Limits to health care are set in all societies. The developed world delivers health care through a complex system that involves institutions, primary care doctors, other healthcare workers, researchers, industry and others. Delivery takes place in the context of stakeholders’ and patients’ interests and rights. The use of health care then becomes an important question of interests, rights, and values, thereby calling for more transparent and explicit processes of priority setting (Reference Daniels and Sabin2;Reference Ham5).
Healthcare expenditure has increased in most countries over recent decades (18). Major drivers of these increases include the rising cost of drug development and thereby drug prices, the emergence of innovative and expensive diagnostics and devices, and the general expansion of potentially treatable conditions (Reference Daniels and Sabin2). This has raised the general need for setting priorities, and has forced funders to take more account of costs and cost-effectiveness in addition to clinical effectiveness and safety when making healthcare decisions (Reference Ham5). The necessary debates on how best to use limited resources in health care are taking place in all countries, regardless of their stage of development.
General discussions and research on priority setting started to take place in the 1980s. In the Nordic countries, the Netherlands, and New Zealand, public commissions were established to provide a framework for setting limits to healthcare expenditure (Reference Ham and Robert6). Within a subnational context, the USA state of Oregon was a forerunner. Whereas the Nordic commissions decided to prioritize the needs of the sickest patients (14;21), in Oregon all Medicaid services were ranked according to their cost-effectiveness only (Reference Kitzhaber10). Later, the importance of the evidence regarding the effectiveness of treatments and their cost-effectiveness was also added to the rationing framework in the Nordic countries (15;Reference Norheim16;21). What processes can then produce the evidence necessary for informing decision making and achieve greater legitimacy for priority setting? Health technology assessment (HTA), which also includes cost-effectiveness analyses, forms a key part of healthcare research and evaluation, and supports the work of decision makers in health care in many countries (Reference Banta1;Reference Drummond, Schwartz and Jönsson3;Reference Oortwijn, Vondeling and Bouter19).
The present article aims to describe the recently established national system for priority setting in Norway, with particular attention to the importance of transparency in the process, and the necessary support mechanisms such as HTAs (Reference Oortwijn, Vondeling and Bouter19). The introduction of human papillomavirus (HPV) vaccination is presented as an illustration of how HTAs can provide valuable decision-making support.
THE NORWEGIAN HEALTHCARE SYSTEM
The Norwegian healthcare system is often described as a decentralized national health service (NHS) with universal coverage. Its fundamental aim is to give residents equal access to health services, irrespective of their location, gender, age, or financial status, and to prioritize those who have the greatest need.
The provision of primary care services (including general practitioners and nursing care) is the responsibility of the 430 municipalities (see Figure 1).
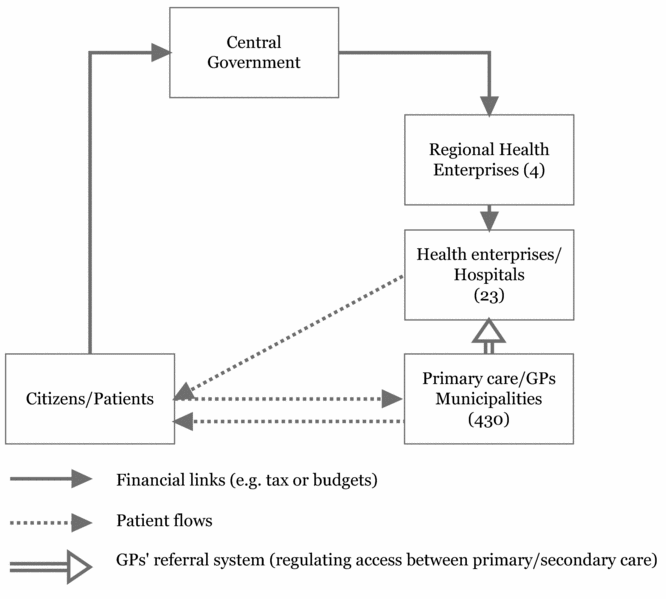
Figure 1. Overview of the Norwegian healthcare system.
Following the hospital reforms that took place in 2002, specialist care has been the responsibility of the state, but this has been delegated to four regional health authorities (RHAs) (see Figure 1). Regional health authorities oversee all hospitals in their region, and are led by an executive Board, appointed by the Minister of Health. Provision of health services is thus based on two separate tiers: state-owned health authorities and municipalities (local authorities). The most important regulatory mechanisms for the healthcare system in Norway are thus the government financing and presenting aims and working plans to the hospitals, and the municipalities with similar responsibility for local primary care.
The providers of health care have freedom when treating individual patients. They must, however, take into account decisions from the Medicines Agency, which is responsible for regulating and reimbursing drugs for primary care, as well as the Directorate for Health, which is responsible for National Clinical Guidelines. These guidelines are advisory only, and cover broad topics, such as handling of diabetes, pregnancy care, etc. The introduction of new technologies (procedures, devices, drugs in hospitals) is essentially unregulated, except for the technical CE marks for devices and standard market approval processes for drugs. Consequently, individual clinicians and manufacturers often promote these technologies into the health services. It has been claimed that the lack of a national regulatory system for introducing technologies into hospitals is responsible for the high rise in the Norwegian healthcare budget (17). The division of responsibilities between the state and municipalities for primary and specialist health care also suggests a further challenge for systematic priority setting at a national level.
Setting Priorities in Norwegian Health Care
Discussion on prioritizing health care began in Norway during the 1980s (Reference Norheim16). A milestone in the debate was the report from the “Lønning commission” (named after the former Member of Parliament and professor Inge Lønning) (14), leading to the first national guidelines for priority setting in health care (see Box for historical details).
Box: Key dates in the Norwegian priority setting debate
1987 The first expert report on priority setting published (‘Lønning I’)
1997 The second expert report on priority setting published (‘Lønning II’)
1998 The first HTA-centre established (Senter for medisinsk metodevurdering)
2000 The first National council for priority setting is set up
2001 The Patient Rights Act comes into effect (includes criteria for priority setting)
2002 The Directorate of health established
2002 The Hospital reform – central government resumes responsibility of hospitals
2004 The Norwegian Knowledge Center for the health services established (HTA)
2006 The first National council for priority setting closed down
2007 The Current National health plan (2007–2010) comes into effect
2007 The National council for quality improvement and priority setting established
The main feature of the guidance provided was that priority should be given to the sickest patients, who are the most vulnerable. A problem with these guidelines was, however, that they did not take into account the effectiveness of interventions, nor opportunity cost, which is the benefit one may forgo for others.
This was partly why a new report “Prioritering på ny” (“Priority setting revisited”) was drawn up and published (15). The revised framework recommended priority to be given according to three core values: the severity of the condition, the expected outcome from the intervention, and a reasonable cost-effectiveness ratio of the intervention. These three values have since been included in the Patient Rights Act of 2001. By its length and contents, it is the most comprehensive Nordic patient rights act (Reference Spongberg, Ringard, Magnussen, Vrangbæk and Saltman20). In accordance with what had already been proposed on theoretical grounds (Reference Daniels and Sabin2), the 1997 report also underlined the importance of a fair and open process in setting priorities. More recently, yet another principle has been proposed regarding the quality of the evidence used when establishing priorities (Reference Johanson, Miljeteig and Norheim9;Reference Norheim16).
The 1997 report also described the use of some “tools” such as the development of national clinical guidelines based upon the best available evidence regarding both treatment and importance to patients. The guidelines were to be created by specialty-specific groups. To further achieve the open process described by the second “Lønning-commission,” a National Priority Council was set up in 2000. The Council was to identify and advise health authorities on priorities to be set in primary and secondary care and public health care, and initiate a debate on priorities in the healthcare system. Members of the Council were appointed individually from different fields of the healthcare sector, together with researchers in ethics, health economics, and related disciplines. After some years, the Government, however, wanted a Council with closer links to the responsible management levels (personal communication from former leader of secretariat), and brought the work of the Priority Council to an end.
Evidence-Based Decisions: Establishing HTA in Norway
Internationally, policy makers are increasingly expected to underpin their decision-making processes with evidence. HTA has attained importance in the United States, Canada, the United Kingdom, and Australia as a base for decisions on priority setting in health care, a tool for avoiding differences in practice, and a tool for optimizing use of resources (Reference Banta1;Reference Maddern11).
The first Norwegian health technology assessment organization, Senter for Medisinsk Metodevurdering, was established in 1998 (Reference Mørland12). Initially, the important issue was the clinical effectiveness of the technology. At the same time, healthcare expenditure had increased to levels beyond those previously seen by Norwegian governments, and cost-effectiveness assessments became increasingly important in obtaining more information and control. Over time, Norwegian HTA users have spread from the clinical micro level to include managers (meso level). Furthermore, the goal of evidence-based work has also been more prominent at the macro level of national policy making.
A recent European survey shows, however, that the use of HTA is not as broad among those with provision responsibilities or regulators of healthcare services as may be desired (Reference Garrido, Kristensen, Nielsen and Busse4). In a recent article, Drummond and associates (Reference Drummond, Schwartz and Jönsson3) assess existing and future uses of HTA. They underline in general the importance of considering the link between HTA and the decision that will follow.
LINKING PRIORITY SETTING AND HTA
The closure of the first Council for Priority Setting in 2006 left an organizational void, suggesting that national prioritization processes were only handled in a systematic manner through the drug reimbursement system for primary care. At the same time, the introduction of other technologies (procedures, devices, drugs in hospitals) remained largely unregulated or was buried within complex internal budgetary processes. Thus, the fundamental challenges of limit setting were still present within the health system.
The Ministry of Health and Care Services issued a national health plan in 2007 that underlined the need for a more comprehensive approach to important issues of priority setting and quality (7). The plan marked out how the health service faces considerable future challenges from increasing numbers of senior citizens, the shift from acute to chronic conditions, and continuing development of increasingly expensive new medicines and treatment methods. The National Health Plan for Norway reflected the need for an arena and processes by which the different actors could collectively assess conditions and challenges.
The Norwegian Council for Quality Improvement and Priority Setting in Health Care
A new Council was established in April 2007 for an initial period of 2007–2010. It was intended to combine members who had leadership responsibilities at the level of hospitals, primary health care, and national authorities with professional and patients’ representatives, and to promote discussions of vital questions for the system as a whole based on the best evidence available. Three major aims were formulated for the Council. First, it was to help clarify the roles and responsibilities of agents responsible for the work on quality and prioritization. Second, it was to provide an arena to improve interaction between actors on different “levels” of the healthcare sector (see Figure 1). Finally, it was to produce more scope and transparency around the national work on quality and prioritization issues. It currently consists of twenty-five members, and is supported by its own secretariat located at the Norwegian Knowledge Centre for the Health Services (NOKC).
The Council does not derive authority from these institutions, but offers an arena for actors with joint responsibilities for priority setting and quality in health care. Its task is to create legitimacy around difficult issues. According to its mandate (8), the Council is to focus on the following five topics: (i) unacceptable inequalities/differences in healthcare services: socially, geographically, etc; (ii) introduction of new (and costly) treatment options; (iii) division of work and functions, that is, national services and centers of excellence/competence; (iv) national clinical guidelines; and (v) coordination of primary and specialist healthcare services.
An explicit aim of the Council is to establish transparency in its workings. Topics for discussion are brought forward by the Council members themselves or by the secretariat, and the problems most often represent concrete decisions to be taken (Figure 2). The Council selects the topics in plenary and consider the documentation needed. A thorough assessment of the problem is prepared by the secretariat, often in collaboration with external experts. Most often, one or more HTAs are identified or commissioned as rapid reviews from the NOKC as the main mechanism for informing the deliberations and the decision making. It is important for the legitimacy of the process that the HTAs include considerations on organizational and ethical consequences of the issue that often illustrate the dilemmas and elements of uncertainties. The documents for the meetings are made public on www.kvalitetogprioritering.no 3 weeks before the meeting. There is no fixed time schedule for the process, but the approximate time used is included in Figure 2. Time variations may depend upon needs for documentation, the difficulty of the problems discussed and type of outcomes.

Figure 2. Overview of the Norwegian council's work process.
All meetings are publicly accessible to the media, industry, clinicians, and patients.
During its first 3 years, the Council has discussed and made recommendations on approximately seventy cases, of which approximately fifty has been on substantial issues. Its intention has been to cover all five areas of the mandate. However, questions predominate in relation to the introduction of new (and costly) technologies in the hospital sector.
Table 1 gives an overview of the cases discussed by the Council between 2007 and 2009. The technology cases have been related to drugs (cancer drugs, biological drugs), devices (positron emission tomography, cochlear implants, ventricular assist devices), and procedures (trans-catheter valve implantation, genetic testing). In addition, issues of importance to public health, such as programs of routine screening and vaccination, have also been debated. Table 2 provides a more detailed picture of the decisions in which HTAs have played a pronounced role in supporting the discussions and decisions.
Table 1. Overview of Cases Discussed by the Council and Cases Supported by HTA

HTA, health technology assessment.
Table 2. Examples of Cases Discussed and the Kind of Information Provided by HTA Documents

aDetailed cost (micro-costing) data available, but due to lack of information of efficacy no explicit cost-effectiveness evaluation performed.
bDeemed to be cost-effective (due to low costs), but no explicit cost-effectiveness analysis performed.
C/E, cost-effectiveness analysis; AMD, age-related macular degeneration; MABS, monoclonal antibodies.
All Council recommendations have been based on consensus, except for the one on introducing HPV vaccination (see below). The recommendations have been implemented through national clinical guidelines (cancer drugs), hospital management (establishment of PET facilities) or the ordinary national policy process (HPV vaccine). Initially it was thought that the Council was solely to provide advice, while the subsequent binding decisions was to be taken by the members (and others) responsible for regulation and provision of care. The conclusions of the Council debates have, however, over time come to be regarded as proper decisions. Varying Ministers of Health has also emphasized that it is their expectation that managers within the system should remain loyal and following up the Councils advice.
An Example of the Priority-Setting System at Work: The Introduction of HPV Vaccination
An HPV infection is a necessary but insufficient condition for development of cervical cancer. Two vaccines against HPV have been developed over the past 2 decades, and many countries started to discuss the clinical effect of vaccination on development of cervical cancer. Parallel HTA initiatives to assess clinical effectiveness and cost-effectiveness of the vaccines were also taken. Introduction of HPV vaccination into the national vaccination program was, even before the establishment of the Council, a highly contested topic among professionals, administrators, and politicians. As the case also contained general problems related to priority setting, the Directorate of Health decided to present HPV vaccination as a case for the Council in the fall of 2007. To guide and support the discussions, a range of documents was commissioned (see Table 2 for details). The most important of these were four HTA reports covering (i) efficacy and safety aspects of the vaccines, (ii) cost-effectiveness analyses, (iii) questions of organizational consequences, and (iv) ethical considerations (13).
The Council debated HPV vaccination in November 2007 and March 2008. In the latter meeting, the Council recommended, albeit in the absence of full consensus (16 versus 3 members), to introduce the vaccine for 12-year-old girls. In its recommendations, the majority conclusion was that sufficient evidence existed on the protective effect of HPV vaccines on cervical cancer. All members expressed concerns about safety aspects (especially the unknown long-term effects of the vaccine). In particular, the minority emphasized the problem of unknown side effects. With respect to cost-effectiveness, costs were judged substantial, but not sufficiently high to not recommend the vaccine. The vaccine was, however, to be financed within existing healthcare budgets, thereby supporting a shift toward more focus on preventive services. It was also recommended that a future evaluation of the existing screening program be carried out to ascertain the vaccine's effect. Ethical concerns were also raised throughout the discussions (i.e., concerning the information given to 12-year-old girls).
After having received the NC's advice, the Norwegian Ministry of Health proposed to include HPV vaccine into the national vaccination program for children. The Norwegian Parliament subsequently decided to fund the vaccination in a school-based program for 12-year-old girls. The program started operating in the fall of 2009.
DISCUSSION
The Norwegian healthcare system was, in addition to being decentralized, until recently quite fragmented when it came to setting priorities. The National Council, set up by the Ministry of Health in 2007, was an attempt to create a common arena for making such decisions. The creation of the Council led to putting the missing piece of the priority-setting jigsaw puzzle in place. The result is a comprehensive system in which complex problems (input), scientific support mechanisms (e.g., HTAs), and values (priority-setting criteria) are brought together to create viable solutions (outputs) for the health system. In other words, a transparent system of collective decision making, including all relevant stakeholders is now in place.
The National Council is still a new entity. The Council has thus far based its debates and subsequent advice on the best available clinical evidence, but has also sought assessments of financial, organizational, and ethical issues that illustrate the dilemmas and elements of uncertainty. The Council has succeeded in setting limits, and thus shown that setting priorities also means restricted (new cancer drugs, cochlear implants) or postponed (mammography for 40- to 49-year-olds) decisions under a fixed budget.
The members of the Council with executive responsibilities for the regulation or provision of health care have largely been able to take necessary initiatives in following up in their respective portfolios. Many problems have been raised on concrete decisions to be taken on the introduction of new and costly interventions in the hospital sector, which is not surprising, because such introductions are not regulated in a standardized and systematic way in Norway. Thus, one important outcome of the Council's discussions was to recommend such a system. Another somewhat related initiative was to propose a system for public financing of clinical trials, related to the decision makers’ need for evidence-based documentation.
The Council members have performed a SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis on the Council's objectives, composition, working methods, etc. An external evaluation is presently conducted among users of the Council's recommendations. The general view is that the establishment of the Council has been useful; the composition of a team of key stakeholders makes the decisions relevant, and the openness of the working methods based on best evidence is appreciated. However, challenges and weaknesses remain. The most important of these may be that the debates have generated a general awareness and interest in priority setting among the public or at the clinical (micro) level. The Council has not been sufficiently engaged in primary health care or the care-giving sector, or issues related to coordination between the healthcare sectors in Norway. This was given as a clear mandate to the Council, but it has been difficult to achieve results because of the split responsibility for primary care (by municipalities) and secondary care (by the State). To partly solve this difficulty, a separate process has proceeded within the Ministry of Health, resulting in an Integrated Care Reform to be debated in the Parliament in 2010.
POLICY IMPLICATIONS AND FURTHER STEPS
The Council was established as part of the present Norwegian health plan. We believe that a similar model may function well in other countries, provided a uniform (public) healthcare system, with common set of values and access to supporting mechanisms in the form of HTA or similar organizations. It seems that the most effective implementation of NC's recommendations depends on discussions on concrete problems that can be based on high-quality HTA reports, and with explicit responsibility for further actions.
As shown in the present publication (Tables 1 and 2), questions relating to introduction of new technologies have often been based on HTA assessment reports on efficacy, safety, and cost-effectiveness. The broader aspects of HTA reports (ethical, organizational, societal consequences) have been presented less frequently. When available, this additional information has been very useful, especially when giving advice on public health issues such as genetic testing and screening. A new national health plan that is under preparation will put more emphasis on primary care, prevention, and health promotion. This should be reflected in the future problems brought up for Council discussions. It follows that it will also raise a challenge to future evidence-based tools for priority setting such as HTAs and comparative effectiveness programs. Hence, an important topic for future discussion will be how HTA, as an academic and practical field, should evolve to provide support for decisions within the realms of public health, health promotion, and health systems issues. We also see a great need for broader public debates and awareness of priority setting based on common values in our society. This may promote political debates as well as the challenges experienced at the clinical level in meetings with individual patients.
CONTACT INFORMATION
Berit Mørland, DDS, Dr philos (berit.morland@kunnskapssenteret.no), Fagdirektør; Ånen Ringard, MA (rin@nokc.no), Senior Advisor, John-Arne Røttingen, MD, PhD, MSc (jor@nokc.no), Chief Executive, Norwegian Knowledge Centre for the Health Services (NOKC), PO Box 7004, St. Olavs plass, N-0 130 Oslo, Norway
CONFLICT OF INTEREST
All authors report having no potential conflicts of interest.






