Diabetes mellitus (DM) is rapidly becoming one of the most common chronic diseases globally. The International Diabetes Federation estimates that 285 million people around the world suffer from diabetes, and this number is projected to rise to approximately 440 million by 2030 (Reference Schaper, Apelqvist and Bakker1). Characterized by impaired glycemic control due to insulin deficiency (Type 1 DM) or resistance to insulin action (Type 2 DM), diabetes is associated with complications such as peripheral neuropathy and peripheral vascular disease. Combined with lower limb trauma, these complications increase the likelihood of diabetic patients developing lower limb ulceration with a lifetime risk estimated to be 15 percent (Reference Reiber, Edward, Smith, Harris, Cowie, Stern, Boyko, Reiber and Bennett2). In these patients, wound healing is compromised due to poor circulation, and a foot injury or infection may not be detected due to poor sensory function in the lower limbs and feet (Reference Rakel, Huot and Ekoe3). Without early treatment, a foot ulcer may aggravate until it becomes infected and chronic. Chronic wounds are difficult to heal, despite medical and nursing care, and may lead to impaired quality of life and functioning, amputation, or even death (Reference Reiber, Edward, Smith, Harris, Cowie, Stern, Boyko, Reiber and Bennett2;Reference Mayfield, Reiber and Sanders4).
Diabetic foot ulcers can be classified on the basis of severity through a widely used wound classification system called the Wagner Grade Scale (Reference Wagner5). This tool ranges from grades zero (cellulitis) to five (foot gangrene), and encompasses the depth of the ulcer and whether the ulcer is infected. Wagner Grades 2, 3, and 4 characterize deep ulcers, deep abscess, osteomyelitis, and foot gangrene, respectively (Reference Rakel, Huot and Ekoe3;Reference Mayfield, Reiber and Sanders4). Severe, chronic lower limb ulcers often require amputation. For example, people with diabetes have a 15-fold greater risk of lower extremity amputation than those without diabetes (Reference Reiber, Edward, Smith, Harris, Cowie, Stern, Boyko, Reiber and Bennett2). Amputations are classified as either major (an amputation of the leg above or below the knee) or minor (which involves the amputation of toes or the forefoot) with differing impact on quality of life and health (6).
The standard of care for treating diabetic ulcers involves adequate nutrition, proper glycemic control, regular debridement, dressing changes, use of antibacterial agents, foot pressure relief, and in some cases amputation (Reference Bowering, Ekoe and Kalla7;Reference Frykberg8). In addition, adjunctive therapeutic interventions such as hyperbaric oxygen therapy (HBOT) have been shown to improve the rate of wound healing and reduce the risk of lower extremity amputation (Reference Abidia, Laden and Kuhan9–Reference Faglia, Favales and Aldeghi11). HBOT has been suggested to increase plasma oxygen levels and improve wound healing through the inhalation of 100 percent oxygen at 2.0–2.5 atmospheres absolute (ATA) pressure in a compression chamber (Reference Tibbles and Edelsberg12). HBOT has been in use for more than 50 years, it is thought to aid healing by supplying oxygen to the wound (Reference Rakel, Huot and Ekoe3).
Previously published systematic reviews evaluating adjunctive HBOT to good wound care have reported mixed results using varying methods and inclusion criteria. In 2005, Roeckl-Wiedmann et al. (Reference Roeckl-Wiedmann, Bennett and Kranke13) reviewed randomized controlled trial (RCT) data and were unable to confirm any significant benefit on ulcer healing or need for minor amputation with HBOT treatment. The systematic review presented statistically significant evidence that HBOT decreased the risk of major amputation (based on fixed effects model). A review that included seven RCTs found that 11 percent of patients treated with HBOT and 32 percent of patients in control groups had major lower extremity amputations and 83 percent versus 43 percent of wounds healed in the HBOT and control groups, respectively (Reference Hailey, Jacobs and Perry14). The authors combined the event rates from each paper but did not perform any statistical tests.
A recently published systematic review of the published RCT data by the Cochrane Collaboration (Reference Kranke, Bennett and Martyn-St James15), reported a significant improvement in wound healing in the short-term (i.e., 6 weeks), but found no statistically significant difference in the rates of wound healing, major or minor amputation favoring HBOT.
There are reviews that considered data from both RCTs and observational studies (Reference Goldman16–Reference Londahl, Fagher and Katzman18). Goldman (Reference Goldman16) reported that the pooled odds for major amputation decreased with the administration of HBOT and that the odds of healing are also better with HBOT. Other reviews that looked at the randomized and nonrandomized evidence have also concluded that HBOT is associated with improved wound healing and a reduction in the rate of amputation (Reference Wang, Schwaitzberg and Berliner17;Reference Londahl, Fagher and Katzman18). However, Wang et al. (Reference Wang, Schwaitzberg and Berliner17) did not pool the data from the individual trials and Londahl et al. (Reference Londahl, Fagher and Katzman18) combined the event rates from each paper by study design but did not perform any statistical tests.
The objective of this study is to systematically review the clinical evidence from both RCT and observational studies of HBOT for adults with nonhealing diabetic ulcers of the lower limb to ascertain whether adjunctive HBOT decreases the rate of amputation, improves wound healing, safety, and quality of life compared with standard care alone (i.e., debridement, dressings, antibiotics, and minimization of pressure on the wound) or sham. Additionally, this review synthesizes important study outcomes using meta-analytic techniques where possible.
METHODS
Literature Search Strategy
A systematic search was undertaken for the purposes of locating clinical studies assessing HBOT for lower limb diabetic ulcers. The search strategy was developed by an Information Specialist (K.C.) with input from the project team. All search results were imported into a Reference Manager Version 12 database for the purposes of de-duplication and title/abstract screening.
A search strategy with controlled vocabulary and keywords focusing on the concepts of “HBOT” and “lower limb diabetic ulcers” was executed. No year or language limits were used but the human limit was applied when available (see Supplementary Table 1, which can be viewed online at www.journals.cambridge.org/thc2013113). The following bibliographic databases were searched: Ovid Medline (1946-present; In-Process & Other Non-Indexed Citations) and EMBASE (1980-present); PubMed (for non-Medline records only); Wiley's Cochrane Library (Issue 4, 2012); and Thomson's Biosis Previews (1995-present). OVID and PubMed search alerts were set up to send monthly updates with any new literature until November 1, 2012. Gray literature (literature that is not commercially published) was identified by searching the websites of health technology assessment and related agencies, the websites of relevant professional associations, and their associated databases. The Google search engine was used to search for additional Web-based materials and information. These searches were supplemented by reviewing the bibliographies and abstracts of key papers.
Inclusion Criteria
Following the initial search, articles were selected based on the following criteria: randomized controlled clinical trial or comparative observational study comparing systemic HBOT as the intervention to standard wound care (i.e., debridement, dressings, antibiotics, and minimization of pressure on the wound) or sham therapy; human participants (age ≥ 18 years old) suffering from Type 1 or Type 2 diabetes; patient group with nonhealing lower limb ulcers unresponsive to standard wound care (including debridement, glycemic control, antibiotic therapy, and revascularization if necessary); relevant outcomes: rate of wound/ulcer healing, wound size reduction, rate of major amputation (amputation of the lower limb proximal the ankle), rate of minor amputation (amputation of the distal end of foot), safety, and quality of life.
Exclusion Criteria
Studies were excluded for the following reasons: reviews (systematic or narrative), conference abstracts, case reports, comments, or editorials; animal studies; patients did not suffer from diabetes or lower limb ulceration; studies on topical HBOT or interventions other than systemic HBOT; no comparison group; irrelevant outcomes of interest; or non-English language publications.
Study Selection Method
Study selection was performed in two phases: title/abstract review and full-text review. Independent reviewers conducted the title and abstract review, using prespecified inclusion and exclusion criteria. Based on the titles and abstracts (when available), studies were excluded at this stage if both reviewers agreed that they did not meet the inclusion criteria. Studies that were not excluded based on title and abstract screening were retrieved in full text and assessed by two reviewers to determine if they should be included for data abstraction.
Data Abstraction Strategy
Data from all included studies were abstracted into predesigned data extraction forms. Information on the following data items was abstracted from the papers: study design; single/multicenter; country; patient characteristics; follow-up period; sample size; type of intervention (e.g., type, dose, durations, and frequency of treatment) versus control intervention; any adverse events attributable to treatment, study outcomes and study results. In cases where data were presented in graphical form, data were abstracted from the graphs where possible. Authors were contacted if further details were required to clarify reported results. The data abstraction was verified by a second reviewer.
Quality Assessment
The methodological quality of all included randomized controlled clinical trials was assessed using the Jadad scale (Reference Jadad, Moore and Carroll19). The Jadad scale is a three-item scale covering the randomization method, the blinding method, and withdrawals/dropouts. A study is assigned one point if it was described as randomized or as double blind or had described withdrawals/dropouts, respectively. If the randomization method or blinding method described was judged to be appropriate one additional point was awarded for that item. However, if the randomization method or blinding method described was judged to be inappropriate one point was deducted for that item. The final quality score for each article could range from 0 (lowest quality) to 5 points (highest quality).
For comparative observational studies, quality assessment was conducted using the Newcastle-Ottawa scale (Reference Wells, Shea and O'Connell20). The Newcastle Ottawa Quality Assessment Scale for Cohort Studies measures the quality of three categories: selection of cohorts; comparability of cohorts; and assessment of outcome for a maximum score of 9. The quality scores for the studies were used to summarize and describe the studies. However, no sensitivity or subgroup analyses were performed based on the quality of the included studies.
Data Analysis
Analysis was grouped by study type (e.g., RCT, observational) as well as by outcome (e.g., amputation, wound healing). Comparability of the studies was assessed by careful review of the population, intervention, comparator, and outcomes. Relative risk and 95 percent confidence interval were used to summarize effect sizes for dichotomous outcome measures (i.e., proportion of those requiring amputation, proportion of wounds unhealed) using the “per protocol” data. When possible, a pooled estimate of effect was assessed using meta-analytic techniques using Review Manager 5.0 to explore the clinical efficacy of HBOT. A fixed-effect model was used where there was no evidence of significant heterogeneity between studies and a random effects model was used when such heterogeneity was likely. Statistical heterogeneity was assumed to be significant if the I2 analysis suggested more than 50 percent of the variability in the analysis was due to differences between trials. I2 is a statistic ranging from 0 percent to 100 percent which reflects the proportion of total variation across studies due to heterogeneity (Reference Khan, Kunz, Kleijnen and Antes21). Ninety-five percent confidence intervals (CIs) were used for all effect size estimates for both individual trials and pooled estimates. As an estimate of clinical relevance of any difference between HBOT and control, the number-needed-to-treat (NNT) with 95 percent CI as appropriate was calculated.
Sensitivity Analysis
If appropriate, we planned to use sensitivity analyses using different approaches to imputing missing data.
RESULTS
Literature Search Results
A total of 654 potentially relevant citations were identified through the literature review. Of these citations, 157 articles were retrieved for full text screening and 145 of these were excluded. This resulted in twelve relevant studies for review and final data abstraction. Reasons for exclusion of articles are outlined in the PRISMA flowchart (Figure 1) of the process used to identify and select studies for the review. Overall, six randomized controlled trials (RCTs) (Reference Abidia, Laden and Kuhan9;Reference Doctor, Pandya and Supe22–Reference Londahl, Katzman, Nilsson and Hammarlund26) and six comparative observational studies (Reference Faglia, Favales and Aldeghi10;Reference Baroni, Porro and Faglia27–Reference Zamboni, Wong, Stephenson and Pfeifer31) were identified that provided data on the efficacy of using HBOT compared with standard wound care for the treatment of nonhealing ulcers of the lower limb in patients with diabetes.

Figure 1. PRISMA flowchart of reasons for exclusion of articles.
Study Characteristics
Randomized Controlled Trials
All six RCTs that met the inclusion criteria for this review were single-center trials, with sample sizes ranging from 16 to 100 patients and follow-up from 1 to 24 months. The studies are summarized in Table 1. The number of patients randomized to HBOT and control/sham therapy was reported in all trials except the trial by Doctor et al., which only reported the total number of study participants (Reference Doctor, Pandya and Supe22). The trial by Duzgun et al. was the largest with 100 patients and also had the longest follow-up of approximately 2 years (Reference Duzgun, Satir and Ozozan23).
Table 1. Study Designs and Findings (Randomized controlled trials and observational studies)

ATA – absolute atmosphere, min – minutes, NCO – Newcastle-Ottawa, NR - not reported, pts – patients, SD – standard deviation, TcPo2 – transcutaneous oxygen pressure, wk - week
In terms of the patient population, Kessler et al. (Reference Kessler, Bilbault and Ortega24) and Abidia et al. (Reference Abidia, Laden and Kuhan9) included patients with ulcers classified as Wagner Grades 1 or 2. On the other hand, Faglia et al. (Reference Faglia, Favales and Aldeghi25) and Duzgun et al. (Reference Duzgun, Satir and Ozozan23) recruited patients with more severe ulcers classified as Wagner Grade 2, 3, or 4. The study conducted by Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) recruited 94 patients with Wagner grade 2, 3, or 4 ulcers, which had been present for >3 months.
The trials by Abidia et al. (Reference Abidia, Laden and Kuhan9) and Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) included sham therapy in the control group while in the other studies the control group received standard wound care. While the definition of standard wound care was not provided in every study, Kessler et al. (Reference Kessler, Bilbault and Ortega24) described it as the stabilization of hyperglycemia through subcutaneous insulin administration, antibiotics for local infection, offloading measures to avoid mechanical stress on the foot, and debridement.
The HBOT regimen also varied across trials. In the trial by Doctor et al. (Reference Doctor, Pandya and Supe22), patients underwent only four sessions of HBOT over 2 weeks at 3 ATA with each session lasting 45 minutes while in the study by Abidia et al. (Reference Abidia, Laden and Kuhan9), patients underwent 30 sessions of HBOT over 6 weeks at 2.4 ATA with each session lasting 90 minutes. In the trial by Faglia et al. (Reference Faglia, Favales and Aldeghi25), treatment with HBOT was variable, starting at 2.5 ATA for 7 days a week in the first phase for 90 minutes per session to 2.2–2.4 ATA for 5 days a week in the second phase for 90 minutes per session. In the study by Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26), patients were subjected to 2.5 ATA of pressure with each session lasting 85 minutes, 5 days per week for 8 weeks. Wound healing and amputation rate were the most commonly reported outcomes in the studies (Table 1).
Comparative Observational Studies
Six comparative observational studies met the inclusion criteria for this review (Reference Faglia, Favales and Aldeghi10;Reference Baroni, Porro and Faglia27–Reference Zamboni, Wong, Stephenson and Pfeifer31). Follow-up times ranged from 2 months to approximately 3 years, and the largest sample size was 115 patients (Reference Faglia, Favales and Aldeghi10). A summary of the included studies’ characteristics is provided in Table 1. All studies were prospective cohort studies with the exception of two that were retrospective chart reviews (Reference Faglia, Favales and Aldeghi10;Reference Lyon29). In one study, the treatment regimens included variable oxygen dose in the HBOT chamber (Reference Faglia, Favales and Aldeghi10), while two studies did not report details regarding HBOT sessions (Reference Baroni, Porro and Faglia27;Reference Lyon29) and three studies maintained a consistent oxygen pressure (2 or 2.5 ATA) for each HBOT session (Reference Kalani, Jorneskog and Naderi28;Reference Oriani, Meazza and Fava30;Reference Zamboni, Wong, Stephenson and Pfeifer31). Also, there was substantial variability in the frequency of treatment sessions and the duration of treatment among the studies.
In terms of outcomes, the proportion of healed wounds was reported by four studies (Reference Baroni, Porro and Faglia27;Reference Kalani, Jorneskog and Naderi28;Reference Oriani, Meazza and Fava30;Reference Zamboni, Wong, Stephenson and Pfeifer31). Five studies reported amputation as an outcome (Reference Faglia, Favales and Aldeghi10;Reference Baroni, Porro and Faglia27;Reference Kalani, Jorneskog and Naderi28;Reference Oriani, Meazza and Fava30;Reference Zamboni, Wong, Stephenson and Pfeifer31), and one study reported ulcer size reduction, in terms of volume, as the primary outcome (Reference Lyon29).
Quality of Included Studies
Study quality scores based on the Jadad and Newcastle-Ottawa scales are presented in Tables 1 and 2. Of the six RCTs included in this review, study quality varied from low to high. One of the trials was assessed as being poor quality (score of 1 on the Jadad scale) due to the lack of blinding or use of sham therapy, no description of withdrawals or dropouts, and no description of the method of randomization used (Reference Doctor, Pandya and Supe22). Three other trials were assessed as being moderate quality (score of 3 on the Jadad scale) due to the lack of double-blinding or method of blinding (Reference Duzgun, Satir and Ozozan23–Reference Faglia, Favales and Aldeghi25). In the absence of blinding, decisions around debridement and wound care in trials may be affected by the knowledge of the treatment allocation and this may contribute to significant bias in these studies. Only the studies by Abidia et al. (Reference Abidia, Laden and Kuhan9) and Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) trials scored 5 on the Jadad scale due to appropriate randomization and appropriate randomization method being described, a description of dropouts, and appropriate patient blinding through the use of sham therapy in the control group.
Table 2. Summary of Effects of Outcomes using Different Models

Of the six comparative observational studies included in this review, three were assessed as being high quality (scores of 7 or 8 of 9 on the Newcastle-Ottawa scale) (Reference Kalani, Jorneskog and Naderi28–Reference Oriani, Meazza and Fava30). The three other studies were of moderate quality with scores of 5 or 6 (Reference Faglia, Favales and Aldeghi10;Reference Baroni, Porro and Faglia27;Reference Zamboni, Wong, Stephenson and Pfeifer31).
Treatment Effects
Proportion of patients requiring major amputation
Randomized controlled trials
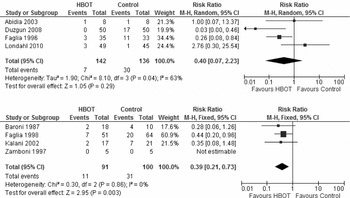
Five of the six RCTs reported the proportion of patients requiring major amputation for distinct follow–up time periods ranging from 12 months to 2 years (Reference Abidia, Laden and Kuhan9;Reference Doctor, Pandya and Supe22;Reference Duzgun, Satir and Ozozan23;Reference Faglia, Favales and Aldeghi25;Reference Londahl, Katzman, Nilsson and Hammarlund26). Because the trial by Doctor et al. (Reference Doctor, Pandya and Supe22) failed to report the number of patients randomized to intervention or control groups, this study was not included in the pooled analysis. However, it is worth noting that of the 30 patients included in the study, two patients in the HBOT group and seven in the control group required major amputations. As a result, four trials (Reference Abidia, Laden and Kuhan9;Reference Duzgun, Satir and Ozozan23;Reference Faglia, Favales and Aldeghi25;Reference Londahl, Katzman, Nilsson and Hammarlund26) contributed 278 patients to the analysis, with 142 randomized to HBOT and 136 randomized to the control group. Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) and Duzgun et al. (Reference Duzgun, Satir and Ozozan23) contributed approximately 70 percent of the patients to the analysis. Pooling the data revealed that there were seven (5 percent) major amputations in the HBOT group and 30 (22 percent) major amputations in the control group. There was significant heterogeneity among trials for the primary outcome of major amputation (I2 = 63 percent; p = .04). As such, a random effects model was used and the results showed that there was no significant difference in major amputation between the two groups (RR = 0.40; 95 percent CI, 0.07–2.23; p = .29) (Figure 2a and Table 2). This translates into a relative risk reduction of 77 percent with a NNT to avoid one amputation equals 5 (chance of benefit is 1 in 5 or 20 percent).

Figure 2. (a) Meta-analysis of randomized controlled trials indicates no significant difference in major amputations between the two groups. (b) Meta-analysis of observational studies indicates a significant difference in major amputation between the two groups.
Sensitivity analysis
This result was not sensitive to the inclusion of the trial by Doctor et al. (Reference Doctor, Pandya and Supe22) (we assumed an allocation of 15 patients to HBOT group and 15 patients to the control group).
Comparative observational studies
Major amputation was reported in four comparative observational trials (Reference Faglia, Favales and Aldeghi10;Reference Baroni, Porro and Faglia27;Reference Kalani, Jorneskog and Naderi28;31). Oriani et al. (Reference Oriani, Meazza and Fava30) reported amputation rates by group however, they did not distinguish between major and minor so it was decided to include them in the minor amputation estimate to be conservative. The four studies contributed 191 patients to this analysis, with 91 in the HBOT group and 100 in the control group. Because the percentage of variability in effect estimates due to heterogeneity was 0 percent, a fixed effect model was estimated. As shown in Figure 2b and Table 2, HBOT significantly reduced the rate of major amputation (RR of major amputation with HBOT was 0.39; 95 percent CI, 0.21–0.73; p = .003). The percentage of the variability in effect estimates due to heterogeneity was 0 percent based on the I2 statistic.
Proportion of patients requiring minor amputation
Randomized controlled trials
The rate of minor amputation was reported by five RCTs. Because the trial by Doctor et al. (Reference Doctor, Pandya and Supe22) did not report the number of patients randomized to the treatment and control arms, it was not included in the primary pooled analysis. Thus, the trials by Faglia et al. (Reference Faglia, Favales and Aldeghi25), Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26), Abidia et al. (Reference Abidia, Laden and Kuhan9), and Duzgan et al. (Reference Duzgun, Satir and Ozozan23) contributed 278 patients to this analysis, with 142 randomized to HBOT and 136 randomized to the control group. There were fewer minor amputations in the HBOT group (n = 30) compared with the control group (n = 40), heterogeneity between the studies was high and accounted for approximately 84 percent of the variability between trials (I2 = 84 percent; p = .0003). The random effects meta-analyses showed no significant difference in minor amputation between the two groups (RR = 0.79; 95 percent CI, 0.19–3.30; p = .75) (see Table 2 and Supplementary Figure 1, which can be viewed online at www.journals.cambridge.org/thc2013114).
Sensitivity analysis
The trial by Doctor et al. (Reference Doctor, Pandya and Supe22) found that there were four minor amputations in the HBOT treatment arm and two minor amputations in the control arm. Including these results in the meta-analysis, assuming 15 patients allocated to each treatment group, the results did not change (RR of amputation with HBOT was 0.78; 95 percent CI, 0.53–1.15).
Comparative observational studies
Three comparative observational studies reported the proportion of minor amputations, with 128 patients contributing to the analysis; 84 in the HBOT group and 44 in the control group (Reference Kalani, Jorneskog and Naderi28;Reference Oriani, Meazza and Fava30;Reference Zamboni, Wong, Stephenson and Pfeifer31). In the pooled analysis, the trial by Oriani et al. (Reference Oriani, Meazza and Fava30) accounted for 63 percent of the data. Similar to the situation observed for the major amputation, there was no heterogeneity in the data (i.e., I2 = 0 percent) and a fixed effects model was used. As shown in see Supplementary Figure 2, which can be viewed online at www.journals.cambridge.org/thc2013116, and Table 2, HBOT significantly reduced the rate of minor amputation (RR = 0.23; 95 percent CI, 0.09–0.59;p = .002).
Proportion of Wounds Healed at End of Study period
Randomized controlled trials
The proportion of healed wounds was reported by four RCTs (Reference Abidia, Laden and Kuhan9;Reference Duzgun, Satir and Ozozan23;Reference Kessler, Bilbault and Ortega24;Reference Londahl, Katzman, Nilsson and Hammarlund26). The four studies included in the pooled analysis contributed 233 patients to this analysis, with 120 in the HBOT and 113 in the control groups. The trial by Duzgun et al. (Reference Duzgun, Satir and Ozozan23) accounted for 43 percent of the sample in this analysis. The pooled estimate was calculated for the negative outcome (i.e., the number of unhealed wounds as opposed to the number of healed wounds at follow–up). There was a reduction in the proportion of unhealed wounds with HBOT application (32 percent) compared with usual care (60 percent). Given the high degree of heterogeneity (I2 = 87 percent), a random effects model was used and the results showed a non-significant reduction in the proportion of unhealed wounds with HBOT treatment (RR in unhealed wounds with HBOT was 0.54; 95 percent CI, 0.26–1.13; p = .10) (see Supplementary Figures 2 and 3, which can be viewed online at www.journals.cambridge.org/thc2013117). The heterogeneity may be attributed to the differences in baseline ulcer severity between the studies (Wagner Grade 1 and 2 included in the Abidia trial versus Wagner Grade 2, 3, 4 included in the study by Duzgun et al.), the use of sham therapy in the trials by Abidia et al. and Londahl et al. that was absent in the study by Duzgun et al., and the difference in follow up (1 year versus 2 years).
Comparative observational studies
The study by Lyon (Reference Lyon29) did not report the proportion of wounds healed but the percent change in wound volume (length × width × depth). The authors found that the wounds of patients receiving standard care were reduced by 15 percent and the wounds of patients receiving HBOT plus standard care were reduced by 30 percent. Four comparative observational studies reported the proportion of healed wounds as an outcome (Reference Baroni, Porro and Faglia27;Reference Kalani, Jorneskog and Naderi28;Reference Oriani, Meazza and Fava30;Reference Zamboni, Wong, Stephenson and Pfeifer31). The studies contributed 156 patients to this analysis, with 102 in the HBOT group and 54 in the control group. The study by Oriani et al. accounted for 51 percent of the sample in this analysis. Supplementary Figure 4, which can be viewed online at www.journals.cambridge.org/thc2013118 and Supplementary Table 2 show that there was a significant reduction in the proportion of ulcers unhealed with the application of HBOT (RR of an unhealed ulcer with HBOT was 0.24; 95 percent CI, 0.13–0.43; p < .00001). Due to the low degree of heterogeneity (i.e., I2 = 8 percent), a fixed effects model was used.
Wound size reduction
Two RCTs reported on wound size changes (Reference Abidia, Laden and Kuhan9;Reference Kessler, Bilbault and Ortega24). The results of the trial by Abidia et al. (Reference Abidia, Laden and Kuhan9) indicated a statistically significant difference in the reduction in wound size between the HBOT and control groups at 6 weeks (48 percent, p = .027); however, no difference was found between the two groups at 6 months. Similarly, Kessler et al. (Reference Kessler, Bilbault and Ortega24) observed a statistically greater reduction in wound size for HBOT, at 2 weeks after treatment (20 percent; p = .037), with no statistically significant difference between the groups in measurements that took place after 4 weeks.
Safety
There was limiting reporting of adverse events in the available studies. Overall, three of the included RCTs and one observational study reported at least one adverse event that was associated with HBOT. Three studies (two RCTs and one observational) collected data on death as a study outcome (Reference Faglia, Favales and Aldeghi25;Reference Londahl, Katzman, Nilsson and Hammarlund26;Reference Kalani, Jorneskog and Naderi28). In the study by Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26), the rate of mortality was 2 percent (1/49) in the HBOT group, as compared with 7 percent in the control group (3/45). The trial by Faglia et al. (25) reported one death in the control group (3 percent) but no mortality in the HBOT-treated patients. In the observational study, 12 percent (2/17) and 14 percent (3/21) of patient died in the HBOT and control groups, respectively (Reference Kalani, Jorneskog and Naderi28). HBOT was associated with barotraumatic otitis in two studies (Reference Kessler, Bilbault and Ortega24;Reference Londahl, Katzman, Nilsson and Hammarlund26), one case in each trial. The study by Kalani et al. (Reference Kalani, Jorneskog and Naderi28) reported that one patient in the HBOT group complained of pain in their ears. Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) also reported one case of treatment-related dizziness and one of worsening of cataract in the HBOT group. The observational study also noted that one patient developed a cataract that was assumed to be caused by HBOT (Reference Kalani, Jorneskog and Naderi28).
Hypoglycemia, as a complication of HBOT, was described in two studies (Reference Faglia, Favales and Aldeghi25;Reference Londahl, Katzman, Nilsson and Hammarlund26). Faglia et al. (Reference Faglia, Favales and Aldeghi25) described HBOT-induced hypoglycemia in 5 percent of the patients, with no treatment-related hypoglycemic events in the control group. Whereas Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) found this rate to be lower in HBOT group (4 percent) than in the control group (8 percent).
None of the studies reported oxygen toxicity, or any lung or sinus damage.
Quality of life
Quality of life outcomes were measured in one RCT (Reference Abidia, Laden and Kuhan9) using the SF-36 Short Form survey and Hospital Anxiety and Depression (HAD) Scale. In this study, statistically significant differences were found in favor of HBOT in terms of general health (p = .012) and vitality (p = .018) domains of SF-36. HBOT-treated patients showed a significant improvement in depression scores (p = .011), but not anxiety score in HAD scale. Whereas, both depression and anxiety scores improved significantly in the control group (p = .023, and p = .042, respectively). None of the observational studies measured any quality of life outcomes.
DISCUSSION
This systematic review and meta-analysis evaluates and synthesizes the most recent published evidence pertaining to HBOT for treating diabetic lower limb ulcers; however, it differs from previous reviews, because this review was not limited to RCTs but considered data from both clinical trial evidence and observational studies. The article also goes beyond the clinical outcomes of HBOT in treatment of diabetic foot ulcers and describes reported quality of life and safety data from the included studies.
Results from a small number of randomized trials suggest that adjunctive HBOT results in a nonsignificant reduction in the proportion of patients with chronic diabetic foot ulcers requiring amputation and improvements in wound healing. The results of the nonrandomized studies showed statistically significant reductions in major and minor amputations as well as proportion of wounds healed.
A recently published systematic review by the Cochrane Collaboration (Reference Kranke, Bennett and Martyn-St James15), which was limited to RCTs, analyzed data from the same six RCTs included in our review plus one additional study (Reference Lin, Chen and Niu32), which has been excluded from our review because it was published in abstract form. This review reported a pooled relative risk of 5.2 (95 percent CI, 1.25–21.66; p = .02) for wound healing in short-term (6 weeks), but found no statistically significant difference in the rates of wound healing, major or minor amputation favoring HBOT. These findings are consistent with the results of our meta-analyses of RCT data. However, using data from observational studies our review found a statistically significant reduction in the risks of unhealed wound, and major and minor amputations for HBOT.
Other systematic reviews that looked at the randomized and nonrandomized evidence have also concluded that HBOT is associated with improved wound healing and a reduction in the rate of amputation (Reference Wang, Schwaitzberg and Berliner17;Reference Londahl, Fagher and Katzman18). However, Wang et al. (Reference Wang, Schwaitzberg and Berliner17) did not pool the data from the individual trials and Londahl et al. (Reference Londahl, Fagher and Katzman18) combined the event rates from each study by study design but did not perform any statistical tests. In 2005, Roeckl-Wiedmann et al. (Reference Roeckl-Wiedmann, Bennett and Kranke13) reviewed the RCT data and were unable to confirm any significant benefit on ulcer healing or need for minor amputation with HBOT treatment. The systematic review presented statistically significant evidence that HBOT decreased the risk of major amputation (based on fixed effects model). Another review by Goldman (Reference Goldman16), that combined data from three RCTs, one prospective and three retrospective cohort studies, reported a pooled odds ratio of 0.24 (95 percent CI, 0.13–0.42) for major amputations favoring HBOT. In addition, the authors meta-analyzed data from three RCTs and three observational studies and calculated an odds ratio of 11.64 (95 percent CI, 3.5–39.2) favoring HBOT for wound healing.
There are several limitations with the current review. First, there were a small number of studies meeting the inclusion criteria (six RCTs and six comparative observational studies). Only two RCTs were deemed to be of high quality (Reference Abidia, Laden and Kuhan9;Reference Londahl, Katzman, Nilsson and Hammarlund26). One of these studies had a very small sample size of 16 patients, while the study by Londahl et al. (Reference Londahl, Katzman, Nilsson and Hammarlund26) randomized 94 subjects. It should be noted that the one large RCT of high quality (Reference Londahl, Katzman, Nilsson and Hammarlund26) tended to favor the control group with respect to major amputation but favored HBOT for wound healing compared with the other studies. This may be a sign that many of the studies overestimate the clinical effect of HBOT due to methodological problems. In addition to the small number of trials available, there was heterogeneity between trials in terms of patient inclusion criteria (ulcer severity), the intervention (oxygen dose, length, and frequency of HBOT sessions), and the nature and timing of outcomes. However, we could not carry out subgroup analyses, to examine the effect of ulcer severity or HBOT protocol on the study outcomes, due to the lack of sufficient number of studies in each subgroup. While most trials used standard wound care for the control group, the definition of “standard” was poorly described in papers, if at all. As such, the results of the meta-analyses should be interpreted with caution.
Given that the RCT evidence was limited, it was decided to include the observational data as well. The authors acknowledge that observational studies, in general, may overestimate the treatment effect because patients who would likely benefit from the intervention are likely to be selected. Thus, the synthesis of observational study data using meta-analytic techniques presents particular challenges because of inherent biases; however, it was believed that observational data has merit in that it provides evidence of the effectiveness of HBOT in everyday practice as opposed to the special setting of a controlled trial.
CONCLUSIONS
To the best of our knowledge, this systematic review includes the most recent published RCTs and comparative observational studies published in this area. Based on our findings, there is limited evidence in the form of rigorous RCTs and observational studies that suggest that HBOT application reduces the rate of major and minor amputations, and improves the rate of wound healing in nonhealing diabetic ulcers of the lower limb. The results of the largest high quality RCT found no significant effects on amputation and some benefit for wound healing (albeit nonsignificant). There was statistically significant reduction in amputation and improvement in wound healing when the data from the observational comparative studies are combined but not significant when the RCT data are combined.
Following the appraisal of study quality, it can be concluded that there are methodological flaws in many studies related to patient blinding through the use of an appropriate sham therapy, and blinding of outcome assessors, surgeons, and data analysts. In some cases, the reporting of study design elements such as method of randomization, allocation concealment, blinding, description of any dropouts and withdrawals, and assessment of adverse events is missing or inadequate. Thus, it appears that the widespread use of HBOT as a treatment for diabetic foot ulcers over the past decades has been founded on weak scientific ground (i.e., few randomized trials with methodological flaws). However, the consistency in positive outcomes in these trials with respect to amputation reduction and ulcer healing is important (Reference Londahl, Fagher and Katzman18). Rigorously conducted clinical trials are needed to ascertain the efficacy of HBOT for nonhealing ulcers of the lower limb in patients with diabetes. Special consideration should be given in these trials to maintain patient blinding, and explore issues such as the appropriate dose of oxygen and frequency of treatments, and response to treatment based on varying ulcer severity. Quality of life outcomes and the cost-effectiveness of HBOT as an adjunct compared with standard wound care also warrant consideration in future studies.
SUPPLEMENTARY MATERIAL
Supplementary Table 1: www.journals.cambridge.org/thc2013113
Supplementary Figure 1: www.journals.cambridge.org/thc2013114
Supplementary Figure 2: www.journals.cambridge.org/thc2013116
Supplementary Figure 3: www.journals.cambridge.org/thc2013117
Supplementary Figure 4: www.journals.cambridge.org/thc2013118
CONTACT INFORMATION
Daria O'Reilly, PhD, MSc Assistant Professor, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Programs for Assessment of Technology in Health (PATH) Research Institute, Centre for Evaluation of Medicines, St. Joseph's Healthcare Hamilton
Kaitryn Campbell, BA(H), BEd, MLIS Research Coordinator, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph's Healthcare Hamilton
Anjori Pasricha, Msc, BHSc, MD candidate Faculty of Medicine, University of Ottawa Natasha Burke, MSc; Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph's Healthcare Hamilton
Nazila Assasi, MD, PhD Research Associate, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph's Healthcare Hamilton
James M. Bowen, BScPhM, MSc Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Program Manager, Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph's Healthcare Hamilton
Jean-Eric Tarride, PhD Associate Professor, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Programs for Assessment of Technology in Health (PATH) Research Institute, Centre for Evaluation of Medicines, St. Joseph's Healthcare Hamilton
Ron Goeree, MA Professor, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Sciences, McMaster University; Programs for Assessment of Technology in Health (PATH) Research Institute, Centre for Evaluation of Medicines, St. Joseph's Healthcare Hamilton
CONFLICTS OF INTEREST
Daria O'Reilly, Natasha Burke, and James Bowen have received a grant to their institution from Ontario Ministry of Health and Long-Term Care. Ron Goeree has received a program of research grant and has similar grants pending to his institution from Health Quality Ontario. The other authors report they have no potential conflicts of interest.