Introduction
It is imperative to effectively control invasive plants. An integrated regime for invasive plant control is a treatment in which one control method is followed by another. An increasing number of recent studies have shown that an integrated regime has higher control efficacy than a single method (Hammond Reference Hammond2001; Hedge et al. Reference Hedge, Kriwoken and Patten2003; Patten Reference Patten2002, Reference Patten2003; Roberts and Pullin Reference Roberts and Pullin2007; Shaw et al. Reference Shaw, Hymanson and Sasaki2016; Strong and Ayres Reference Strong and Ayres2016; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). For example, some studies have found that mowing followed by application of glyphosate can inhibit the growth and infestation of invasive species of the genus Spartina, common name cordgrass, more significantly than when either method is used alone (Hedge et al. Reference Hedge, Kriwoken and Patten2003; Patten Reference Patten2002, Reference Patten2003; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). ((Box 1))
Box 1 Management Implications

The high control efficacy of an integrated regime is believed to occur because such regimes incorporate dual treatments (Hammond Reference Hammond2001; Hedge et al. Reference Hedge, Kriwoken and Patten2003; Shaw et al. Reference Shaw, Hymanson and Sasaki2016; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). However, while many studies have indicated the importance of different types of methods on the control efficacy of an integrated regime, the effects of the intensity of an integrated regime and the interval between the applied methods have been mostly ignored and are still unclear.
The intensity of a control regime is the energy released per unit area and time (Chapin et al. Reference Chapin, Matson and Mooney2002). For example, fire intensity can be defined as the temperature that invaders experience, which may determine their probability of surviving the fire (Chapin et al. Reference Chapin, Matson and Mooney2002). Thus, the intensity of an integrated regime could influence the control efficacy. The stress tolerance and resilience of a plant varies at different life-history stages and can be altered by disturbances (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009; Tang et al. Reference Tang, Gao, Wang, Wang, Li, Chen and Zhao2009), implying that the interval between the methods used in an integrated regime may influence the regime’s control efficacy. Therefore, for an integrated regime to effectively control invaders, the appropriate combination of interval and intensity should be used.
Invasive species of the genus Spartina have invaded many marshes, from the Udale Gulf (57.61°N) in northern England to Amazon estuaries (near the Equator), and have changed the vegetation patterns and the structure of the trophic functional groups in these invaded marshes (Morgan and Sytsma Reference Morgan and Sytsma2013; Nieva et al. Reference Nieva, Castillo, Luquea and Figueroa2003; Strong and Ayres Reference Strong and Ayres2016; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). Alien Spartina competitively excludes the native plants on nearly every coast in China (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). Because these invaders have a higher root density than natives, such as Phragmites australis (Cav.) Trin and Cyperaceae, crabs in the invaded populations are usually smaller in size, and consequently, the depth, length, diameter, branch number, and degree of sinuosity of their burrows are also lower (Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010).
Many efforts have been made to control these invaders, and most studies have focused on improving the control efficacy of a single method (Evans Reference Evans1986; Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009; Mateos-Naranjo et al. Reference Mateos-Naranjo, Redondo-Gómeza, Coxb, Cornejob and Figueroaa2009; Patten Reference Patten2002, Reference Patten2003; Pritchard Reference Pritchard1996; Ranwell and Downing Reference Ranwell and Downing1960; Roberts and Pullin Reference Roberts and Pullin2007; Strong and Ayres Reference Strong and Ayres2016). Some studies show that the tide has a substantial influence on the ecophysiology and population dynamics of invasive Spartina (Abbas et al. Reference Abbas, Rubio-Casal, Cires, Figueroa, Lambert and Castillo2012; Nieva et al. Reference Nieva, Castillo, Luquea and Figueroa2003; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). In our previous studies, the regrowth of smooth cordgrass (Spartina alterniflora Loisel) after mowing decreased from the dike to the seaward side of Dongtan marsh, located on the Chinese coast. This is because tidal water limits the respiration of the mowed invader, and mowing therefore has greater control efficacy in low marsh than in high marsh (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009; Tang et al. Reference Tang, Gao, Wang, Wang, Li, Chen and Zhao2009, Reference Tang, Gao, Wang, Wang, Li, Chen and Zhao2010, Reference Tang, Gao, Wang, Li, Chen and Zhao2013). These findings imply that an integrated control regime of continuous submergence after clear mowing, with an appropriate interval and intensity, may work well for eradicating S. alterniflora.
The interval of continuous submergence after clear mowing is the time between mowing and submergence. The intensity of mowing is the energy consumed by people or machines during the removal of S. alterniflora ramets; therefore, it does not directly influence the regrowth of the invader. The submergence depth can represent the energy that is released by anaerobic respiration of mowed S. alterniflora in water and can substantially influence the regrowth of mowed S. alterniflora (Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014). Thus, the intensity of the regime is represented by submergence depth.
To apply the integrated control regime of continuous submergence after clear mowing and subsequently find a proper combination of intensity and interval, ecological engineering was conducted in Dongtan marsh. This engineering consisted of creating earth cofferdams. These cofferdams enclose the invasive populations and retain water. Thus, submergence can be performed after mowing.
Recovery of crab populations can be considered an objective of invasive S. alterniflora management. Crabs are omnivorous crustaceans that consume diverse foods, including fresh plant material, litter, and carrion (Bortolus and Iribarne Reference Bortolus and Iribarne1999; Gittman and Keller Reference Gittman and Keller2013). Moreover, crab burrows can significantly modify microtopography; improve soil aeration, oxidation, and penetrability; increase soil carbon and nitrogen; and decrease soil bulk density (Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010). Thus, crab populations and, subsequently, the distributions of their burrows, are associated with the transfer of matter and energy flow in wetlands; as a result, they can significantly influence nutrient availability for plants, including invasive S. alterniflora and native species (Bortolus and Iribarne Reference Bortolus and Iribarne1999; Gittman and Keller Reference Gittman and Keller2013; Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010). Therefore, if practices for the control of invasive S. alterniflora can simultaneously improve the recovery of crab populations, the recovery of the components, structures, and functions of invaded wetlands will also be improved.
We predict that a proper combination of intensity and interval exists and will enable the integrated regime of continuous submergence after clear mowing to substantially reduce S. alterniflora populations, while also providing mudflat-like habitat conducive to crab recovery. We also predict that the ecological engineering of cofferdams will provide the proper conditions required to implement the regime. If so, this study would offer a method for controlling invasive cordgrass and restoring habitat for native crabs.
Materials and Methods
Invader Control
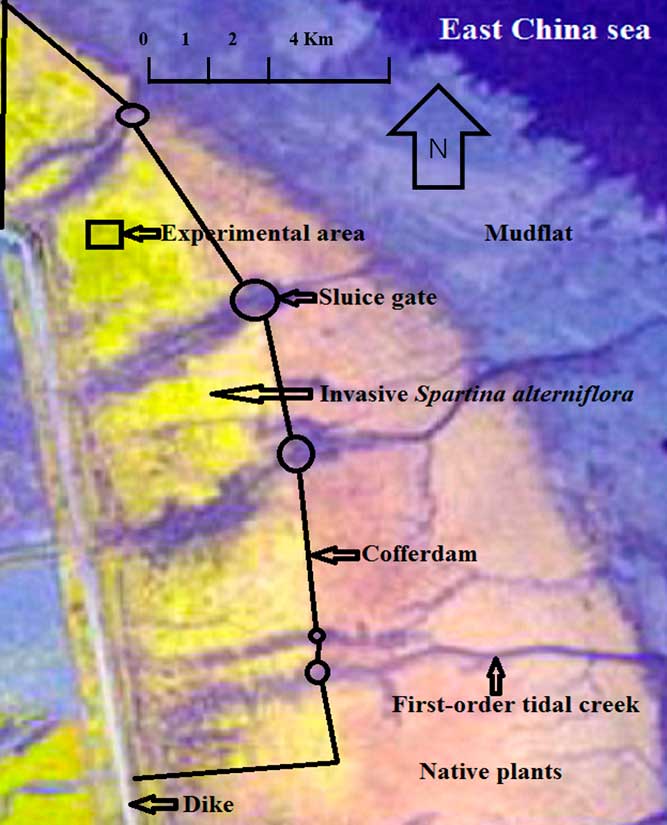
The experiment was conducted in Dongtan marsh (31°25′11.57″ N to 31°37′27.25″N, 121°51′13.41″E to 122°01′54.64″E), located on Chongming Island in the Yangtze River estuary, Shanghai, China (Figure 1). The sediment type in Dongtan marsh is clay. From the dike to the seaward side of Dongtan marsh, the ground is submerged by tidal water for ca. 6 h/tidal cycle to ca. 40 h/tidal cycle (Tang et al. Reference Tang, Gao, Wang, Li, Chen and Zhao2013). In this wetland, the canopy height of the S. alterniflora population varies considerably and, depending on growing conditions, can be as high as 250 cm; moreover, S. alterniflora can produce large amounts of aboveground dry biomass, for example, 1,640±176 (g m−2) (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009).

Figure 1 Diagram of the experimental area and the cofferdam construction locations in Dongtan marsh.
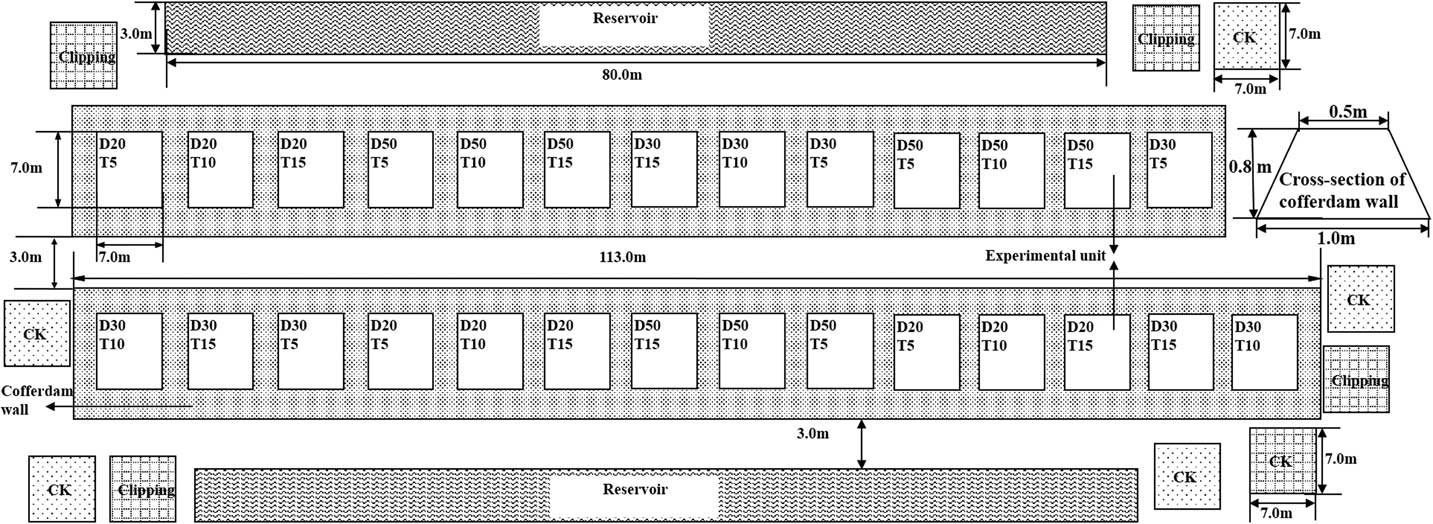
The experiment was conducted as a two-factor design (n=3), with three interval levels between clear mowing and continuous submergence (5, 10, and 15 d) and three depth levels for continuous submergence (20, 30, and 50 cm), which represent the intensity of the integrated regime.
These treatments were randomly distributed among the 27 earthen cofferdams that enclosed S. alterniflora populations. The cross section of the cofferdam wall was in the shape of an isosceles trapezoid of 0.5 m by 1.0 m by 0.8 m (top length by bottom length by height), and the area of each cofferdam was 7.0 m by 7.0 m (length by width) (Figures 2 and 3).

Figure 2 Experimental processes: A, cofferdams; B, experimental units; C, Spartina alterniflora eradication and gradual collapse of cofferdams.
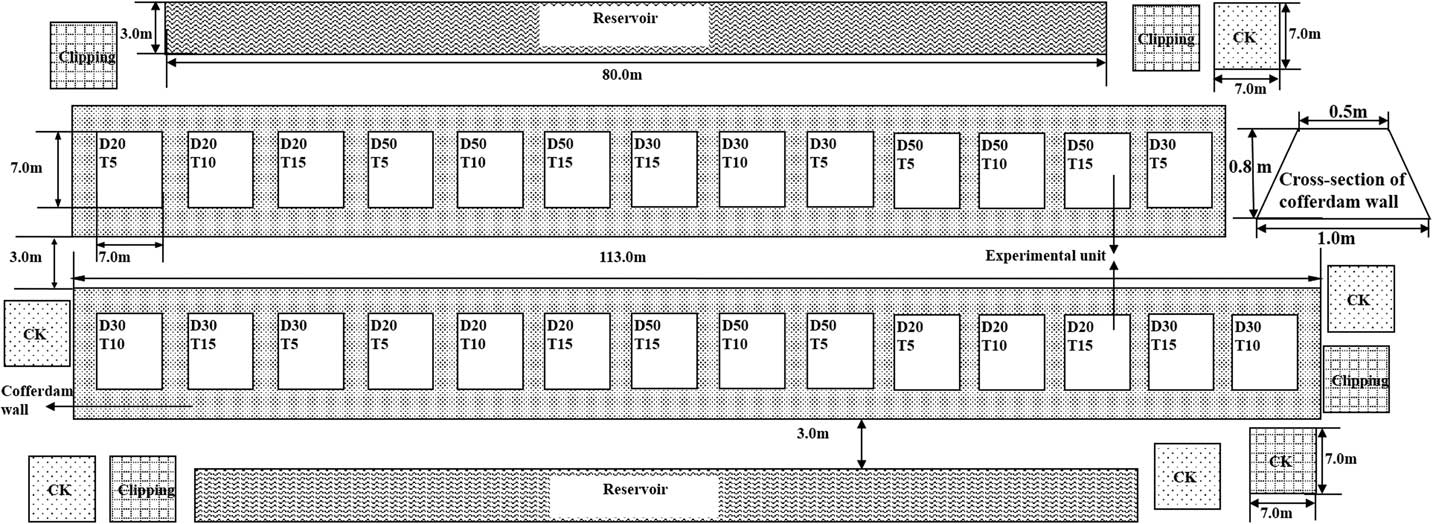
Figure 3 Schematic representation of the experimental layout in the study area. Submergence depths (D): D20, 20 cm; D30, 30 cm; D50, 50 cm. Interval between submergence and mowing (T): T5, 5 d; T10, 10 d; T15, 15 d. ![]() , Experimental unit in cofferdam,
, Experimental unit in cofferdam, ![]() , reservoir created by digging the mud to build the cofferdams;
, reservoir created by digging the mud to build the cofferdams; ![]() , earth dam;
, earth dam; ![]() , mowing quadrats;
, mowing quadrats; ![]() , control quadrats.
, control quadrats.
Spartina alterniflora ramets in the cofferdams were mowed using sickles, as close as possible to the ground, and then their roots were submerged. We mowed S. alterniflora in all cofferdams in early July, which we previously reported to be effective (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009). Subsequently, we pumped reservoir water into the cofferdams for the continuous submergence treatment. The reservoirs include natural pools and puddles created when digging the mud to build the cofferdams. During the experiment, we monitored the water level every 2 wk and pumped water into the cofferdams when the water level dropped below the level required by the experimental design.
At the same time, 10 quadrats (7.0 m by 7.0 m) were randomly established in the populations of S. alterniflora near the cofferdams. The populations within five quadrats were mowed once, and populations in the other five quadrats were not treated; thus, they served as a control (Figure 3).
We tried to eradicate S. alterniflora populations. Therefore, the ramet density of treated S. alterniflora was the best and most important index to estimate the efficacy of control. Spartina alterniflora is a perennial geophyte. Our previous studies showed that even though some populations of this plant suffered from high-frequency mowing treatments and could not sprout in the same year, they were able to grow the following year (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009; Tang et al. Reference Tang, Gao, Wang, Wang, Li, Chen and Zhao2009, Reference Tang, Gao, Wang, Wang, Li, Chen and Zhao2010). Moreover, a study on the seasonal growth pattern of S. alterniflora showed that the ramet density of the plant did not significantly increase due to the onset of sexual reproduction (Gao et al. Reference Gao, Tang, Wang, Wang, Liang, Li, Chen and Zhao2009). Thus, we determined that mowed S. alterniflora can be considered to be dead only if it has no ramets at the end of July in the following year. Therefore, we measured the ramet density of S. alterniflora in each treatment and the control on July 30 of the following year, when the roots of mowed S. alterniflora had been submerged for 12 mo.
Because the densities of S. alterniflora in some treatments were zero, the probability distributions of the data were not normal, and the variances were heterogeneous. Thus, the differences in density among the ecologically engineered (i.e., integrated regime), the single-mowing treatment, and the control were tested using the Kruskal-Wallis nonparametric test, with a post hoc test. Friedman’s two-way analysis, with a post hoc test, was used to test the effects of submergence depth and interval on plant density. To conduct the post hoc test, we used an SAS (SAS Institute, Cary, NC) macro implementation of a multiple-comparison test, based on significant Kruskal-Wallis results from the SAS NPAR1 WAY procedure (Elliott and Hynan Reference Elliott and Hynan2011). A P-value lower than 0.05 was considered to be statistically significant.
Crab Distribution
When we tested control efficacy, we documented crab species and estimated their abundances and biomasses among three habitats: areas in the cofferdams, S. alterniflora populations, and mudflats. The crabs were sampled using pitfall traps. The traps were cylindrical plastic buckets (45-cm diameter, 50-cm depth) buried in the soil so that the upper part was level with the soil surface. Five holes (2-cm diameter) were drilled in the bottom of each bucket to allow tidal-water flow.
After the floodwaters in the cofferdams were drained away, a trap was set in each cofferdam. At the same time, 27 traps were set in S. alterniflora populations, and 27 traps were set in mudflats. The distance between adjacent traps was 7.0 m. Thus, there was a trap in each 49 m2 (7.0 m by 7.0 m) of the S. alterniflora population and mudflat. Preliminary tests showed that crabs were unable to escape from the traps, and after 1 d, the crabs in the traps were collected.
The abundance and biomass of each captured crab species were measured. The biomass and abundance data could not be normalized by transformation. Therefore, Friedman’s two-way analysis with a post hoc test was used to test the effects of habitat and crab species on biomass and abundance. Differences in biomass and abundance of each crab species among the cofferdams, invasive plant populations, and mudflats were tested using the Kruskal-Wallis nonparametric test, with a post hoc test. The post hoc test was the same as was used for analysis of invader control. A P-value lower than 0.05 was considered to be statistically significant.
Results and Discussion
Effects of Interval and Intensity
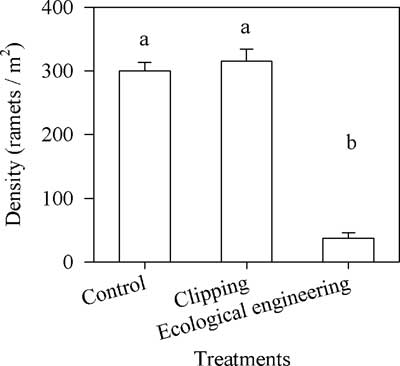
There was no significant difference in the density of S. alterniflora between the control and single-mowing treatment, and the density was ca. 307±35 ramets m−2 (average±SE); however, the density of S. alterniflora treated with submergence after mowing was much lower compared with densities in the control and the single-mowing treatment (Kruskal-Wallis nonparametric test, df=2, H=22.09, P<0.01) (Figure 4).
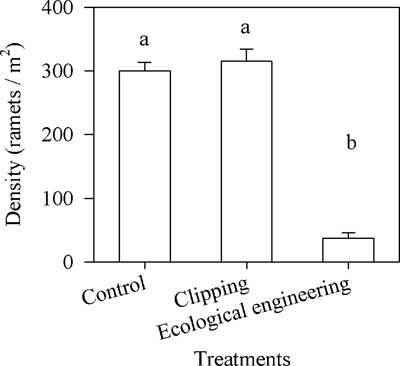
Figure 4 Effects of mowing and the integrated regime of continuous submergence after clear mowing (i.e., ecological engineering) on the density of Spartina alterniflora. Vertical bars indicate standard errors. The letters above the columns denote significant differences (Kruskal-Wallis nonparametric test, 5% significance level).
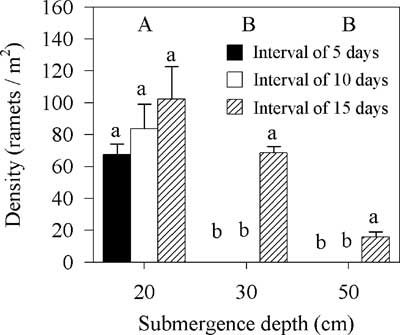
Spartina alterniflora density declined when treated with submergence after mowing with a decrease in the interval between mowing and submergence (Friedman’s two-way analysis, χ2=25.91, df=2, P<0.01); moreover, the density significantly declined with an increase in submergence depth (Friedman’s two-way analysis, χ2=35.22, df=2, P<0.01) (Figure 5).
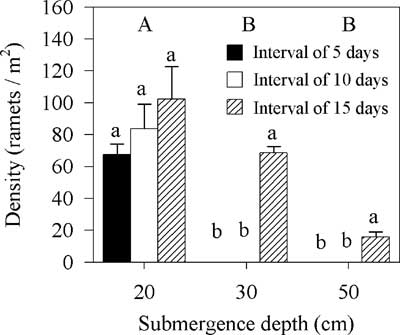
Figure 5 Effects of submergence depth and interval between mowing and submergence on the density of Spartina alterniflora. Vertical bars indicate standard errors of three replicates. Capital letters denote significant differences among submergence-depth groups; lowercase letters denote significant differences among interval groups (Friedman’s two-way analysis for submergence depth and interval, 5% significance level).
There were some ramets in the treatment with an interval of 15 d and in the treatment with a submergence depth of 20 cm (Figure 5). After evaluating different combinations of interval and intensity, we determined invasive S. alterniflora could be eradicated using the integrated regime with a submergence depth of 30 or 50 cm and an interval (time between mowing and submergence) of 5 or 10 d (Figure 5).
Abundance and Biomass of Crabs
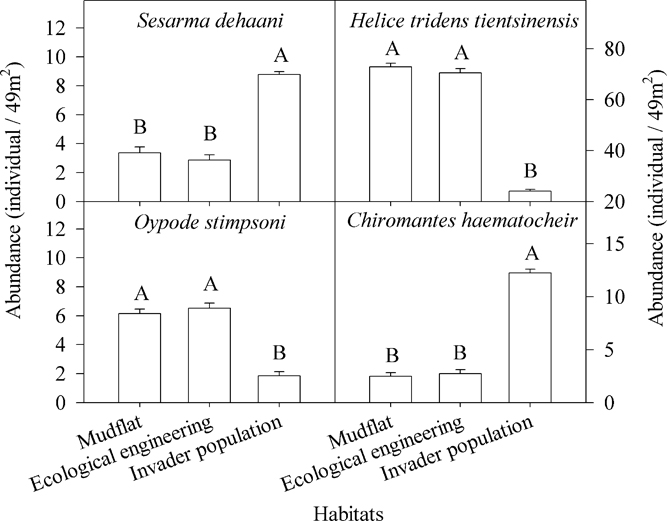
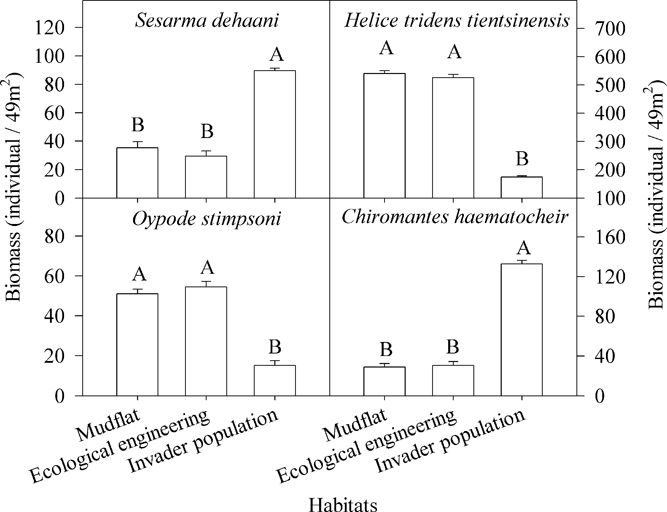
Four species of crabs were captured: Sesarma dehaani H. Milne-Edwards (Sesarma picta), Helice tridens tientsinensis Rathbun, Ocypode stimpsoni Ortmann (Uca arcuata), and Chiromantes haematocheir de Haan (Sesarma haematocheir) (Figures 6 and 7).
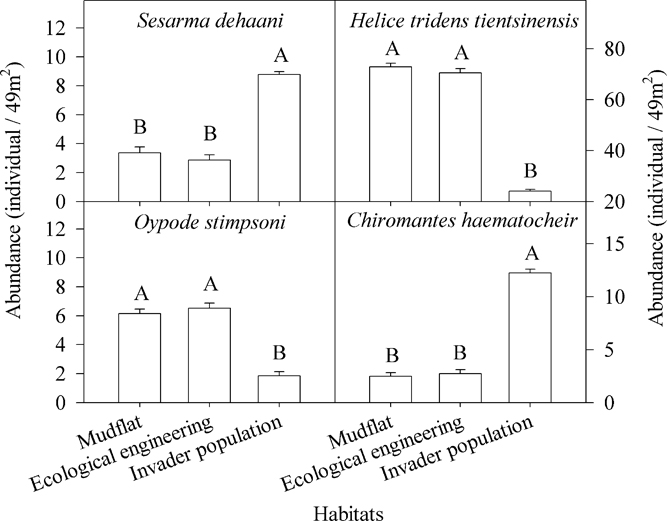
Figure 6 Differences in crab abundance among invader Spartina alterniflora populations, areas within the cofferdams (i.e., ecological engineering), and mudflats. The letters above the columns denote significant differences. Vertical bars indicate standard errors (Kruskal-Wallis nonparametric test, 5% significance level).
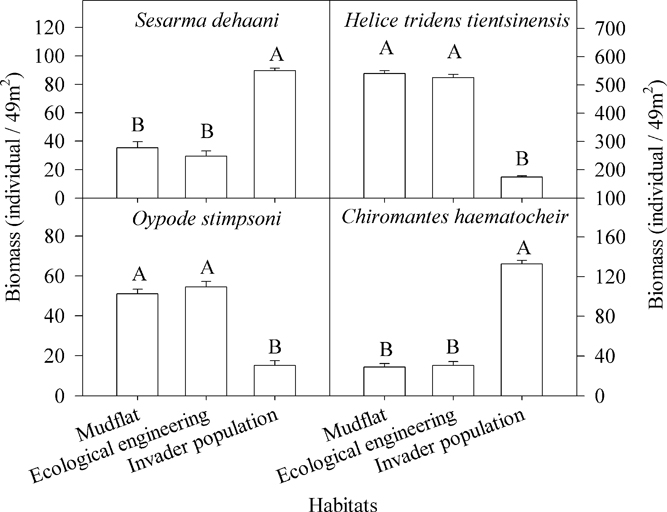
Figure 7 Differences in crab biomass among invader Spartina alterniflora populations, cofferdams (i.e., ecological engineering), and mudflats. The letters above the columns denote significant differences. Vertical bars indicate standard errors (Kruskal-Wallis nonparametric test, 5% significance level).
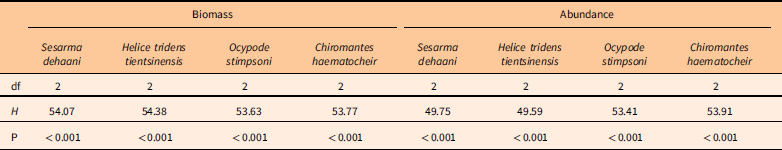
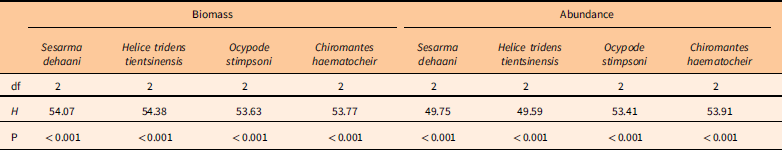
There were significant differences among the biomass and the abundance of the four crab species (Table 1; Figures 6 and 7). The biomass and abundance of H. tridens tientsinensis was ca. 413.7 g 49 m−2 and ca. 55 individuals 49 m−2, respectively, representing the highest biomass and abundance among the four crab species. Those of C. haematocheir were ca. 64.1 g 49 m−2 and ca. 6 individuals 49 m−2, respectively; moreover, the biomass of S. dehaani was 51.5 g 49 m−2 and that of O. stimpsoni was 40.2 g 49 m−2, and they had a similar abundance, which was ca. 5 individuals 49 m−2 (Figures 6 and 7).
Table 1 Summary of Friedman’s two-way analysis for the effect of habitat and crab species on the biomass and the abundance of captured crabs.

The biomass and abundance of each species in S. alterniflora populations significantly differed from those in the mudflats and in the cofferdams, and there were no significant differences between the abundance and biomass of species found in the mudflats and the cofferdams (Table 2; Figures 6 and 7). The biomasses of S. dehaani and C. haematocheir in S. alterniflora populations were ca. 127% and 342% higher, respectively, than those in the mudflats or the cofferdams, and the abundances of these two crab species in S. alterniflora populations were ca. 192% and 390% higher, respectively; moreover, the biomasses of H. tridens tientsinensis and O. stimpsoni in S. alterniflora populations were ca. 67.3% and 70.7% lower, respectively, than those in the mudflats or the cofferdams, and the abundances of these two crab species in S. alterniflora populations were ca. 66% and 70.6% lower, respectively (Figures 6 and 7).
Table 2 Summary of Kruskal-Wallis nonparametric test on the difference in biomass and abundance of crab species among Spartina alterniflora populations, areas within the cofferdams (ecological engineering), and mudflats.
Importance of a Proper Combination of Intensity and Interval in Control Efficacy
Plants will allocate resources from remnants to buds or meristematic tissue for regrowth after defoliation (Briske and Richards Reference Briske and Richards1995; Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014). Spartina alterniflora has developed rhizomes that store large amounts of oxygen and energy (Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). These resources can be diverted to the buds or other aboveground parts for ramet regrowth after mowing (Briske and Richards Reference Briske and Richards1995; Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014). Several days later, the resources in the rhizomes will be recovered due to anabolism and photosynthesis of the regrowing ramets (Briske and Richards Reference Briske and Richards1995; Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014).
Thus, if the continuous submergence depth is too low, even if submergence occurs shortly after mowing, the regrowing ramets of S. alterniflora can grow out of the water using the stored resources in the rhizomes. On the other hand, if the interval between mowing and submergence is too long, even if the submergence depth is sufficient, the regrowing ramets can grow out of the water using the recovered resources (Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014; Tang et al. Reference Tang, Gao, Wang, Li, Chen and Zhao2013). Once the ramets grow out of the water, they can provide oxygen and photosynthates that enable the whole clone to survive (Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014). This may be why there were some ramets when the treatment submergence depth was 20 cm and the treatment interval was 15 d. In contrast, when the submergence depth and the interval are appropriate, there may be too few resources left in the rhizome to support ramet growth out of the water; thus, the mowed S. alterniflora is asphyxiated (Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014).
Previous studies have shown that invasive species of the genus Spartina can be efficiently controlled through an integrated regime of aboveground biomass removal followed by herbicide application (Hammond Reference Hammond2001; Hedge et al. Reference Hedge, Kriwoken and Patten2003; Wang et al. Reference Wang, An, Ma, Zhao, Chen and Li2006). The interval of the regime is the time between ramet removal and herbicide spraying. The intensity can be represented by the concentration of herbicide, as it reflects the energy that is released from the chemical reaction between the herbicide and the molecules in the plant cells. Some ecologists suggest that glyphosate should be applied at a concentration of 8.8 kg ha−1 when the new ramets of mowed Spartina reach heights of 30 to 45 cm; otherwise, this dual-treatment approach is time-consuming and expensive, with costs ranging from US$1,700 to US$3,700 ha−1 (Hammond Reference Hammond2001; Hedge et al. Reference Hedge, Kriwoken and Patten2003). Both these findings and our results highlight the importance of using a proper combination of intensity and interval between treatment methods to maximize the control efficacy of an integrated regime.
Ecological Engineering
Ecological engineering can be used to create the required conditions for implementing an integrated regime (Bergen et al. Reference Bergen, Bolton and Fridley2001). In this study, the cofferdams changed the topography and hydrology of the invaded regions in the Dongtan salt marsh, thereby creating a hypoxic environment for the mowed S. alterniflora (Gao et al. Reference Gao, Yan, Li, Zhao, Li, Li and Tang2014). Thus, cofferdams should be built to enclose invasive S. alterniflora and retain water.
We recommend the use of this technique across large areas. The construction of cofferdams is a civil engineering project. The geotextile-encased sand-column method is recommended, because it is suitable for clay and sand sediment and is economical compared with other methods, such as the rear-anchored raking-pile solution (Yang et al. Reference Yang, Bi, Han and Liao2014). Geotextile columns are sunk into the sediment ca. 0.5 m, with a distance between the neighboring columns of ca. 15 m (Yang et al. Reference Yang, Bi, Han and Liao2014). Biodegradable, disposable, nonwoven cloth bags filled with clay are placed among the columns to complete the installation of the cofferdams.
To reduce costs, the height of the cofferdam should be as low as possible. According to our results, the height can be slightly more than 30 cm, which was an adequate depth for the continuous submergence treatment to function. Moreover, to facilitate water storage, the cofferdams should be constructed based on the distribution of tidal creeks along invaded coasts.
Wang et al. (Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010) show that the tidal creeks of a coast can often be divided into three classes. First-order creeks, with a width of tens of meters, develop in high-tidal marshes and low-tidal mudflats and enter the subtidal zone (Figure 1). The width of second-order creeks is smaller but may be up to 10 m; these end at the outer margins of high-tidal flats. Third-order creeks, with a width of only approximately 1 m, are mainly distributed in the mudflats. The first-order tidal creeks are the main channels of tidal flow along coasts.
On the coastal section where experiments are conducted, some cofferdams should be built perpendicular to the first-order tidal creeks (Figure 1). These cofferdams should enclose the S. alterniflora populations that are closest to the sea. A sluice gate is needed at a first-order tidal creek to connect these cofferdams. Moreover, two cofferdams are needed to connect the dike and those cofferdams that are perpendicular to the first-order tidal creeks, and these two cofferdams should enclose the S. alterniflora populations that are located at the two ends of the coast (Figure 1). Thus, all invasive populations will be enclosed in the cofferdams.
According to our results, S. alterniflora ramets enclosed within a cofferdam need to be removed within 10 d. Then, water should be poured into the cofferdam, and the water level should be maintained until June of the following year. The sluice gates should be opened when the tidal level is higher than the water level in the cofferdams, and gates should be kept closed to retain water when the tidal level is low. During the submergence period, any leaks observed in the cofferdams should be repaired. Additionally, there may be higher regions in the microtopography of large areas. Thus, the submergence depth will be lower than 30 cm, and S. alterniflora may have some regrowing ramets that need to be removed.
After invasive S. alterniflora is eradicated, the ecological engineering provides a mudflat-like habitat for crabs. Thus, the biomasses and abundance of four crab species in the area of ecological engineering were similar to those in the mudflats. Notably, the performances of S. dehaani and C. haematocheir were greater in S. alterniflora sites than in the mudflats or ecologically engineered sites. The analysis of feeding preference proves that these two crab species consume invasive S. alterniflora more than twice as much as native plants such as P. australis and Cyperaceae (Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010). Interestingly, some invasive plants such as Impatiens glandulifera Royle have a particular trait of pollen abundance; this invader can attract a higher visitor species richness, visitor abundance, and flower visitation than native plants (Lopezaraiza-Mikel et al. Reference Lopezaraiza-Mikel, Hayes, Whalley and Memmott2007). This implies that greater abundance of native animals in invader sites may simply be a result of decreased animal abundance in native plant sites rather than an absolute increase in their numbers. Thus, the reason for the greater abundance of those two crab species in S. alterniflora sites may just be that S. alterniflora offers a more suitable food source for the crabs, not that crab abundance increases after the invasion.
If so, the population sizes of S. dehaani and C. haematocheir would decrease over time, because S. alterniflora can have a negative impact on the crabs' development and reproduction (Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010). Moreover, the function of the two crab species will be limited. Because crabs burrow, they can entirely mix the surface and deeper layers of sediment over a period of 1 to 4 yr and accelerate litter decomposition (Gittman and Keller Reference Gittman and Keller2013; Silliman and Bortolus Reference Silliman and Bortolus2003; Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010). However, the size and the density of crab burrows in S. alterniflora sites, as mentioned in the “Introduction,” are lower than those in native plant sites or mudflats (Wang et al. Reference Wang, Zhang, Jiang, Bertness, Fang, Chen, Hara and Li2010). Thus, the crabs in S. alterniflora sites have a low effect on transfer of matter and energy. Consequently, the crabs have a limited ecosystem benefit in the entire marsh, because too many of them are in S. alterniflora sites.
After ecological engineering projects are implemented, natural processes take over, and the species composition is dominated by those that are best suited to respond to the conditions imposed upon the system (Bergen et al. Reference Bergen, Bolton and Fridley2001). In this study, when the invader is eradicated by the ecological engineering, that particular food source is eliminated, and the distributions of these crabs in the entire marsh will be recovered. Thus, the nutrients available to native vegetation and subsequently to other native species will be increased, prompting their recovery.
While the crab population distribution recovered, our cofferdams were gradually eroded by tides (Figure 2C). Similarly, if the cofferdams are constructed of biodegradable, disposable nonwoven cloth bags filled with clay, they will disappear over time. Cofferdams can also be constructed using encapsulated-air plastic, which can be easily collected. Managers have applied a similar ecological engineering effort in the Dafeng wetlands, Jiangsu province, China, to control Spartina populations and to prompt the recovery of native species and coastal topography, which was changed by invasive S. alterniflora.
Implications
Considerable resources have been invested in controlling invasive species of the genus Spartina, and management practices have evolved since the 1960s, from small-scale field trials to a large-scale integrated pest management programs (Evans Reference Evans1986; Mateos-Naranjo et al. Reference Mateos-Naranjo, Redondo-Gómeza, Coxb, Cornejob and Figueroaa2009; Patten Reference Patten2002, Reference Patten2003; Pritchard Reference Pritchard1996; Ranwell and Downing Reference Ranwell and Downing1960; Roberts and Pullin Reference Roberts and Pullin2007; Strong and Ayres Reference Strong and Ayres2016). At present, the most effective Spartina control technique is a single-mowing event followed by glyphosate application (Hedge et al. Reference Hedge, Kriwoken and Patten2003). The integrated regime of submergence after mowing applied in cofferdams can be expected to control invasive S. alterniflora in a single growing season and subsequently provide a habitat similar to bare soil that promotes the recovery of native crabs. More broadly, invasive species of the genus Spartina may be controlled using the integrated regime of submergence after mowing. These integrated regimes in which one control method is followed by another are efficient for controlling invasive plants when the regime includes the proper combination of intensity and interval between treatment methods; additionally, such an efficient regime subsequently provides habitat similar to bare soil, which promotes the recovery of some native consumers. Relevant ecological engineering can be conducted to create the conditions required for implementing an integrated regime.
Acknowledgments
This work was funded by the National Natural Science Foundation (no. 31670548) and the Fundamental Research Funds for the Central Universities. We thank the anonymous reviewers, editor Antonio DiTommaso, and the associate editor for important comments on the article. No conflicts of interest have been declared.