To be successful, invasive species must generate and disperse large numbers of propagules in their introduced range (Baker and Stebbins Reference Baker and Stebbins1965; Burns et al. Reference Burns, Pardini, Schutzenhofer, Chung, Seidler and Knight2013; Hawkes Reference Hawkes2007; Pyšek and Richardson Reference Pyšek and Richardson2007). The density of propagules produced by an introduced species is termed its “propagule pressure” and is viewed as a major factor influencing the outcome of species introductions (Blackburn and Duncan Reference Blackburn and Duncan2001; Carlton Reference Carlton1996; Lockwood et al. Reference Lockwood, Cassey and Blackburn2005). At low propagule pressures, propagule densities may not be sufficient to overcome biotic resistance in the introduced range (e.g., from competitors, consumers, or disease) (Elton Reference Elton1958; Mack et al. Reference Mack, Simberloff, Lonsdale, Evans, Clout and Bazzaz2000), and at high pressures, propagule densities become saturating. Thus, it is thought that establishment probability varies most greatly within a critical window of propagule pressures, termed the “invasion cliff” (Davis Reference Davis2009).
Box 1 Management Implications
Johnsongrass is an invasive weed that presents a continuous threat to croplands and natural ecosystems throughout much of the southern and central United States and temperate and subtropical regions worldwide. It is noteworthy among weeds for having dual reproductive modes, and its capacity to propagate via both extensive seed production and underground clonal growth have been implicated in its ability to invade a variety of ecosystems. At two Virginia farms, we find that both forms of reproduction are efficient both in the presence and absence of competition. Our results suggest that a single Johnsongrass plant, if left unattended, produces enough seeds and rhizomes to establish an area exceeding a hectare. Although on a per propagule basis rhizomes reproduce more efficiently than seeds, on a per unit of carbon basis seeds are more efficient. Our data strongly indicate that successful management of Johnsongrass requires controlling its reproduction from both rhizome and seed.
Plants produce many different types of propagules that are suited for different environments (Grime Reference Grime1977). For example, invasive plants producing large numbers of small, mobile seeds may have high fecundity and dispersal ability and may perform well in disturbed environments. In contrast, species that use vegetative reproduction are hypothesized to be suited for perennial environments with strong grazing pressure and intense intraspecific competition (Baker Reference Baker1974). Research has shown that invasive species are often, but not always, distinguished from native species and from noninvasive exotic species by large size, fecundity, and a tendency toward vegetative reproduction (Burns et al. Reference Burns, Pardini, Schutzenhofer, Chung, Seidler and Knight2013; Hawkes Reference Hawkes2007; Pyšek and Richardson Reference Pyšek and Richardson2007).
Many invasive weeds are capable of both clonal and sexual reproduction. Seeds and vegetative organs used for clonal propagation (e.g., rhizomes, stolons, or stem fragments) differ in their construction, maintenance, and opportunity costs (Bell Reference Bell1980; Obeso Reference Obeso2002) and their dispersal dynamics. The relative benefits of sexual reproduction versus clonal propagation are therefore likely to depend on both the abiotic and biotic environments (Campbell Reference Campbell2000; Dorken and Eckert Reference Dorken and Eckert2001; Obeso Reference Obeso2002). Sexual reproduction is hypothesized to be more beneficial than clonal propagation for plants growing at high density, because it promotes dispersal (Abrahamson Reference Abrahamson1975). However, clonal spread may also help plants survive competition caused by crowding, and in some situations vegetative organs can also disperse (Loehle Reference Loehle1987). For example, flooding of riparian areas and tillage of agricultural fields can disperse clonal fragments (Catford and Jansson Reference Catford and Jansson2014), potentially favoring vegetative spread as a mechanism of improving both local persistence and dispersal.
Johnsongrass [Sorghum halepense (L.) Pers.] is a perennial C4 grass that has strong negative effects on the biodiversity of natural systems and the yield of agricultural systems, where it is a common weed globally (Warwick and Black Reference Warwick and Black1983). Johnsongrass is frequently cited as one of the world’s worst weeds (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977). Its invasive success is attributed to its competitive ability, reproductive vigor, and ability to reproduce both sexually and clonally, producing up to 1 kg of shattering seeds per year (100,000 to 250,000 seeds) and growing 60 to 90 m of rhizomes with millions of nodes in a single season (McWhorter Reference McWhorter1961; Warwick and Black Reference Warwick and Black1983). This flexibility has enabled Johnsongrass to colonize much of the United States since its North American introduction 140 yr ago (McWhorter Reference McWhorter1971). Due to its dual reproductive modes, ability to colonize a variety of habitats, and global threat to food and biosecurity, Johnsongrass is a useful species for exploring how differential modes of reproduction influence the success of invasive weeds.
In this study, we investigate how reproduction via seeds and rhizomes influenced the establishment and growth of Johnsongrass in both the presence and absence of interspecific competition. Specifically, we ask: (1) What amount of vegetative propagules versus seeds are needed for Johnsongrass establishment? (2) How does establishment probability and aboveground biomass production differ between seeds and rhizomes on a per propagule basis when accounting for their carbon content (as a proxy for investment cost)? (3) How does competition affect growth and establishment of both seeds and rhizomes? Answers to these questions have the potential to shed significant light on how dual reproductive strategies influence the success of invasive species in general and Johnsongrass specifically.
Materials and Methods
Propagules
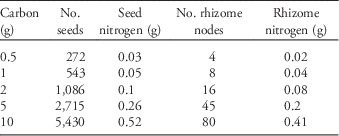
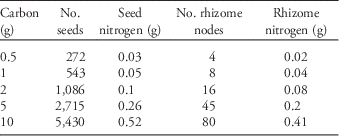
We obtained seeds from a commercial supplier (Azlin Seed Service, 112 Lilac Drive, Leland, MS 38756). Some of those seeds we planted directly into the common garden and others we used to generate rhizomes by planting them in an adjacent field the year prior to initiation of the experiment. Because resources are finite and plants must allocate among competing needs (e.g., flowers, roots, defenses), we held the amount of tissue carbon constant among seeds and rhizomes and then varied their planting densities. To determine the tissue carbon content of seeds and rhizomes, we dried three 10-g samples of seeds and 20 single-node rhizome fragments at 70 C for 5 d. The dried tissue was then homogenized and ground to 1-mm size and analyzed for total elemental carbon and nitrogen. From this analysis we determined that 1 g C corresponded to 543 seeds (=0.05 g N) and eight single-node rhizomes (=0.04 g N), which was used to calculate the amount of both seeds and rhizomes containing 0.5, 1, 2, 5, and 10 g C (total propagule carbon content; Table 1). We chose these amounts because they span an ecologically realistic range of possible propagule densities in a given area of ground. We were careful to use rhizomes fragments that were as similar as possible in size.
Table 1 The number of seed and rhizome propagules corresponding to the amount of total propagule carbon, as well as the amount of corresponding nitrogen.
Study Sites
We conducted the experiment over a single season at two locations in the Blacksburg, VA, area: Kentland Farms (37.1933°N, 80.5728°W) and the Glade Road Research Facility (37.2335°N, 80.4370°W). The site at Kentland was established in a low-elevation floodplain containing Ross series soils (fine-loamy, mixed, superactive, mesic cumulic Hapludolls) and was historically used for agronomic research. The site at Glade Road was a higher-elevation, less productive site used historically as both a pasture and a farm. Glade Road had a complex of Duffield series (fine-loamy, mixed, active, mesic Utic Hapludalfs) and Ernest series (fine-loamy, mixed, superactive, mesic Aquic Fragiudults) soils (data from USDA Web Soil Survey, http://websoilsurvey.nrcs.usda.gov/app/WebSoilSurvey.aspx [accessed December 4, 2016]). These sites are representative of two common habitats for Johnsongrass in southwestern Virginia: floodplain croplands and gently sloped fields. At each location, the experiment consisted of a split-block design with eight replicates. We planted plots in a grid of 16 rows of 10 plots. In each row, all propagule type and density treatments were randomized. Each of the eight pairs of rows constituted one block, with one full row in the block sprayed for the weed-removal treatment and the other being left untreated. Within each block the main effect was with or without the resident plant community (i.e., +/– competition), and the subplot was the propagule type (seed or rhizome) and propagule carbon (0.5, 1, 2, 5, or 10 g). In sum, there were 20 plots in each block: 2 communities by 2 propagule types by 5 carbon levels (Supplementary Figure S1). We tilled both study locations in April and then applied glyphosate (RoundUp ProMax®, 2% v/v, Monsanto, St. Louis, MO 63141) to remove all standing plant material. We maintained the competition-free treatment using biweekly application of broadleaf herbicide (2,4-D amine, 2% v/v, distributed by Universal Crop Protection Alliance, Eagan, MN 55121) and hand pulling. In plots containing resident plants, competition was intense at both sites. Propagules were planted in mid-May into a 1-m2 area centered in a 2-m2 treated area to account for edge effects. This planting date may have been slightly late for Johnsongrass, which germinates with the spring flush (Warwick and Black Reference Warwick and Black1983), but it was necessary in order to apply our treatments, to avoid a late frost, and to obtain all necessary rhizome fragments. Rhizomes were planted in a 10-cm grid at a 1-cm depth, while seeds were evenly sown across the same area on the soil surface.
Data Collection
At the end of the growing season in early October, we counted the number of culms in each plot and harvested, dried, and weighed all Johnsongrass aboveground vegetative biomass (i.e., stems and leaves) and reproductive biomass (i.e., panicles).
Data Analysis
Establishment was fitted using a generalized linear mixed model with a binomial distribution and a complementary log-log link, because this link function most accurately models plot establishment as a function of log-transformed planting density (Barney et al. Reference Barney, Ho and Atwater2016). Under this model, the regression coefficient (B) for log-transformed planting density represents the strength of density-dependent effects on per capita germination probability. A value of B=0 means no density dependence (per capita germination probability is the same at all densities), and a value of B=−1 indicates strong density dependence (in which per capita germination probability drops so sharply as densities increase that all propagule densities produce the same absolute number of germinating propagules in a given area). The regression coefficients for the factor variables competition and site indicate the effect of biotic resistance and site suitability, respectively. A negative regression coefficient (B) for competition would mean that interspecific competition increases the resistance of the community to invader establishment.
We considered a plot to be “established” if at least one Johnsongrass persisted through the end of the experiment. Establishment was modeled as a function of community type, propagule type, log-transformed carbon content (hereafter, “ln-carbon”), and site, with only main effects specified. Carbon content was log transformed to linearize the relationship between propagule density and Johnsongrass growth and establishment, because establishment and growth are predicted to have a log-log relationship with propagule density (Barney et al. Reference Barney, Ho and Atwater2016). We restricted the complexity of the establishment model because some treatments had zero variance (e.g., all or no propagules germinated), giving us low confidence in our ability to estimate higher-order effects accurately. For plots that established, we analyzed the effects of propagule type, ln-carbon, and community type on cube-root-transformed aboveground biomass, square-root-transformed culm number, and cube-root-transformed biomass produced per culm. Reproductive biomass was not modeled because of a tight correlation with total biomass (r=0.872). In each model, community type, propagule type, and site were modeled as fixed factors, while log-transformed propagule carbon was a continuous predictor, and block was a random effect using R package ‘lme4’ (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). It was not possible to accurately estimate random effect variance for site, as it was a two-level factor, so site was modeled as a fixed rather than random factor (Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). We note that this restricts our scope of inference to the two study sites. For each model, all possible second- and third-order interactions were specified for factors other than site. Degrees of freedom for F-tests were performed using Kenward-Roger’s approximation (Kenward and Roger Reference Kenward and Roger1997) with R packages ‘lmerTest’ (Kuznetsova et al. Reference Kuznetsova, Brockhoff and Christensen2015) and ‘pbkrtest’ (Halekoh and Hojsgaard Reference Halekoh and Hojsgaard2014).
Finally, using McWhorter’s (Reference McWhorter1961) and Warwick and Black’s (Reference Warwick and Black1983) data on Johnsongrass productivity, we used the models described earlier to calculate the area established by a single Johnsongrass plant whose propagules were evenly distributed over a 1,000-m2 area and the total yield following a year of growth. To do this, we estimated that a typical large year-old Johnsongrass produces roughly 250,000 seeds and 90 m of rhizomes in a single growing season. Given a 4-cm internode distance, this equates to roughly 2,250 nodes. This is perhaps a conservative estimate; Anderson et al. (Reference Anderson, Appleby and Weseloh1960) observed 5,200 nodes in one 18-wk-old container-grown Johnsongrass. If these 5,200 nodes were evenly spread over a 1,000-m2 area, it would result in a density of 9 rhizome nodes m−2 (1.13 g C m−2).
Results and Discussion
Propagule pressure is often cited as a key factor influencing invasion success (Baker and Stebbins Reference Baker and Stebbins1965; Burns et al. Reference Burns, Pardini, Schutzenhofer, Chung, Seidler and Knight2013; Hawkes Reference Hawkes2007; Pyšek and Richardson Reference Pyšek and Richardson2007). In general our data suggest that propagule pressure is important; while establishment was much higher at Kentland than at Glade, with the former being the more productive site overall (Table 2; Figure 1), when averaged across treatments and propagule types, overall establishment probability increased as more propagules were added (Table 2). The effect size estimate of ln-carbon provides an estimate of the degree to which establishment was affected by density dependence. We found a value significantly less than 1 (B=0.69, 95% confidence interval: 0.431 to 0.988; Table 2), suggesting that establishment was hampered somewhat at high propagule densities by weak negative density–dependent per capita recruitment (Barney et al. Reference Barney, Ho and Atwater2016). However, we note that establishment probability saturated at very low seed densities, possibly interfering with our ability to accurately estimate B or to estimate differences in B between propagule types.

Figure 1 Establishment probability for each propagule type (seed and rhizome) and community type (with or without competition) as a function of propagule carbon content.
Table 2 Effects of treatment on establishment.

It has been suggested that the probability of establishment success increases with propagule pressure until a critical point is reached. At propagule densities beyond this invasion cliff (i.e., densities at which establishment probability is close to 100%), population dynamics may not respond to increases in propagule pressure (Barney et al. Reference Barney, Ho and Atwater2016; Davis Reference Davis2009). At two study sites we found that Johnsongrass establishment probabilities saturated at the lowest seed densities used in this study; even if the seeds of a typical plant were spread out over a 1,000-m2 area, the propagule pressure was intense enough to ensure establishment throughout the entire area (Table 3). These results indicate that dispersal—and not biotic resistance or variation in propagule pressure—was the principal factor preventing expansion of Johnsongrass in our study sites. Our results also suggest that establishment was uninhibited by dormancy, which can be high in Johnsongrass (Taylorson and McWhorter Reference Taylorson and McWhorter1969). However, while establishment from seed saturated at low propagule densities, the yield of established plots continued to increase with increasing propagule pressure (Figure 2A and B). Thus, propagule pressure likely plays a role in Johnsongrass population dynamics, even for populations with propagule pressures sufficient to ensure establishment.

Figure 2 Total aboveground biomass (A and B), number of culms at 7 wk (C and D), and mass per culm (E and F) for each propagule type (seed and rhizome) and community type (with or without competition) as a function of propagule carbon content for each site.
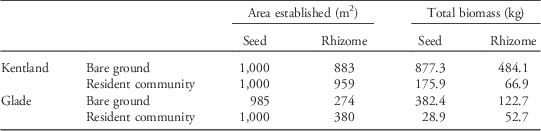
Table 3 Projected result of dispersal of seeds and rhizomes over 1,000 m2, as produced by a typical large Johnsongrass individual.Footnote a
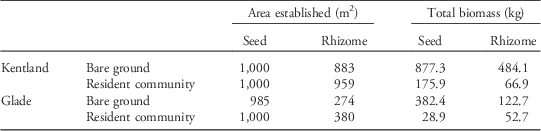
a Total yield reflects the total aboveground dry biomass in kilograms of the entire area established by dispersed propagules after one growing season.
In addition to seeds, dispersal of vegetative propagules is important for the success of many introduced species (Catford and Jansson Reference Catford and Jansson2014) and for Johnsongrass in particular (Anderson et al. Reference Anderson, Appleby and Weseloh1960; Paterson et al. Reference Paterson, Schertz, Lin, Liu and Chang1995; Warwick and Black Reference Warwick and Black1983). In this study, Johnsongrass readily established from rhizome fragments. While seeds produced much more aboveground growth than rhizomes when controlling for carbon content, we found that per propagule biomass was as much as 20 to 70 times higher in the rhizome-propagated plots than in the seeded plots on average (Table 4; Figure 3A and B), albeit with much lower culm densities (Table 5; Figure 2C and D). Natural log–transformed per propagule biomass was significantly higher in the rhizome-planted than in the seeded plots and was also much higher in the weeded plots. However, transformed per propagule biomass for rhizomes was also strongly negatively correlated to propagule density, suggesting that rhizomes experience strong negative density dependence, especially as compared with seeds (Figure 3A and B). The cause of this is not clear, but it corroborates earlier reports that Johnsongrass does not grow as vigorously from rhizomes as from seeds (McWhorter Reference McWhorter1961). Natural log–transformed per propagule culm number responded similarly, although competition had weaker effects, particularly at Glade (Figure 3C and D). Per propagule culm number was 5 to 8 times greater for seeds than for rhizomes on average. Overall, rhizomes produced plots with fewer, larger plants with lower culm densities, and seeds produced densely grown plots with numerous culms.

Figure 3 Total aboveground biomass (A and B) and number of culms at 7 wk (C and D) expressed per propagule planted for each propagule type (seed and rhizome) and community type (with or without competition) as a function of propagule carbon for each site.
Table 4 Effects of competition (Comp.), propagule type (Prop.), carbon added (i.e., propagule density), and site on per propagule biomass and per propagule culm number.Footnote a

a Degrees of freedom, estimated using the Kenward-Roger approximation, are shown as subscripts. ANOVA sums of squares (SS), estimated F-statistics, and P-values are shown. Bold text indicates significance (P<0.05).
Table 5 Effects of competition (Comp.), propagule type (Prop.), carbon added (i.e., propagule density), and site on biomass, culm number, and mass per culm.Footnote a

a Degrees of freedom, estimated using the Kenward-Roger approximation, are shown as subscripts. ANOVA sums of squares (SS), estimated F-statistics, and P-values are shown. Bold text indicates significance (P<0.05).
Interspecific competition appears to have played a major role in the yield of Johnsongrass plots despite having little to no effect on establishment probability (Table 5; Figure 2), although effects of competition were complex and depended on propagule type and density. At Kentland, competition had strong negative effects on cube-root-transformed aboveground biomass in established plots. Transformed biomass was much more sensitive to propagule addition in the seeded plots than in the rhizome-propagated plots, with both propagule treatments having similar biomass in the 0.5-g carbon treatment and nearly 10-fold differences in the 10-g carbon treatment. Effects at Glade were similar, although poor establishment in the low-density plots made it difficult to evaluate interacting effects of carbon content and propagule type (Table 5; Figure 2A and B). Square-root-transformed culm number was responsive to propagule input, particularly of seeds. As a result, seeded plots produced many more culms than rhizome-propagated plots. This relationship was stronger in the weeded plots than in those experiencing competition (Table 5; Figure 2C and D). Cube-root-transformed per culm biomass was much greater in the seed-propagated plots than in the rhizome-propagated plots, particularly in the weeded treatments and at Glade. There was no overall relationship between propagule density and transformed per culm biomass; however, there was a weak negative relationship between carbon content and transformed per culm biomass in the bare ground plots and a slight positive relationship in the resident-community plots. (Table 5; Figure 2E and F). Our discovery that competition had little effect on establishment but major effects on yield is significant, because it means that even intense competition may not provide a barrier to Johnsongrass dispersal. Rather, competition simply reduces the growth rate, and perhaps final size, of Johnsongrass populations at these sites.
While rhizomes were much more successful than seeds on a per propagule basis, when their carbon content was accounted for, rhizomes were more sensitive to competition than seeds and were also less able to overcome interspecific competition at increased propagule densities. These findings are surprising, because large, high-cost propagules are typically thought to aid recruitment under competition (Leishman et al. Reference Leishman, Wright, Moles and Westoby2000). However, the data also support the hypothesis that reproduction via seed is more beneficial than vegetative propagation for plants experiencing competition (Abrahamson Reference Abrahamson1975; Loehle Reference Loehle1987). The carbon content of a propagule is only one aspect of the total cost of producing a propagule (Jurik Reference Jurik1985), and rhizomes are important for more than just dispersal (Loehle Reference Loehle1987). Thus, we do not know enough about the total costs of both modes of reproduction to speculate which reproductive strategy might be favored by selection under these conditions. However, our results suggest that seeds likely play a greater role than vegetative propagation of Johnsongrass under competition, provided sufficient dispersal of both propagule types. Future studies that include complete construction costs of each propagule type (i.e., the production of support structures and the metabolic costs of tissue construction and maintenance) (Jurik Reference Jurik1985) would fill important gaps in the circumstances that favor either reproductive scenario.
Johnsongrass is a globally invasive species that poses an enormous threat to biodiversity and food production (Monaghan Reference Monaghan1979; Rout et al. Reference Rout, Chrzanowski, Smith and Gough2012; Warwick and Black Reference Warwick and Black1983). Its invasive success has been attributed to both its prolific seed production and its ability to propagate via an extensive underground rhizome network (Paterson et al. Reference Paterson, Schertz, Lin, Liu and Chang1995; Warwick and Black Reference Warwick and Black1983; Warwick et al. Reference Warwick, Phillips and Andrews1986). This may be especially true in riparian habitats, rights-of-way, and tilled agricultural fields, where mechanical disturbance of the soil both fragments and distributes viable vegetative propagules (Catford and Jansson Reference Catford and Jansson2014). Our results from two sites in western Virginia suggest that the reproductive potential of Johnsongrass is extreme regardless of its mode of reproduction and that dispersal limitation is likely to be the major factor controlling Johnsongrass abundance on the landscape. While more research is needed to understand how sexual and clonal propagation influence the population of invasive species in the field, our results highlight the necessity of considering both sexual and vegetative reproduction when trying to understand the factors that influence the population dynamics and spread of invasive species such as Johnsongrass that are capable of multiple reproductive modes. Our study was also limited by occurring at two sites over a single season. Although these sites were chosen to be representative of habitat variation in western Virginia, more work is needed to determine how environmental variation influences the reproductive ecology of Johnsongrass at larger scales.
Acknowledgments
This work was partially supported by a Virginia Tech Fralin Summer Undergraduate Research Fellowship to W Kim and by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture, and USDA Controlling Weedy and Invasive Plants program (2013-67013-21306).
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/inp.2017.4