Nowadays, regulators, funding bodies, and other financing parties are exercising pressure on researchers to provide proof on the cost-effectiveness of an innovative intervention in a (too) short time frame. The question is to what extent outcomes obtained in such a short time frame do justice to the actual cost-effectiveness of a new intervention. The majority of innovations in health care are complex in nature, meaning that they require considerable time and effort to implement (Reference Reelick, Faes, Esselink, Kessels and Olde Rikkert1;Reference Faes, Reelick, Esselink and Rikkert2). Even for rather common drugs such as statins, and (novel) anticoagulant agents it is clear that “time to benefit” is a crucial, although highly neglected characteristic in medical decision making (Reference Holmes, Hayley, Alexander and Sachs3). Hence, there might be reason to believe that a discrepancy exists between short- and long-run cost-effectiveness outcomes (Reference Adang, Voordijk, Van der Wilt and Ament4). As the outcomes of decision analytical modeling are largely dependent upon clinical trials that use a short time frame for their evidence generation, researchers should make a strong effort to provide insight into the usability of these short-run outcomes for funders and decision makers when determining the cost-effectiveness of an innovation. The aim of this paper is to provide insight in the mechanisms by which short-run inefficiencies arise and how a (too) short study duration influences the reliability of cost-effectiveness (CE) outcome in studies of new health technologies.
Short-Run Inefficiencies in Cost-Effectiveness Analysis
Cost-effectiveness analysis (CEA) as part of the evaluation of medical innovations, both in drug and nondrug interventions, has become well accepted and widely applied in several high-income countries. For example, in the United Kingdom, the National Institute of Clinical Excellence (NICE) uses cost-effectiveness outcomes as a criterion for coverage recommendations to the National Health Service. Theoretically standard CEA should be performed in a common practice setting and aims to collect all relevant costs and effects for all alternative interventions (mutually exclusive) over a representative time period. However evidence on cost-effectiveness is often gathered alongside clinical trials or is derived from available evidence by decision analytical modeling. Cost-effectiveness of a new intervention relative to current practice is often expressed in a decision rule relating the incremental cost-effectiveness ratio (ICER) to some societal willingness to pay for a unit of effect like for example a quality-adjusted life year gained. In using this ICER, costs and effects collected during the trial period are averaged and assumed to be constant over and representative for the economic lifetime of the intervention. However, this assumption is not always realistic as it neglects possible inefficiencies that occur during the implementation phase, where additional costs are often unanticipated and clinical effectiveness for the innovation is often not yet optimal (Reference van de Wetering, Woertman and Adang5). As most innovations are in a transitional state when evaluated, inefficiencies are likely to occur, and the resulting ICER might be an incorrect reflection of the actual cost-effectiveness of the innovation. The impact of these inefficiencies on the outcomes of economic evaluations in health care is strongly related to the timeframe available for evaluation. It is generally assumed that if the timeframe is sufficiently long than the CEA outcome is a reliable reflection of the “steady state”. However, as argued before this is often not the case. In order to be able to estimate the impact of short-run inefficiencies, and thus assess the expected accuracy of a CEA, there needs to be transparency about the underlying factors that might cause a discrepancy between the estimated ICER and the “true” steady state ICER. Here, three major drivers of short-run inefficiencies are identified and discussed: learning effects, capacity constraints, and delayed time to benefit. The choice for these three factors is based on micro economic theory (short-run versus long-run cost behavior) and research methodological reasons (a timeframe representative for the long-term CE outcome). Also, when one or more of these factors are present, the consequences for the outcomes of an economic evaluation often become clinically relevant, as the actual decision whether or not to adopt an innovation is easily changed by the presence of these factors (Reference van de Wetering, Woertman and Adang5).
Learning Effects
Learning can both take place at an individual as well as at an organizational level (Reference Argote and Epple6). Learning effects are particularly visible in interventions where human actions have significant influence on procedural outcomes, for example when performing innovative surgical procedures or a switch from a 2D to a new 3D diagnostic modality. Learning effects arise because initially clinical personnel involved in a new and complex procedure will perform suboptimal. This suboptimal performance will manifest itself in longer procedure times, higher complication rates, and lower quality of life outcomes and survival rates for patients, as compared to the performance of the innovation when the learning curve has reached a plateau (Reference Anzanello and Fogliatto7). As the usual care is usually free of any learning effects, not correcting for learning can lead to an inaccurate estimation of the incremental cost-effectiveness of the innovation. A well-known example is laparoscopic surgery, in which learning tends to exert a large influence on the results of clinical trials (Reference Ramsay, Grant and Wallace8). For example, Broeders et al. studied the impact of surgeon experience on 5-year outcome of laparoscopic Nissen fundoplication (LNF) for gastroesophageal reflux disease (Reference Broeders, Draaisma and Rijnhart-de Jong9). This study found a significant decrease in operating time, in-hospital complications, early dysphagia, and conversions from laparoscopic to conventional surgery. Here the impact of learning on trial outcomes and its consequences can be directly related to the original randomized controlled trial (RCT) which was prematurely terminated because seven patients in the LNF group had developed dysphagia versus none in the control group (Reference Bais, Bartelsman and Bonjer10). The study of Broeders et al. confirmed the criticism on the RCT that not taking into account learning had introduced bias causing inferior outcomes for the laparoscopic treatment arm. Researchers should be careful when performing a trial in a multicentre setting consisting of centers of excellence and less experienced centers. Depending on the distribution of the inclusion of patients between these centers, as well as the absolute number of patients treated in these centers, the resulting ICER might not be representative for the steady state ICER.
Capacity Constraints
When substituting one intervention for another, it is often assumed that the old intervention can be scaled down at the decision maker's own discretion or be fully replaced without suffering financial losses due to excess capacity (Reference Smet11). This is, however, dependent on the rate of implementation and whether the technologies under evaluation are convex (perfectly divisible). When an innovation is rapidly implemented, not all resources of the old intervention can immediately be freed up to finance the innovation. For example, medical equipment not yet at the end of their economic life (e.g., older generation CT scanners) decrease the anticipated savings in the short run (Reference van de Wetering, Woertman and Adang12). This in turn increases the ICER in the short run. On the other hand, when implementation commences in a more gradual manner, for example due to organizational and infrastructural changes that need to be made, the innovation may not have enough capacity to supply all patients at once. This results in the need to keep the old and relatively inefficient intervention operational throughout the implementation period, resulting in both diseconomies of scale and the healthcare provider not reaping the full benefits of the new and more cost-effective intervention. An illustrative example is the conversion from analogue screen film mammography to digital mammography, which is a process that takes place in a gradual manner as the technological substitution takes place on a nationwide level (Reference van de Wetering, Woertman, Verbeek, Broeders and Adang13).
Delayed Time to Benefit
Even when implementation of an innovation is optimal, a clinically meaningful therapeutic effect is not guaranteed to be immediately present. An example is statins, for which “time to benefit” has emerged as a useful concept to assess efficacy in specific settings over varying periods of time (Reference Ray and Cannon14). For example, treatments that lower LDL cholesterol in patients with coronary artery disease reduce these LDL levels dramatically within months. However, an actual reduction in angiographic disease progression may require 3 years (Reference Buchwald, Moore and Rucker15;Reference Buchwald, Varco and Matts16). This has obvious implications for cost-effectiveness analysis where the main focus lies on final outcome measures such as quality of life and mortality and where the exact functional behavior between the intermediate (LDL level) and final outcome (quality of life, survival) are not precisely known. For innovations with long time to benefit on these outcome measures, cost-effectiveness may be underestimated during the evaluation period when the focus is on the final outcomes or might be imprecise because of misspecification of the relationship between intermediate and final outcome. This particular problem of time to benefit might be alleviated by correcting for the fact that a trial only gives intermediate endpoints. By correctly extrapolating intermediate endpoints in a trial to final endpoints to be used in an economic evaluation, research conclusions can be nuanced while still serving as a solid guide for decision making about policy and further research. However, often clinical trials are not designed to provide input for decision analytical models. Therefore, researchers should pay specific attention to the usability of trial data in their models, especially with regard to the relationship between intermediate trial outcomes and model input parameters, specifically the outcomes over time. The inclusion of intermediate outcomes in health economic models should acknowledge the uncertainty surrounding this relationship. Hence, this problem should be tacked using simple methodology, such as including the model parameters contingent on intermediate outcome measures in deterministic sensitivity analyses.
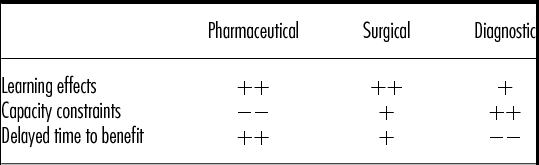
Besides the three drivers presented here, there are other factors that can trigger short-run inefficiencies. However, most of these factors can be traced back to the three drivers presented in this study. When looking at the nature of healthcare innovations we distinguish three main groups in this article, namely pharmaceuticals, surgical interventions, and diagnostic interventions. Table 1 provides an overview of these innovations and a description of the extent in which learning effects, capacity constraints, and delayed time to benefit drive short-run inefficiencies for these particular innovations, based on analysis of the examples given.
Table 1. Influence of Factors Driving Short-Run Inefficiencies on Different Types of Healthcare Interventions
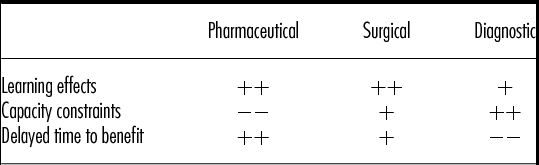
Note. ++ influence can be very strong and/or is prevalent in intervention type; + influence can be strong but is relatively rare in intervention type; −− influence is negligible and/or very rare/non-existent in intervention type.
When comparing these three types of interventions, We see that learning might play a role for all interventions. For pharmaceuticals, learning might both play a role at the introduction (when professionals only slowly learn the rare side effects, that cannot be found in smaller RCTs) and also later on when dosages and packing are adjusted. Unfortunately in several drug developments of recent years (e.g., Cox-2 inhibitors, new oral antidiabetics, the dpp-4 inhibitors), the cost-effectiveness estimation changed considerably from introduction on the market to the appearance of much less positive phase IV surveillance data (Reference Chancellor, Hunsche, de Cruz and Sarasin17;Reference Brown, Hooper and Elliott18). Regarding capacity constraints, these do not play a role for pharmaceuticals, as their cost behavior is mostly variable, but are a concern when adopting innovations in the surgical or diagnostic area. Delayed time to benefit can obviously impact pharmaceuticals, but also plays a role in surgical interventions, as described by Buchwald et al. (Reference Buchwald, Moore and Rucker15;Reference Buchwald, Varco and Matts16).
Ideally, all these factors should be modeled in one overarching algorithm that gives, depending on the nature of the innovation, a correction to the cost-effectiveness outcome. Due to the variety between innovations, this might be rather difficult. However, existing outcomes research might be improved to better capture foreseen short-run inefficiencies. For now, the most important task for health economic researchers is to raise awareness between themselves and decision makers about the possible implications of these short-run inefficiencies.
Consequences of Biased Cost-Effectiveness Outcomes
The failure to address the short-run–long-run discrepancy in standard CEA can have important consequences. Most decision makers and funders tend to focus on short-term results and demand these results on short notice (Reference Drummond, Cooke and Walley19). This myopic attitude reduces the length of trial periods and outcome measurements, as researchers feel pressured to shorten the time frame for their studies. Additionally, researchers may display myopic behavior themselves. An example is when health economic models are populated with data that are generated within a too short timeframe with regard to cost-effectiveness, and are hence not representative for the steady state health economic outcome. As short-run inefficiencies are most prevalent and impactful during an innovation's earliest stage of operation, reducing the length of evaluation periods will result in cost-effectiveness outcomes being biased even more against the innovation. If no attempt is made to correct for this by using for example intermediate outcome measures or taking potential short-run inefficiencies into account in the interpretation of study results, this might lead to outcomes that seriously underestimate the cost-effectiveness of a new intervention, potentially resulting in second thoughts about the implementation of an in essence cost-effective innovation. Worst-case scenario is that such a “lag-time bias” in cost-effectiveness analysis will result in denying patients access to more efficient health care. Of course the opposite may also be true, in the sense that cost-effectiveness will decrease later on, for example by extending the indication to other patient groups with less benefit. This is for example suspected in the recent case of transcatheter aortic valve replacement, but as far as we know, it has not been demonstrated in cost-effectiveness data (Reference Van Brabandt, Neyt and Hulstaert20).
Careful health technology assessment (HTA) of new medical interventions is increasingly important and more and more considered as obligatory for further implementation and reimbursement of healthcare innovations. Coming back to the research question, we argue that the combination of a myopic focus of decision makers and the discrepancy between study outcomes and long-term (steady state) cost-effectiveness of an innovation can lead to misguided decisions about the implementation of innovations in health care, due to short-run inefficiencies surrounding the substitution of the old technology. It should be acknowledged that if the dynamics in a certain health market are large and innovations follow in rapid succession a long-run steady state of a particular innovation will probably not be reached. However, such a market is unlikely to exist for a long period as, in the context of large discrepancies in efficiency between the short run and long run, healthcare providers will strive to produce their products on the long-run efficient frontier because this gives them a competitive advantage over those that do not exploit economies of learning and scale. The mechanism by which short-run inefficiencies manifest themselves is dependent on a multitude of factors, most importantly the type of innovation and the characteristics of the to-be-substituted technology. The role of short-run inefficiencies in the evaluation of an innovation offers ample opportunities for further research. We, therefore, urge policy makers and researchers to expand research in this important, but relatively neglected topic. HTA studies that extrapolate short-term outcomes may fail to deliver good estimates of cost-effectiveness, often underestimating the real effects of an innovation. Therefore, we advocate to carefully take into account the different factors that may cause this lag-time bias in the design and analysis of cost-effectiveness studies. Here lies a crucial responsibility for researchers in the field of health economics, as they are the ones being able to raise awareness among decision makers on these methodological issues. Therefore, it is essential that information on potential short-run inefficiencies is reported alongside the results of an economic evaluation. If this information is communicated to decision makers, together with options to deal with these inefficiencies, it can help to prevent pitfalls in implementation of innovations, and guide important decisions on the implementation strategy.
CONTACT INFORMATION
Gijs van de Wetering, PhD, Department for Health Evidence, Radboud University Medical Center, Nijmegen 6500 HB, The Netherlands
Marcel Olde Rikkert, Prof.dr, Department of Geriatrics, Radboud University Medical Center, Nijmegen 6500 HB, The Netherlands
Gert Jan van der Wilt, Prof.dr, Eddy Adang, PhD (eddy.adang@radboudumc.nl), Department for Health Evidence, Radboud University Medical Center, Nijmegen 6500 HB, The Netherlands
CONFLICTS OF INTEREST
The authors declare no conflict of interest.