Uterine leiomyomas, or fibroids, are common benign smooth muscle tumors originating from myometrial uterine cells and are estimated to affect up to 80 percent of perimenopausal women (Reference Janus, White, Dottino, Brodman and Goodman1–Reference Pritts, Parker, Brown and Olive11). Many fibroids cause debilitating symptoms, such as pain, pressure symptoms, subfertility, abnormal uterine bleeding, dysmenorrhea, and menorrhagia, incurring great costs to patients and healthcare systems (Reference Cardozo, Clark, Banks, Henne, Stegmann and Segars12;Reference Soliman, Yang, Du, Kelkar and Winkel13). Traditional treatment options for fibroids include myomectomy or hysterectomy. Recently, management has also trended toward more conservative techniques, such as temporary reduction with hormonal medical therapy, volumetric radiofrequency ablation, uterine artery embolization, and magnetic-resonance imaging (MRI) guided focused ultrasound. While these are associated with reduced perioperative morbidity and shorter hospital inpatient stays, histopathological diagnosis is generally impractical. A malignancy may be missed, given also the difficulty in accurate preoperative diagnosis to guide management choices, resulting in potential delayed diagnosis or inadvertent upstaging of disease.
Laparoscopic fibroid resection has become much more commonplace. Morcellators enable removal of a large tumor by means of a laparoscopic port site, reducing postoperative morbidity with shortened operating times and smaller incisions. The Food and Drug Administration released a statement in 2014 discouraging use of laparoscopic power morcellation following a case of inadvertent morcellation of a leiomyosarcoma, and subsequent malignant upstaging secondary to dissemination within the abdominal cavity (Reference Wallis14). This has prompted many clinicians to consider the therapeutic challenges posed by current treatment techniques.
Uterine sarcomas represent approximately 3–7 percent of uterine cancers (Reference del Carmen15;Reference Gadducci16). Leiomyosarcomas are the most common histological variation of these, accounting for a total of 1–2 percent of uterine malignancies (Reference del Carmen15). Annual incidence is estimated to range from 0.5 to 7 in 100,000 women (Reference del Carmen15;Reference Toro, Travis, Wu, Zhu, Fletcher and Devesa17–Reference Sahdev, Sohaib, Jacobs, Shepherd, Oram and Reznek20). The great majority of leiomyosarcomas are believed to arise de novo from the uterine myometrium or connective tissue surrounding the uterine vasculature, and very rarely, from pre-existing fibroids (Reference Tinelli18;Reference Rha, Byun and Jung21–Reference Rockall, Freeman, Mitchell, Sala and Reinhold25). Leiomyosarcomas are characterized by their aggressive behavior, with high recurrence rates once pelvic tissues are involved (Reference Tinelli18;Reference Hayashi, Horiuchi and Aburatani19;Reference Santos and Cunha24;Reference D'Angelo and Prat26).
Reliable differential diagnosis of fibroids from uterine malignancies such as leiomyosarcomas, is increasingly important due to popular use of conservative treatment modalities for fibroids. There is no scientifically validated screening process to reliably diagnose leiomyosarcomas preoperatively, with no clear consensus or international guidelines available to describe pathognomonic radiological features.
There is no atudy in the literature that has systematically reviewed the diagnostic value and explored statistically significant diagnostic features of leiomyosarcomas in MRI modalities in differentiation from fibroids. This investigation aims to identify and discuss possible significant indicators of diagnosing uterine leiomyosarcoma in a key preoperative diagnostic modality.
METHODS
A systematic literature review was performed according to guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Reference Moher, Liberati, Tetzlaff and Altman27). The review was not registered with PROSPERO International Prospective Register of Systematic Reviews. The systematic review process for this report is illustrated in Figure 1.
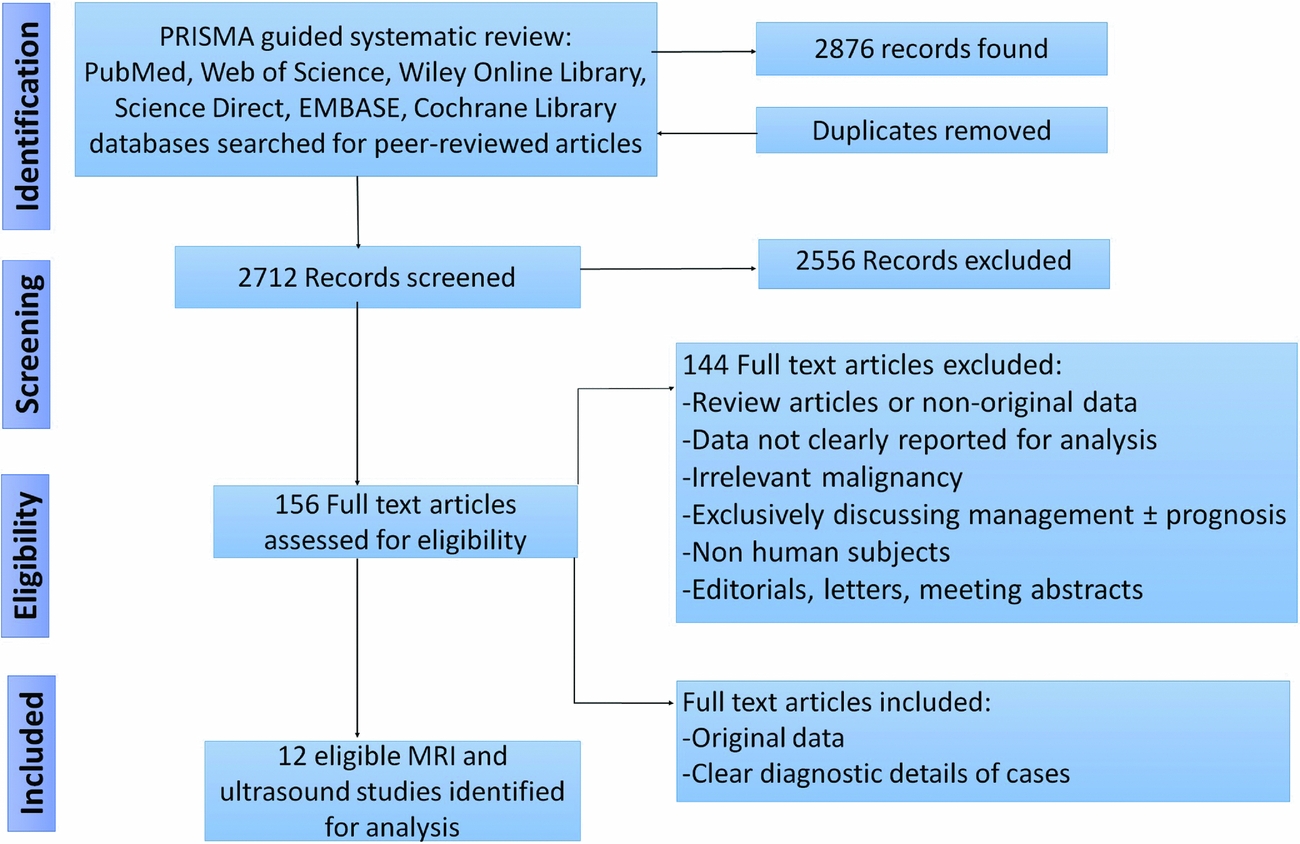
Figure 1. PRISMA guided flow-diagram of the systematic review process.
Literature Search Strategy
PubMed, Web of Science, Wiley Online Library, Science Direct, EMBASE, Cochrane Library databases were comprehensively searched up to March 2016. Search terms were: “uterine sarcoma”, “uterine leiomyosarcoma”, “leiomyosarcoma”. These terms were additionally searched in combination with “MRI”, “ultrasound” “computed tomography”, “positron emission”, “histopathology” and “biopsy”. All articles available in English incorporating pre- and perioperative diagnosis of uterine leiomyosarcoma discussing and comparing imaging techniques and markers were reviewed. Duplicate studies were removed. An updated search was run subsequently to November 2017, which did not yield relevant studies for inclusion in the review.
Screening
Studies were assessed on criteria of malignancy, specifically histopathological “leiomyosarcoma”. Studies mentioning uterine “sarcomas” were examined for histopathological subtypes of leiomyosarcomas.
For evaluation of the diagnostic performance, prospective studies reporting diagnosis of uterine leiomyosarcoma, as well as retrospective studies, where at initial reporting the radiologists were blinded or unaware of the diagnosis. Given the rarity of the malignancy, case reports detailing imaging findings were also included. Studies focusing on radiological diagnostic interventions (ultrasound, MRI, computed tomography, and positron-emission technology scans) were reviewed for relevance. In addition to the electronic search, reference lists of all relevant articles were reviewed for potential inclusion of other studies.
Inclusion Criteria
All types of studies were examined for inclusion, including case reports. No relevant randomized-control trials were identified. Studies including women diagnosed with uterine leiomyosarcomas, regardless of menopausal status, before treatment interventions, with clear descriptions of ultrasound and MRI imaging characteristics were included. If the study data described more than one imaging modality, exclusion and inclusion criteria were applied to each technique. Studies reporting on diagnostic features of recurrent uterine leiomyosarcomas were included in the review and recurrence post treatment was noted.
Exclusion Criteria
Non-English publications, letters to the editor, conference or meeting abstracts, animal studies, nonoriginal data, and malignancies that were not uterine leiomyosarcomas, or studies exclusively discussing treatment of leiomyosarcomas were excluded.
Extraction Process
Where listed, the parameters of the extracted data included: patient features (age, menopausal status), lesion size in centimeters, benign uterine leiomyoma pathology (classified as benign, degenerate, infarcted, cellular, or lipoleiomyoma), and MRI features (type of machine, T1 and T2 signal intensity, enhancement profile, component visualized, apparent diffusion coefficient, contrast enhancement and diffusion weighted imaging appearance). Following removal of duplicates, data from included studies were entered into a standardized database.
Statistical Analysis
R (Version 3.2.2, August 2015) (28) statistical software was used for statistical analysis. “Exact” R package® was used for Barnard's exact test. Microsoft Excel 2016® was used for statistical analysis and graph construction. There were three investigative modalities that were reported across MRI studies to enable analysis: T1 and T2 signal intensities and ADC values.
Four questions were posed for statistical analysis: (i) Is histopathological type (leiomyosarcoma versus benign fibroids) independent of T1 intensity? (ii) Is histopathological type (leiomyosarcoma versus benign fibroids) independent of T2 intensity? (iii) Can T1 and T2 MRI signal intensities accurately diagnose the presence of leiomyosarcoma? (iv) Do ADC values vary significantly by histopathological type?
Chi-square tests of independence, likelihood ratio tests and Barnard's exact tests were applied to examine the first two research questions. For the third, a J48 algorithm classifier model (Reference Patil29) was used to construct a decision tree based on T1 or T2 signal intensities using 10-fold cross validation. The last research question was examined using standard tests for normality of a continuous variable with application of a Welch Independent two sample t-test. The significance value was set at p < .05.
RESULTS
The systematic literature review identified 2,855 peer-reviewed records total from PubMed, Web of Science, Wiley Online Library, Science Direct, and Cochrane Library databases. After duplicate removal, 2,679 records remained to be screened. A total of 156 full text articles were assessed for eligibility. Of these evaluated articles, eleven were derived from references that were hand-searched for potential relevance. Three studies investigating ultrasound diagnostic features of uterine leiomyosarcomas were identified (Reference Kurjak, Kupesic, Shalan, Jukic, Kosuta and Ilijas22;30;Reference Hata, Hata, Maruyama and Hirai31), but due to scarcity and heterogeneity of reported data, these were excluded from this report. Nine studies investigating MRI diagnostic features of uterine leiomyosarcomas met the criteria and are summarized in Tables 1 and 2 (Reference Janus, White, Dottino, Brodman and Goodman1–Reference Lin, Yang and Huang9).
Table 1. Characteristics of MRI Studies Reporting Findings on Uterine Leiomyosarcomas

Table 2. Characteristics of MRI Studies Reporting Findings on Uterine Leiomyosarcomas

Reports on parameters were invariably inconsistent and contributed to data heterogeneity. Additionally, paucity of studies and data available resulted in these series being unsuitable for meta-analysis.
The first variables examined in MRI studies were T1 and T2 signal intensities, and whether the histopathological type of lesion influenced signal intensities. A comparison was then made between T1 and T2 signal intensities, and observed frequency (in percent) by histopathological types. The results are summarized in Supplementary Figures 1 and 2, respectively.
A high incidence of high T2-signal intensities (96 percent) was observed for both leiomyosarcomas and benign fibroids. Comparatively, there was a very low incidence (4 percent) of low T2-signal intensities for leiomyosarcoma cases.
The data were reviewed to identify whether histopathological type is independent of T1 signal intensity. A Chi-squared test was conducted, with test statistic of 14.75 and p value of < .05, therefore, rejecting the null hypothesis that histopathological type is independent of T1 intensity. Likelihood G-squared, Barnard's exact test further indicated a statistically significant level of p < .05. This appears to indicate a strong positive relationship between leiomyosarcoma histopathology and high T1 signal intensities on MRI, and a strong negative relationship between leiomyosarcomas and low T1 signal intensities on MRI.
From this dataset, leiomyosarcomas are 3.44 times (95 percent confidence interval [CI], 1.67 to 7.08) more prevalent among lesions with high T1-intensity signals, in comparison to benign lesions. There are 7.38 times (94 percent CI, 2.53 to 21.55) the risk of a leiomyosarcoma if MRI T1-signal intensity is high, in comparison to benign pathology in this study.
The same methodology was applied for determining whether histopathological type is independent of T2 intensity. Chi-squared analysis, Likelihood G-squared, Barnard's exact test all indicate a p value of < .050. There appears to be a significant relationship between histopathological type and T2 intensity signals. The greatest contributions to the Chi-squared statistic derive from low T1 intensity readings. Therefore, from this dataset, there appears to be a negative relationship between leiomyosarcomas and low T2-signal intensities on MRI. Only one leiomyosarcoma was observed with a low T2-signal intensity. From this dataset, leiomyosarcomas are 6.41 times (94 percent CI, 0.95 to 43.22) more prevalent among lesions with high T2-intensity signals, in comparison to benign lesions. The odds of observing a low T2-signal intensity with a leiomyosarcoma was 7.69 and 84.38 for a high-T2 signal intensity (95 percent CI, 1.34 to 89.35).
As the range of the odds ratio CI is wide and crosses the null value, there is insufficient evidence to conclude that histopathology provides statistically significantly different readings in high versus low T2-signal intensities on MRI. This result is largely driven by high T2-signal intensities being often observed by both benign fibroids and leiomyosarcomas in this dataset.
The third research question addressed was whether T1 and T2 MRI signal intensities are useful indicators of diagnosing leiomyosarcomas. To determine this, a J48 decision tree algorithm classifier statistical model (Reference Patil29) was used to classify the 73 cases. The dependent variable was histopathological type (leiomyosarcoma or benign pathology). Figure 2 illustrates the structure of the model.
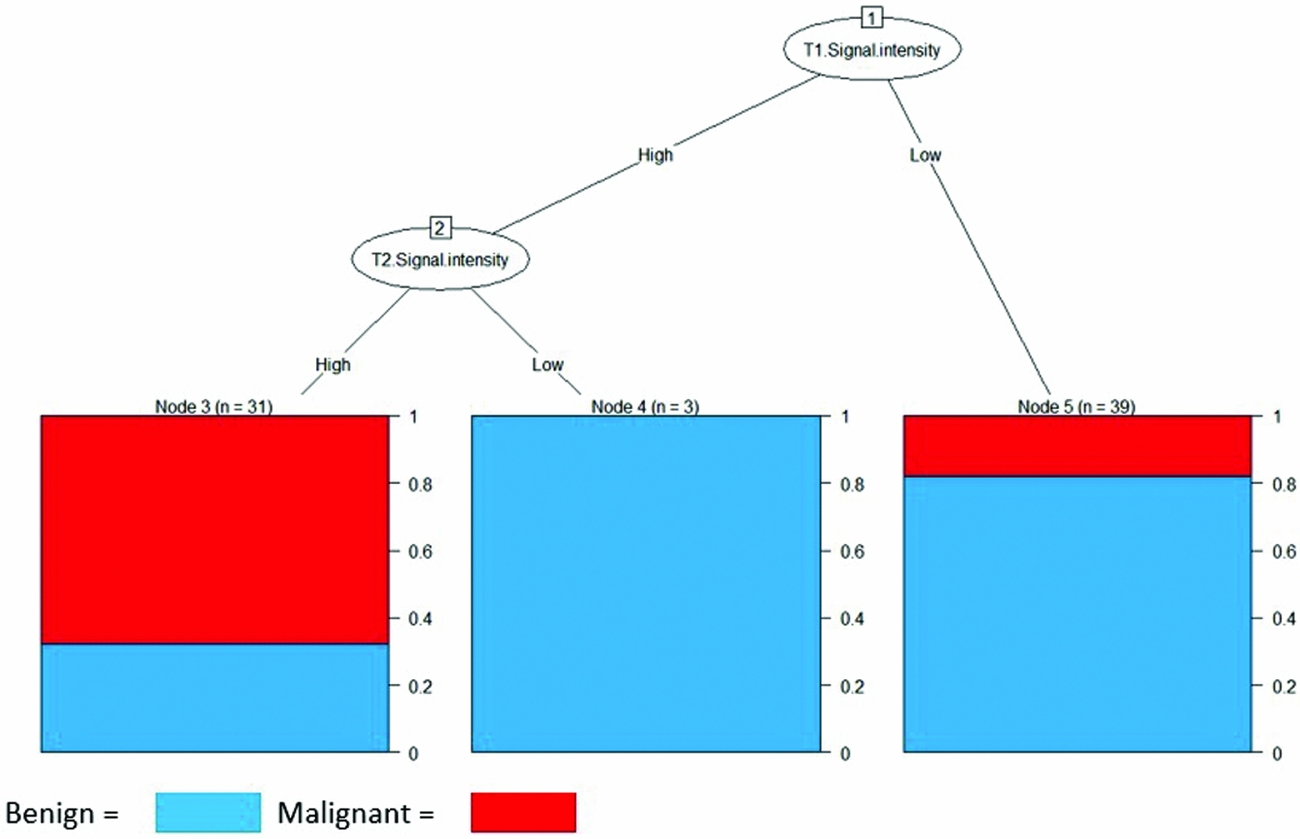
Figure 2. Decision Tree based on histopathological type classified by T1 and T2 signal intensities on MRI.
The decision tree diagram indicates that low T1- and T2-signal intensities are most commonly associated with benign pathology, whereas both high T1 and T2 signal intensities are good indicators for presence of leiomyosarcoma. The J48 tree begins with sequential interpretation of initially T1-signal intensity, and subsequently T2-signal intensity, whereby lesions are grouped by high or low signals. This results in three separate outcomes from the decision tree: T1-low intensity classifies as benign, T1-low and T2-low intensity classifies as benign, and T1-high and T2-high intensity classifies as leiomyosarcomas.
The decision tree model was able to correctly classify histopathology using T1 and T2 signal intensity readings in 76.71 percent of cases with a root mean squared error of 0.4279. The calculated Kappa statistic of 0.52 indicates that the observed accuracy of the model as compared to expected accuracy (or random chance) is of moderate strength.
The positive prediction value of the model is 67.74 percent (CI, 48.63 percent to 83.32 percent), which crosses 50 percent (random chance). This indicates that additional information beyond T1 and T2-signal intensity is required to more accurately identify leiomyosarcoma cases.
However, the negative prediction value of the model of 83.33 percent (CI, 68.64 percent to 93.3 percent) and model specificity of 77.78 percent (CI, 62.91 percent to 88.80 percent) demonstrate that knowledge of just T1 and T2-signal intensities correctly classified benign pathology in approximately eight of ten observed cases.
The fourth research question was addressed by examining ADC values for significant differences between pathological types of leiomyosarcoma and benign pathology. Lin et al. (Reference Lin, Yang and Huang9) and Tamai et al. (Reference Tamai, Koyama and Saga6) reported study findings of mean respective ADC values for leiomyosarcomas to be 1.05 ± 0.37 and 1.24 ± 0.04, and mean ADC values for benign lesions to be 1.20 ± 0.27 and 1.57 ± 0.24, respectively.
Standard tests of normality and homogeneity included Bartlett's K-squared test and Welch Independent two sample t-test. These, respectively, gave p-value of > .05, suggesting that the mean ADC values across groups were not proven to differ significantly by histopathological type. The variation in ADC values by histopathological type is illustrated in Supplementary Figure 3.
DISCUSSION
A major finding in this study was that a statistically significant relationship was observed in high T1-signal intensities on MRI diagnosis of uterine leiomyosarcoma over benign pathology. The study also demonstrated a statistically significant relationship between histopathological type and T2-signal intensities. While high T2-signal intensities are commonly observed with benign and malignant histopathology, the odds of observing a low T2-signal intensity where uterine leiomyosarcoma is very low in this study. This is a finding that has been observed as part of a trend in some previous studies (Reference Tanaka, Nishida, Tsunoda, Okamoto and Yoshikawa4;Reference Goto, Takeuchi, Sugimura and Maruo32), but this has not as yet been substantiated using data collected across multiple studies.
This investigation's results regarding ADC values of leiomyosarcomas are similar to other studies’ reported mean values. With a mean of 1.14, this is most similar to the findings of a mean value of 1.17 by Tamai et al. (Reference Tamai, Koyama and Saga6) and (Reference Lin, Yang and Huang9) a mean value of 1.05 in the study by Lin et al. Studies with lower readings included Zhang et al. (Reference Zhang, Zhang, Tian and Zhang33) reported mean values of 0.93 and Sato et al. (Reference Sato, Yuasa, Fujita and Fukushima34) reported mean ADC values of 0.79. This systematic review did not demonstrate a significant statistical correlation between ADC values of leiomyosarcomas and benign uterine pathology. This may be reflective of the variable and complex nature of the malignancy, for example, higher ADC values may be observed in solid portions of sarcomas or degenerative benign fibroids (Reference Buy and Ghossain35).
Tamai et al. (Reference Tamai, Koyama and Saga6) did also observe overlap in ADC values in benign and cellular fibroids. ADC values may vary on several acquisition parameters, including the magnetic field strength of the MRI unit, variance in the b-values, which may also contribute to the statistical observation in this review (Reference Motoshima, Irie, Nakazono, Kamura and Kudo36–Reference Kim, Lee, Shin, Park and Kim38). The magnetic field strength of the MRI machines differed between the compared study cohorts: Lin et al. (Reference Lin, Yang and Huang9) used 3.0T, whereas Tamai et al. (Reference Tamai, Koyama and Saga6) used 1.5T MRI units. b-Values, factors that signal the strength and timing of the gradients that generate diffusion-weighted images, also varied between the two cohorts, which also likely impacted on interpretation of ADC values (Reference Kim, Lee, Shin, Park and Kim38). Furthermore, with one study prospective (Reference Lin, Yang and Huang9) and the other retrospective (Reference Tamai, Koyama and Saga6) in design, the diagnostic performance of diffusion weighted imaging and ADC interpretation may have also affected reported ADC values.
Although this study is the first systematic review investigating reliable MRI diagnostic features for uterine leiomyosarcomas, the studies included in this review, and subsequently, the data analyzed, has several limitations. Most of the data in the studies was retrospectively collected, with inherent biases arising from gathering and examining data post factum. Moreover, there was a degree of variation in data presentation and incomplete reporting of data variables by researchers. Therefore, meta-analysis of overall sensitivity and specificity of imaging modalities was unable to be performed due to both paucity and heterogeneity of studies. The effects of certain variables were not always able to be sub-analyzed as a result.
There appears to be a degree of risk of selection bias. Inclusion of low-level evidence, such as case reports, in the review, further contributes to selection bias with “cherry picking.” Case reports do not report on other cases that may have been encountered at a particular institution, which may have benign outcomes but clinically presenting similar to a malignancy. Large uterine masses, benign or malignant, may present similarly with bleeding and pelvic pain. Omitting reporting of benign pathology of larger uterine masses prevents comparison from being made on whether there is a significant difference between radiological features for suspicious masses but with different pathologies.
Many of the studies included are case reports or case series. The risk of bias in these studies is likely inherent. The study by Cornfeld et al. (Reference Cornfeld, Israel, Martel, Weinreb, Schwartz and McCarthy8) was considered to have high bias for flow and timing due to reporting on results based on various MRI machines, some of which provided results using intravenous contrast, whereas others were not. Contrast is a medium known to aid diagnosis of pelvic malignancy (Reference Lin, Yang and Huang9,Reference Rockall, Freeman, Mitchell, Sala and Reinhold25,Reference Goto, Takeuchi, Sugimura and Maruo32) and, hence, may have influenced reader interpretation of image results. Current radiological practice for workup of suspicious uterine masses at present almost always uses a contrast medium. The study by Tamai et al. (Reference Tamai, Koyama and Saga6) may also be biased in nonconsecutive design, with exclusion criteria set on a cutoff on lesion size in their particular study (<2 cm). While unlikely, this may potentially miss interpretation of smaller leiomyosarcomas. For all studies, reference standards were consistently based on final histopathological confirmation of diagnosis of leiomyosarcoma, which was also an inclusion criterion for the systematic review.
While all studies examined reported postoperative histopathology of leiomyosarcoma, overall specificity or sensitivity could be determined. It is interesting to note also that many of the studies evaluating MRI did not list whether an ultrasound was primarily used in the initial workup of the patient and if so, what the findings were. It is likely that in clinical practice, ultrasound would have been the initial modality used for investigation of a uterine mass. Therefore, verification bias may be present, whereby the results of a diagnostic test (i.e., first-line potentially suspicious ultrasound) may influence work-up (i.e., how MRI, or subsequent investigation, is interpreted). Indeed, pelvic ultrasound is an important gynecological initial workup modality; the paucity of studies available investigating presentations of leiomyosarcomas with this technology prevented more in-depth analysis. The overall diagnostic ability and superiority of MRI in comparison to ultrasound was not measurable.
Another limitation of the review is that there is a strong risk of sampling bias, with relatively small sample sizes being compared. Uterine leiomyosarcomas are often reported in a series of case reports, which along with case series, were included in the statistical analysis. Despite a broad literature search, only one prospective study was found, reflective of the difficulty in performing prospective studies evaluating rare lesions (Reference Lin, Yang and Huang9). While statistically significant relationships were observed with leiomyosarcomas and T1 and T2-signal intensities, the risk and odds ratio CI ranges were wide, and additional cases may be required to narrow this range and support this finding. Imprecision, or random errors, may occur in smaller studies, due to potential risk of sampling variation. Variations in the imaging technologies used across studies, and user-dependent interpretation, may affect the estimates of the diagnostic test accuracy.
Previous knowledge of the radiological diagnosis of malignancy may also affect accuracy of interpretation, and studies were screened for this potential influence, such as with assessment of recurrence of disease. Furthermore, studies reporting on various diagnostic parameters limit the amount of data available for analysis. Attention was drawn to parameters more commonly reported and, hence, available for statistical comparison and interpretation of diagnostic features of leiomyosarcomas.
The overall heterogeneity of data presented in the studies reflects a great need for a standardized universal protocol when reporting on these rare malignancies. Future considerations may involve use of a combination of imaging and histopathological investigations to aid diagnosis and to assess whether this improves diagnostic sensitivity.
Studies investigating transcervical needle biopsy and intraoperative frozen section samples of suspicious uterine masses appear promising (Reference Shibata, Kawamura and Ito39–Reference Tulandi and Ferenczy42). Such techniques are not widely practiced for workup of uterine masses and their feasibility in wide-ranging practice has not yet been demonstrated. An interesting avenue of research would be to investigate whether combined use of imaging and biopsy for select cases based on a predefined criteria increases the diagnostic odds ratio.
Most studies in this review had a high degree of heterogeneity, suggesting for ideally, high quality prospective studies. Randomized controlled trials may be difficult to organize, however, with the low incidence of uterine leiomyosarcomas and high-cost and difficulty of access to MRI. Clinicians undertaking research in diagnosis of leiomyosarcomas should aim to methodically collect all data available. A national, or even international database may aid in establishing a large enough cohort to derive statistically significant conclusions on diagnostic parameters.
CONCLUSION
The lack of unequivocal preoperative diagnostic criteria in uterine leiomyosarcoma necessitates an ongoing search for more reliable parameters. Due to relatively rare case numbers, and variations in markers of atypia, the diagnosis of leiomyosarcoma is a challenging histopathological and radiological diagnostic dilemma. With statistically significant relationships observed between T1 and T2 signal intensity patterns and uterine leiomyosarcomas, further prospective research into the role of medical imaging in identifying leiomyosarcomas preoperatively will help derive meaningful diagnostic parameters to guide clinicians.
SUPPLEMENTARY MATERIAL
Supplementary Figure 1: https://doi.org/10.1017/S0266462318000168
Supplementary Figure 2: https://doi.org/10.1017/S0266462318000168
Supplementary Figure 3: https://doi.org/10.1017/S0266462318000168
CONFLICTS OF INTEREST
The authors have nothing to disclose.






