsak et al. Reference Vongsak, Kengtong, Vajrodaya and Sukrong2008; Wang et al. Reference Wang, Li, Ding, Peng and Yuan2007; Xue et al. Reference Xue, Li, Lu, Yang and Liu2006). Our study is unlike most examples reported in the scientific literature, in that we tested viable plants capable of spreading into the wild. Most reported studies test nonliving material, which is usually meant for human consumption. However, Honjo et al. (Reference Honjo, Ueno, Tsumura, Handa, Washitani and Ohsawa2008) tested the reported source of stocks of an endangered plant species, Primula sieboldii E. Morren. The authors found that at least 17% of the stocks studied were not derived from the reported source populations and argued that these stocks should not be used for restoration, because they might alter the gene pool of locally adapted populations.
For parties that purchase or propagate Celastrus scandens, including in gardens and native plant community restorations, reproductive structures are the best indication of the true species identity; differences between species include anther color of staminate flowers, inflorescence size and structure, and the color of fruit capsules. In the absence of reproductive structures, the best vegetative structure to differentiate the species is the shape of leaves unfolding from winter buds during spring leaf out (Leicht-Young et al. Reference Leicht-Young, Pavlovic, Grundel and Frohnapple2007a). Emerging C. orbiculatus leaves are conduplicately folded, while C. scandens leaves are involute. However, this characteristic is only visible for a brief period in spring. Other vegetative characteristics, such as mature leaf shape, can provide clues as to the true species identity, but those characteristics are not always conclusive and are not quantified for hybrids.
In the case of Celastrus in North America, what parties are responsible for the mislabeled samples? Can mislabeling be purely accidental? The two species are difficult to distinguish morphologically in the absence of flower or fruits (Leicht-Young et al. Reference Leicht-Young, Pavlovic, Grundel and Frohnapple2007a), and in most cases vendors are selling small plants that have not reached reproductive maturity. Thus, vendors that do not act as their own growers may not be responsible. It is implausible that producers and wholesale growers propagating C. orbiculatus at a large scale and over an extended period of time never observe the axillary inflorescences and yellow fruit capsules that readily distinguish the introduced vine from C. scandens, with its flowers in terminal panicles and orange fruit capsules. Indeed, we were surprised to find labels with our Diana and Hercules samples that included photos of mature plants that clearly had the yellow, axillary fruits of C. orbiculatus. Internet shopping searches for “American bittersweet” or “Celastrus scandens” yield results of products that are clearly C. orbiculatus. Mislabeling may still be unintentional, but it is avoidable.
Plant collectors and botanists interested in novel species started the invasion of C. orbiculatus in North America (Del Tredici Reference Del Tredici2014). The first introducers and propagators did not realize the potential for C. orbiculatus to spread in the wild, altering ecosystems and interfering with successful reproduction in a native congener. Nor did they likely espouse an understanding of biological invasions and the value of native planting that is increasingly the norm among scientists, horticulturalists, and the public at large. Parties responsible for the propagation of C. orbiculatus today are aiding in the invasion of a known problematic weed and exacerbating the decline of a native species that can be used as a horticultural substitute. Any attempt to halt mislabeling of C. orbiculatus must overcome the systematic issues that drive the practice, including intense market pressures, intensifying competition, and a lack of accurate information (Drew et al. Reference Drew, Anderson and Andow2010). One approach to curb the problem of mislabeled horticultural products is to attempt to institute penalties on suppliers through legal means. Another possible approach is to encourage self-policing. Both approaches have limitations, and a lack of proper enforcement may lead to virtually no improvement in the situation. Dissemination of useful information in a manner accessible to a wider public will help to create well-informed consumers and producers, which will in turn help the problem, although it may not eliminate it.
Acknowledgments
We would like to thank Ralph Grundel, Krystal Frohnapple, Boris Igic, Jeremie Fant, Henry F. Howe, John Wilk, Emi Kuroiwa, Janet Backs, Eun Sun Kim, Jason Palagi, Carrie Seltzer, Jenny Zambrano, Greg Spyreas, and two anonymous reviewers for providing helpful comments that improved the study and the article. Bob Streitmatter aided in sample collection. Kevin Feldheim helped with genetic analysis. Financial support was provided by the University of Illinois at Chicago, U.S. Geological Survey, Chicago Wilderness, and the Illinois State Academy of Science. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This article was completed in partial fulfillment of the doctoral degree from the Graduate College at the University of Illinois at Chicago to DNZ. The data for the study were archived on the University of Illinois Data Bank archive at http://https://doi.org/10.13012/B2IDB-3661776_V2.







