Many types of medical devices are labeled and marketed as being for single use only. Some Canadian hospitals reuse such single use devices (SUDs) after reprocessing because of perceived economic benefits. Reprocessing of medical devices may occur in a hospital or health region facility, or be contracted to a third-party reprocessing facility.
The issue of reuse of SUDs is of interest both to the federal agency Health Canada, which is responsible for regulation of the manufacture and marketing of medical devices, and to provincial and territorial governments, which are responsible for the organization and delivery of health services. Concerns remain about possible risks to patients, cost-effectiveness of SUD reprocessing, and legal liability issues.
In 2005, a Health Canada panel proposed advising healthcare facilities and professionals that they should not reprocess SUDs unless the facility has established quality systems; and that healthcare facilities that wished to have their SUDs reprocessed by a third-party reprocessor should ensure that the reprocessor's facilities and procedures have been certified by a regulatory authority or an accredited quality system auditor (12).
There is still uncertainty regarding the safety, effectiveness and economic impact of the reuse of SUDs. In 2004, a New Zealand review concluded that the evidence for the safety and effectiveness of reusing SUDs is anecdotal, with few studies evaluating outcomes directly related to patients (Reference Day4). There is a lack of data on patient exposure to cross-infection, loss of device functionality, and resulting adverse patient outcomes. Much of the literature refers to laboratory contexts, evaluating surrogate outcomes such as contamination and device integrity, so the overall evidence is indirect. There is also uncertainty regarding the current use of this practice in Canada. Earlier surveys had indicated that 41 percent of acute care hospitals regularly reused SUDs, and that most hospitals reusing SUDs did not have a reuse committee or written reuse protocols for most devices (Reference Campbell, Wells and Palmer2;Reference Mahoney14;Reference Miller, Gravel and Paton16).
This article gives an overview of an assessment of the reuse of SUDs, with emphasis on this practice in the Canadian context (Reference Hailey, Jacobs and Ries10;Reference Polisena, Hailey and Moulton20). The intent of the assessment was to provide advice to Canadian decision makers to assist with future policy and administrative arrangements. The assessment included a systematic review of evidence on clinical and economic outcomes after the use of reprocessed SUDs; an economic analysis comparing monetary values for adverse events; a budget impact analysis; an overview of legal and ethical issues; and a survey of Canadian acute healthcare facilities.
METHODS
Literature searches for clinical and economic reviews, using common bibliographic databases, covered studies published from 1996 onward, with no language restrictions. Gray literature was identified from web sites of health technology assessment and related agencies, and Internet search engines. Studies were included for review if they reported outcomes after the use of reprocessed SUDs in humans. The comparator in controlled studies was the one-time use of SUDs. Outcomes considered included infection of patients, other identifiable adverse events, mortality, device damage or failure, and evidence of device contamination. Selection of articles and data extraction were undertaken independently by two reviewers and discrepancies were resolved by consensus. The quality of clinical studies was evaluated using an approach that takes into account study design and study performance (Reference Hailey, Ohinmaa and Roine11), and that of economic studies with an adaptation of the guidelines of Drummond and Jefferson (Reference Drummond and Jefferson8).
In the primary economic analysis, the direct costs and dollar values of adverse health events associated with SUD reuse were measured. The analysis drew on data from two high quality studies included in the clinical review which addressed the reuse of SUDs in laparoscopic cholecystectomy and coronary angioplasty (Reference Colak, Ersoz and Akca3;Reference Zubaid, Thomas and Salman24). Data on costs of instruments and cleaning were obtained from papers included in the review and suppliers and purchasers of equipment. A hospital perspective was used and the time horizon was the physical life of a SUD. The model included the manufacturer's price of the device, reprocessing costs, and costs of adverse events due to reprocessing. Sensitivity analysis related to the probability of an adverse event and was conducted in terms of a break-even value if the savings were just offset by the losses due to adverse events.
A budget impact analysis was conducted for the province of Alberta, where costs per person are similar to those in the rest of Canada. The analysis addressed the question of what additional expenditures would result from eliminating the practice of reuse of SUDs in laparoscopic cholecystectomy and coronary angioplasty. Data were obtained from provincial government sources.
Legal and ethical questions related to the reprocessing and reuse of SUDs were addressed through analysis of duties imposed by law on device manufacturers, re-processors, healthcare facilities, and care providers. Patient rights to give consent and to be informed of treatment-related risks were also analyzed.
A survey was undertaken of acute-care hospitals in all Canadian jurisdictions, using an instrument that drew on information from previous Canadian surveys. Items in the survey included decision making on SUDs, whether SUDs were reprocessed, types of devices reprocessed, how they were reprocessed, incident reporting mechanisms and opinions on the use of third party reprocessors.
RESULTS
Systematic Reviews
From 852 citations in the literature review, 13 articles describing 12 clinical studies met the selection criteria. There were five studies on coronary angioplasty catheters (Reference Browne, Maldonado and Telatnik1;Reference Plante, Strauss and Goulet19;Reference Scherson and Dighero21;Reference Shaw, Eisenberg and Azoulay22;Reference Zubaid, Thomas and Salman24), three on devices used in laparoscopic surgery (Reference Colak, Ersoz and Akca3;Reference Descôteaux, Tye and Poulin6;Reference Gundogdu, Ocal and Çaglikulekci9), two on sphincterotomes (Reference Kozarek, Raltz and Ball13,Reference Wilcox, Geels and Baron23), and one each on external fixation devices for the management of fractures (Reference Dirschl and Smith7) and on phacoemulsification tips (Reference Perry18). There were two high quality randomized controlled trials (RCTs) (Reference Colak, Ersoz and Akca3;Reference Zubaid, Thomas and Salman24). Four of the other studies were of fair quality and the remainder of poor quality. Reprocessing by an external organization was used in only one study (Reference Browne, Maldonado and Telatnik1). None of the comparative clinical studies found a difference in the rates of adverse events between groups treated with reprocessed SUDs and those treated with new devices. The four nonexperimental studies did not identify any problems with the use of reprocessed SUDs.
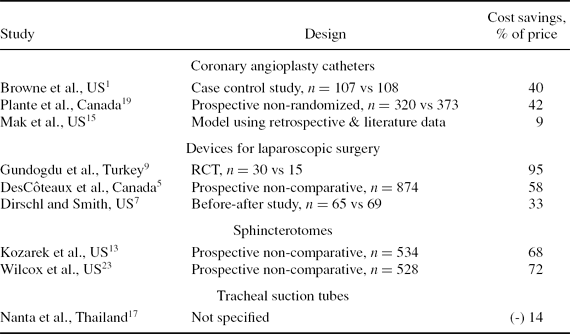
In the review of economic studies, 9 of 374 potentially relevant citations met selection criteria; 6 of these were also included in the clinical review. In all selected papers, savings were calculated as the cost of using new devices for every time the device was used minus the actual cost of reusing devices (i.e., the price paid for the devices and the cleaning costs). Eight of the nine studies showed savings through reuse of SUDs. The average saving was 44 percent of the cost of all new devices. Brief details of the economic studies are shown in Table 1. The economic studies had significant limitations. In two studies, no clinical outcomes were measured, there was no clinical comparator in a third, the method of observation was unclear in a further study and in another, patients in each arm were treated in different hospitals, where practice patterns may have differed.
Table 1. Studies Included in the Economic Review
Primary Economic Evaluation
The population groups used in the analysis were adults undergoing laparoscopic cholecystectomy, and adults undergoing coronary angioplasty. Results are summarized in Table 2. For angioplasty catheters, the results in the base case indicate a cost per patient of $250 for single use of a device. The cost of reuse, when there are no adverse events, is $77 per case, which includes an expected cost of $48 for the catheter. ([$250 × 1.2 catheters per patient] ÷ [6.2 uses per catheter]). The cost per patient of laparoscopic cholecystectomy is $1,233 for single use and $262 for reuse, assuming no adverse events.
Table 2. Results of Primary Economic Analysis

Savings from reuse of SUDs would be offset if there were adverse events. The break-even analysis for angioplasty indicates that the cost per patient is the same between reuse and single use if the probability of an adverse event due to the reuse strategy is 12.6 per 1,000 procedures. The break-even analysis for laparoscopic cholecystectomy indicated that with a probability of an adverse event of 445 per 1,000, the cost of the two strategies would be the same.
Budget Impact
Currently in Canada, <1 percent of each of the procedures are being done with reused SUDs (Reference Polisena, Hailey and Moulton20). In total, there were 1,824 laparoscopic cholecystectomies and 5,199 angioplasties performed in Alberta during 2005. If 1 percent of these were done with reused SUDs, this practice would have been used in 18 laparoscopic cholecystectomies and 51 angioplasties. The additional cost per procedure of the single use of SUDs, compared with the reuse of SUDs, would be $971 for laparoscopic cholecystectomy and $173 for coronary angioplasty. If the practice of reusing SUDs were eliminated, the additional annual cost in Alberta would be $17,500 for laparoscopic cholecystectomies and $8,800 for coronary angioplasties. This would add less than 0.1 percent to the total costs of the procedures.
Legal, Ethical, and Psychosocial Issues
The reprocessing and reuse of SUDs raises several legal and ethical questions; those of major importance in this assessment are shown in Table 3. They focus on matters of law, but ethical and psychosocial issues are intertwined in these problems. In the absence of clear regulation and legal precedents, ethical principles must guide decisions about how to ensure safe healthcare environments, protect patients from undue harm, and communicate risks and benefits. Patients who are exposed to risks—especially undisclosed or poorly understood risks—may experience psychosocial concerns such as heightened anxiety about their health and distrust in care providers, institutions, and regulators.
Table 3. Major Legal Questions Associated with the Reuse of SUDs in Canada

Legal Liability
The reuse of SUDs raises potential liability issues for several entities: the original manufacturer of the device; the reprocessor of the device: the healthcare institution where a patient receives care; and the care provider who treats the patient.
Manufacturers generally seek to avoid liability through the use of labels advising that devices are for single use only. Reprocessors must ensure that their processes are adequate to ensure proper functioning and cleanliness of SUDs intended for reuse. They have an ongoing duty to warn healthcare facilities of new information that reveals heightened risk.
The most relevant responsibilities for healthcare institutions are to provide proper facilities and equipment, and to establish systems necessary for the safe operation of the hospital. A facility may be negligent if its internal processing methods are deficient, or if the facility contracts with a poor quality reprocessor. Healthcare institutions are also vicariously liable for the negligence of their employees. Healthcare providers may be unaware that specific devices have been reprocessed and may not be in a position to judge whether using the device increases risks to patients. It may be burdensome to expect care providers to make independent inquiries about the safety of devices used routinely. Also, off-label use does not automatically amount to negligence without other evidence of deficient practice.
Informed Consent
It is not common practice to inform patients before medical procedures if devices that will be used are reprocessed SUDs. A patient who is harmed through the use of a reprocessed SUD, however, may argue that he or she ought to have been informed that a reprocessed device would be used. Under Canadian law, a healthcare provider must disclose to a patient risk information that a reasonable person in the patient's position would want to know. Canadian courts have not been confronted with a legal claim concerning harm from a reprocessed SUD. If available evidence suggests that the reuse of specific devices may increase the risk of harm, healthcare providers may have a legal obligation to disclose this information to the patient before treatment.
If evidence indicates increased risk, however, it is legally and ethically tenuous to ask patients to accept that risk during the informed consent process. The economic benefit of reusing devices must be balanced against the patient's right to a safe healthcare environment, and care providers may face liability for adopting practices designed to protect budgets rather than patients. If concerns about the safety risks of reusing SUDs are largely theoretical, questions arise about the ethics of informing patients of hypothetical risks.
New Evidence of Risk
In addition to the issue of prospective disclosure of risks to patients before treatment, a duty to notify may arise when newly acquired evidence reveals that patients may have been exposed to harm in the past, for example, from exposure to an infectious disease linked with reuse of a SUD.
Survey of Hospitals
The survey was sent to 572 Canadian acute-care hospitals in all jurisdictions. The response rate was 72 percent, and 398 responses (70 percent) were used in the analysis.
The majority of the hospitals that provided responses (287; 72 percent) do not reprocess SUDs. The most common reasons given by 232 hospitals for discontinuing the practice were concerns about potential legal liability (77 percent) and about patients' safety (74 percent).
Of the 111 hospitals in our sample that reprocess SUDs, 40 percent do not have a written policy, and 12 percent do not have an incident-reporting mechanism, suggesting a need for improved standards of documentation. A significantly greater proportion of academic hospitals reprocess SUDs (37 percent, 32/86) compared with community hospitals (25 percent). Also, larger hospitals are significantly more likely to reprocess SUDs. The SUDs most commonly reprocessed were breast pump kits, ventilator circuits, and burrs.
Ninety-four (85 percent) of the hospitals reprocess SUDs in-house. Thirteen of the seventeen hospitals that use a third-party reprocessor were strongly satisfied or satisfied with the function of the device, cleanliness and sterility of the device, and customer service.
DISCUSSION
This assessment has pointed to the need to consider several factors in providing advice to decision makers on an established practice that may influence the performance of several other types of health technology. The various parties with interest in the reuse of SUDs require information on safety, effectiveness, and economic implications of this practice and on legal and psychosocial aspects.
Our assessment indicated that many uncertainties remain regarding the reuse of SUDs. The results of the clinical review are consistent with the findings of the New Zealand report (Reference Day4) that few studies on the use of reprocessed SUDs have evaluated outcomes directly related to patients. The studies located for our review suggested that reuse of SUDs might be safe and effective for a few types of devices. However, the clinical studies were of variable quality and duration. Even the two high quality RCTs on reuse of coronary angioplasty catheters and instruments for laparoscopic cholecystectomy could not be considered to rule out the risk of adverse events that have a low level of probability.
Although the studies in the economic review provided decisive evidence of cost savings in the absence of adverse events, the methods of observing costs were vague, and the savings calculated did not reflect the marginal savings from SUD reprocessing. If one excluded clinical outcomes, the marginal savings from one extra time that a device is reprocessed would depend only on the original price and the cleaning cost. These savings are greater than those estimated by calculating the price less the average unit cost, which are the measures used in most of the cost studies.
A limitation on the generalizability of the findings from both the clinical and economic reviews is that not all reprocessing is the same. Most studies looked at in-house, nonvalidated reprocessing and are difficult to group with those that considered devices obtained from registered third-party reprocessors. Given that most single-use devices being reprocessed in Canadian hospitals were not represented in these studies and that the reprocessing procedures for these devices are unknown, it is impossible to conclude from the existing literature that the current state of affairs is safe.
The savings identified in the reviewed cost studies would not apply should substantial adverse effects occur as a consequence of reusing SUDs. Our economic analysis gave an illustration of the potential effect of adverse events on costs associated with the practice. In the model used, the cost of an adverse event is higher for coronary angioplasty than for laparoscopic cholecystectomy. Consequently, the break-even value of the probability of an adverse event, where costs of the single-use and reuse strategies are the same, is lower for coronary angioplasty catheters than for laparoscopic instruments (12.6 per 1,000 compared with 445 per 1,000). A complication rate of 12.6 per 1,000 for angioplasties is realistic, but one of 445 per 1,000 for laparoscopic cholecystectomies is high. Therefore, including uncertainty, reuse would generate system-wide savings for laparoscopic cholecystectomies, but would be less likely to do so for angioplasties. The budget impact analysis indicated that with current levels of reuse in Canada of SUDs for these two procedures, consequences of reverting to single use only would be minimal.
The review of legal, ethical, and psychosocial issues identified several issues of importance to healthcare funders, providers and patients. There is a need to balance regulatory and legal responsibilities, and liabilities, with the economic benefits associated with reusing these products. The liability risks associated with the reprocessing and reuse of SUDs may lead to higher costs. Patients may need to be informed about the known risks from using reprocessed SUDs
The survey of Canadian acute-care hospitals indicated that most do not currently reuse SUDs. However, the substantial minority that do so use a variety of reprocessing approaches, and do not always have appropriate documentation.
POLICY IMPLICATIONS
Policies and practices on SUDs vary from one nation to another. France prohibits the reprocessing of all SUDs. The United States, Australia, and Sweden require all reprocessors, including hospitals that reuse SUDs, to comply with the same regulations as original equipment manufacturers. Germany requires the registration of all reprocessors and proof of the reprocessing procedure's suitability. The UK has not instituted a regulatory ban, but a statement against the practice was issued by the UK's Medical Devices Agency.
Those in Canada responsible for policy in respect of SUD reuse are faced with several uncertainties about the practice under current arrangements. Formal studies on SUD reuse give only limited information, and the safety and efficacy of in-hospital reprocessing is unclear. In the absence of comprehensive information on adverse effects, the cost-effectiveness of reusing SUDs is unknown. The liability risks associated with the reprocessing and reuse of SUDs may lead to higher costs, particularly for healthcare facilities, Also, if scientific evidence reveals the risks of reuse, there is an argument that patients ought to be informed of these risks.
The findings of our assessment do not provide support for the reuse of SUDs in the context of Canadian healthcare. Policy options might include taking steps to eliminate reuse of SUDs, restricting the practice to particular types of device or permitting reuse only after reprocessing by a third party organization of acceptable quality. Implementation of any policy changes will require continuing dialogue between the different levels of government and institutions, particularly as the ultimate decision on SUD reuse rests with the hospital sector.
CONTACT INFORMATION
David Hailey, PhD (dhailey@ozemail.com.au), Adjunct Professor, Department of Community Health Sciences, University of Calgary, 3330 Hospital Drive NW, Calgary, Alberta T2N 4N1, Canada; Senior Professor, Institute of Health Economics, #1200, 10405 Jasper Avenue, Edmonton, Alberta, T5J 3N4, Canada
Philip D. Jacobs, DPhil (pjacobs@ihe.ca), Professor, Department of Medicine, University of Alberta, Zeidler Ledcor Centre, Edmonton, Alberta T6G 2×8, Canada; Director, Collaborations, Institute of Health Economics, #1200, 10405 Jasper Avenue, Edmonton, Alberta T5J 3N4, Canada
Nola M. Ries, MPA, LLM (nries@law.ualberta.ca), Research Associate, Health Law Institute, University of Alberta, Law Centre, Edmonton, Alberta T6G 2H5, Canada
Julie Polisena, MSc (juliep@cadth.ca), Research Officer, HTA Directorate, Canadian Agency for Drugs and Technologies in Health (CADTH), 600–865 Carling Avenue, Ottawa, Ontario, K1S 5S8, Canada





