A change in the procedure used in the French National program to screen neonates for cystic fibrosis (CF) was implemented in 2017. The decision for changing the screening process has been explicitly motivated by the results of a health technology assessment reporting joint cost-effectiveness and ethics assessment (1;2). This observation creates the opportunity to make explicit the role played by ethical issues in the decision. Indeed taking into account ethical aspects is increasingly requested (Reference Norheim, Baltussen and Johri3–Reference Saarni, Hofmann and Lampe5) and numerous methods have been developed to integrate and improve ethics assessment of health technology (Reference Hofmann, Lysdahl and Droste6–Reference Scott, Bond, Gutiérrez-Ibarluzea, Hofmann and Sandman8). However, applications remain relatively rare and the role actually played by ethical analyses in the decision-making process is poorly documented.
CF is a serious illness caused by genetic mutations and impairing the CFTR-protein function. It begins mostly in childhood and is expressed by dysfunction of the respiratory, digestive, and reproductive systems. Life expectancy, currently above 50 years for newborns in France, is dramatically improved by care dedicated to improving symptoms (9). Several countries have implemented systematic neonatal screening programs for CF since the early 2000s to identify CF-affected newborns as early as possible and avoid serial misdiagnosis that could delay appropriate care. The screening strategy generally includes an immunoreactive trypsinogen (IRT)-DNA procedure in which newborns are tested for IRT in the first days of life and CF-gene mutations are searched for high IRT measurements. In the case of mutations, a CF dedicated center will perform a sweat test to confirm or reject the diagnosis and will take care of the affected children (Reference Grosse10;11).
The screening program shows abilities to identify affected newborns and costs that are socially well accepted. It produces, however, outcomes that raise ethical questions as they are not within the objectives of the screening program and there is no evidence of their positive benefit-risk balance. They relate to (i) heterozygous status revealed by the newborn screening, and (ii) screening of newborns with a borderline form of CF. Heterozygous newborns will never develop CF and could suffer from stigmatization. Nevertheless in rare cases, if parents plan for another birth or when newborns become parents themselves, the information is worth being disclosed as they may give birth to a CF-affected child if they inherit CF gene mutations from both parents. Newborns with a borderline form of CF have uncertain CF diagnosis, based on mutation identification and additional clinical tests with mitigated results. The relevance of identifying these newborns is debated in the literature and among clinicians, as the uncertain benefit of early intensive care of moderate symptoms may be impaired by the risk of infections and psychological burden associated with frequent visits to specialised care centers.
On that basis, we first supposed that a strategy avoiding the unsought outcomes may be preferred provided that cost-effectiveness is not impaired and no new ethical issue is raised. As these outcomes arise from the use of genetic tests, the possibility of a non-DNA based strategy is an opportunity worth evaluating (11). Since 2005, the dosage of pancreatitis associated protein (PAP) has been studied as a candidate to screen newborns for CF and its role in the screening program, in place of or in addition to the DNA test, has been debated (Reference Sarles, Berthézène and Le Louarn12–14). The choice of this strategy may increase, however, the number of newborns called to perform a sweat test that proves negative (false positive cases) and thus the anxiety for numerous families.
As societal opinions associated with the unsought outcomes of screening strategies (namely identification of heterozygous newborns, of newborns with borderline forms of CF, and false positive cases) are divergent and cannot be balanced straightforwardly, a further evaluation is needed.
Cost-effectiveness evaluation does not handle the interpretation of outcomes toward which societal opinions are not known. For this reason, the French National Authority for Health (Haute Autorité de santé, HAS) developed a cost-effectiveness model in which outcomes were analyzed through an ethical assessment. This assessment was based on a positive description of ethical issues associated with every outcome. The Committee for Economic and Public Health Evaluation (Commission Evaluation Economique et Santé Publique, CEESP) and HAS made a judgement to elaborate its recommendation and support the decision making. The decision maker remains free to implement this recommendation or to take another decision.
This joint analysis intended to (i) increase the cost-effectiveness of the screening program, (ii) describe the ethical issues arising within the context of changing the program of CF screening that are reported in the published literature. In addition, the decision associated with the report of the ethical issues favoring each strategy was observed ex-post. It incidentally unveils what mattered to the decision maker to make them prefer a strategy over another one.
MATERIAL AND METHOD
This section presents the method of the evaluation of cost-effectiveness and ethical dimensions that determined the guidelines issued by HAS. In a first step, the cost-effectiveness analysis is used to eliminate those strategies considered socially unacceptable, that is, simultaneously less efficient and more costly. The priority is given to the cost-effectiveness criterion as this Value explicitly falls into the HAS mandate. In a second step, the ethical analysis is performed to arbitrate the nondominated strategies.
Outcomes
Primary outcomes were total cost and the number of CF cases detected (true positives). Newborns with meconium ileus are identified before knowing the results of the screening and were excluded from the analysis.
Secondary outcomes related to the ethical questions raised by the current or alternative screening programs, that is, the number of heterozygous newborns identified, the number of newborns identified with a borderline form of CF, and the number of newborns called to attend a sweat test without having CF (false-positives).
Cost-Effectiveness Analysis
A decision-analysis model was built (Reference Drummond, Sculpher, Torrance, O'Brien and Stoddart15–Reference Gold18), comparing: (i) the current genetic test-based strategy: IRT-DNA, with failsafe procedure (second IRT dosage) at day 21; (ii) the no genetic test-based strategy: IRT-PAP. Also, two hybrid strategies associating PAP and DNA, namely IRT-PAP-DNA, with and without failsafe procedure at day 21, were added (see Supplementary Figure 1). Performance parameters and sources are detailed in Supplementary Table 1. Unit costs and sources are presented in Supplementary Table 2. References related to cost-effectiveness analysis are listed in Supplementary Table 3.
Table 1. Results of the First Step Cost-Effectiveness Analysis

Note. The table reports the results of the reference analysis and the interval containing 95% of the simulations of the probabilistic analysis, from the 2.5th to the 97.5th percentile. The total number of actual classic CF is 107.
CF, cystic fibrosis; IRT, immunoreactive trypsinogen; PAP, pancreatitis associated protein
Table 2. Results of the Analysis of Uncertainty
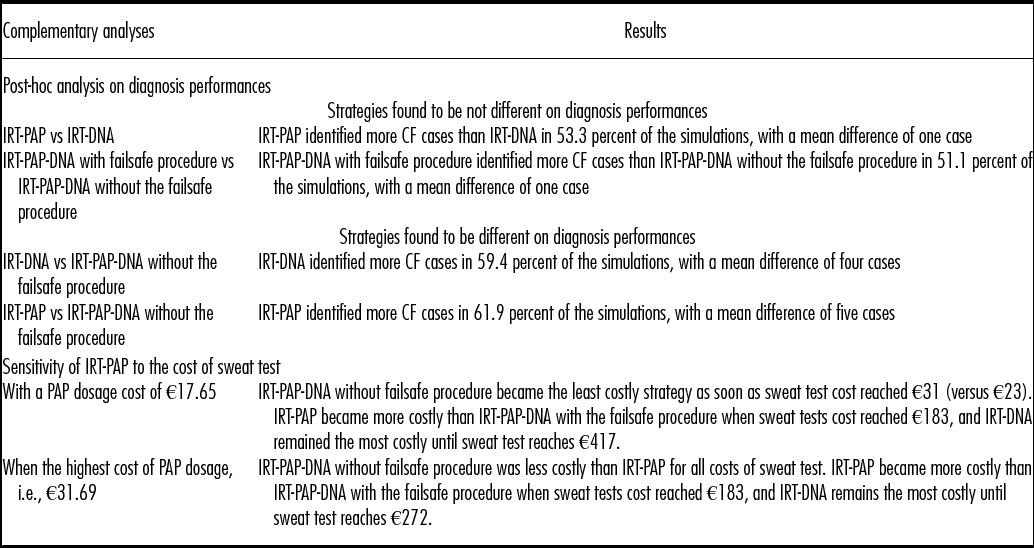
CF, cystic fibrosis; IRT, immunoreactive trypsinogen; PAP, pancreatitis associated protein
Table 3. Results of the second step ethical analysis

Note. The table reports the results of the reference analysis and the interval containing 95% of the simulations of the probabilistic analysis, from the 2.5th to the 97.5th percentile.
a To arbitrate the two dominant strategies, outcomes that differed between them, namely the amount of heterozygous newborns and the number of false positive cases are discussed through the ethical analysis. Secondary outcomes of strategies that are dominated on a cost-effectiveness basis are only presented (in gray) for transparency purpose.
b False-positive, heterozygous identified through screening included.
IRT, immunoreactive trypsinogen; PAP, pancreatitis associated protein.
The decision-analysis model simulating each strategy from the first screening test (IRT on day 3) to the diagnosis test (sweat test) was run for a cohort of 800,000 newborns, that is, the number of annual births in France rounded to the nearest 100,000.
A probabilistic sensitivity analysis was performed, taking into account the uncertainty about the diagnosis performance parameters. A post hoc analysis was performed to estimate the equivalence margins of the strategies found to be similar regarding diagnosis performances, namely IRT-DNA and IRT-PAP-DNA without the failsafe procedure. The cost of the PAP dosage was varied in a one-way sensitivity analysis and a threshold analysis was performed to identify what levels of sweat test cost would modify the hierarchy of the strategies on cost outcome.
Ethical Analysis
Newborns’ blood is collected on the third day of life to be screened for five diseases including CF. Before blood collection, parents are informed of the diseases searched for and asked to give consent for performing a DNA test for CF on the same blood sample in case of elevated IRT dosage. Then most parents will never hear about the screening anymore. If at least one CF-related mutation is identified, parents will be asked to come to a CF-dedicated center to perform a sweat test that will confirm or reject the diagnosis. The heterozygous newborns and newborns with a borderline form of CF will also be identified at that time.
The ethical assessment aims at evaluating whether the change to a strategy without DNA testing has an impact on ethical issues associated with the neonatal screening for CF. The ethical analysis considered several perspectives: newborn, parents, extended family and society. It compared two strategies, namely IRT-PAP and IRT-DNA as the use of DNA tests was the main difference between the compared strategies; if the position of the DNA test in the strategy can change the level of the outcomes, it does not change the nature of the ethical issues. The newborns’ experience of screening does not differ between strategies as they all begin with one blood sampling used for IRT and DNA tests and a sweat test if returns a positive result.
The ethical assessment complies with the HAS guidelines (19). This descriptive analysis rests on an extensive literature review conducted from January 2004 to May 2014 to identify ethical issues described that are then classified according to their explicit or implicit reference to one or more of the Beauchamp and Childress principles, that is, beneficence, nonmaleficence, respect for autonomy, and justice (Reference Beauchamp and Childress20). These principles are used as a framework for classifying the opinions and make them more understandable without being formally used as principles. The approach can be qualified as pragmatic, to enhance the awareness of the ethical issues while taking a decision. The issues related to the screening as it is performed currently, that is, including a DNA test.
They were re-interpreted in reference to a different situation (namely without DNA testing) in the ethical analysis, that is, a reasoning by analogy was performed. The literature search is detailed in Supplementary Table 4. The analysis identifies the ethical issues. All ethical issues reveal the ethical requirements to be considered when implementing the decision, but only some of these issues can be qualified as reasonable disagreements, that is, a competition between different principles that preclude concluding for one strategy without deciding between them.
Despite the reference to principles to classify different observed issues, this analysis may be qualified as a “descriptive systematic review” according to the Scott taxonomy as divergent positions are not weighted in the ethical analysis to favor a strategy (Reference Scott, Bond, Gutiérrez-Ibarluzea, Hofmann and Sandman8). According to the HAS guidelines, the ethical assessment is not expected to arbitrate between different principles. However, the combined presentation of cost-effectiveness outcomes and reasonable disagreements contributes to informing the decision making; bringing them to the attention of the decision maker allows them to weigh the various decision determinants.
A draft of the analysis was submitted to five ethicists, to health economists, health professionals and to the screening stakeholders (health professionals, patients’ associations, and screening organizers) to criticize and complete the description of ethical issues.
RESULTS
IRT-PAP-DNA without failsafe procedure and IRT-PAP dominate IRT-DNA and IRT-PAP-DNA with a failsafe procedure on primary outcomes, that is, cost and number of detected CF cases at first sight. Detailed results of the cost-effectiveness analysis are reported in Table 1.
Uncertainty remains on costs if choosing IRT-PAP and on efficacy, if choosing IRT-PAP-DNA without a failsafe procedure. Indeed, the post hoc analysis showed that IRT-PAP-DNA without failsafe procedure was never inferior to IRT-DNA provided a loss of opportunity to detect at most nine CF cases per year. This means that one case of CF may be missed for every 917 IRT positive tests, knowing that around 8,500 newborns are tested positive for IRT every year in France. Results related to complementary analyses performed to handle the uncertainty are presented in Table 2.
The ethical analysis has been used to evaluate the merits of the secondary outcomes associated with the nondominated strategies. The literature search identified 504 articles, of which 71 were analyzed and 40 eventually referenced. The literature flow-chart is detailed in Supplementary Figure 2 with the references listed in Supplementary Table 5. Ethical issues described usually referred to at least two principles and adopted different perspectives; they are detailed in the Supplementary Table 6. The ethical analysis was used to further document the strategies that were not dominated according to the cost-effectiveness evaluation; thus, it focused on outcomes that differed between the two strategies to be arbitrated, that is, identification of heterozygous newborns and false-positive cases. The issues related to the identification of borderline forms of the disease (forms which do not differ between the two strategies) were not considered in the choice of the strategy. The detailed results of the outcomes considered in the ethical analysis are presented in Table 3.
It is worth noting that parents are not informed of the risk of ancillary results when they consent to the DNA test. In addition, it takes place while a large amount of information is supplied about the child's birth in a highly emotional period. The identification of heterozygous newborns raises a reasonable disagreement about the disclosure of information made available, but not sought for. Positions for providing this information relate to the choice of screening outcomes. They are not legitimate in this context because the neonatal screening will give access to that information only to a very small proportion of actual heterozygous newborns. Thus, switching to a procedure without DNA testing would prevent the identification of heterozygous and avoid the reasonable disagreement.
However, it would bring four times more sweat tests to unaffected newborns and thus reinforce a pre-existing ethical issue related to the anxiety of families while waiting for the tests results in a sensitive period for building the parent-child relationship. The identification of newborns with a borderline form of the disease creates a conflict between the principles of beneficence and nonmaleficence that raises a reasonable disagreement. However, no strategy would modify it as the evaluated strategies did not differ on this last outcome.
In conclusion, once dominated strategies have been excluded, choosing between DNA and non DNA-based strategies requests arbitrating between: (i) raising a reasonable disagreement related to the disclosure of unsought knowledge of the heterozygous status for some newborns and a risk of loss of opportunity that could delay the diagnosis for some other newborns (DNA-based strategy) and (ii) increasing an ethical issue related to the number of false-positive cases that creates anxiety for parents waiting for the definitive diagnosis of newborns (no DNA strategy).
The results of the positive analysis of cost-effectiveness and ethical evaluation were presented to the CEESP and HAS to make recommendations to support decision making.
Taking into account the uncertainty on primary outcomes results and the ethical analysis, HAS recommended that the decision maker gives up the DNA-based strategy for the strategy without DNA test (2). Aside from the choice of the strategy, HAS issued guidelines to accompany any decision to be taken: (i) If the strategy without DNA was to be chosen, to reduce the anxiety that may appear, the parents’ information should be reinforced with the fact that performing a sweat test does not mean, in most cases, having an affected child; and (ii) If the strategy with DNA test was to be chosen, parents should be better informed when informed consent is required about the possibility to identify heterozygosis through the screening.
DISCUSSION
HAS explicitly recommended the IRT-PAP strategy. The decision maker implemented the IRT-PAP-DNA, justifying their choice by the professionals and patients’ attachment to the DNA test (2). As the final decision diverges from the guidelines, it is interpreted ex-post by comparing the motives of the decision with the opinions identified through the ethical analysis. This choice reveals the relative attention given to cost-effectiveness and the identification of heterozygous newborns and false positive cases. The negative consequences of increasing false positive cases, that is, the increased number of families experiencing anxiety in the beginning of the parent-child relationship may have been judged superior to the consequences associated with the identification of heterozygous newborns and the risk of loss of opportunity to detect a limited number of CF cases.
This ex-post reading of the decision must be interpreted considering the scope of our evaluation. Other determinants of the decision that were not taken into account may have played a role in the decision like professional interest or opinions that were not expressed in the literature, and could explain the divergent positions between HAS and the decision maker, in charge of the screening program. Additionally, other dimensions of the evaluation were not in the scope of the study but could have been documented, such as the organisational consequences of switching strategy: if a reduction in the number of DNA tests performed may have been expected while implementing the DNA-PAP strategy, its impact on each laboratory would depend on its case-mix. The expected increase in the amount of PAP tests performed appeared likely manageable (Reference Sarles, Giorgi and Berthézène13). An increase in the number of sweat tests in the specialised care centers would also have been expected with an estimate of approximately 36 annual supplementary tests in each center in France (2).
Our evaluation has some limits worth acknowledging. Regarding cost-effectiveness analysis, the performances of the IRT-PAP-DNA strategies were based on a post hoc analysis as these strategies were not evaluated in the first protocol of the study performed by Sarles et al. (Reference Sarles, Giorgi and Berthézène13). The uncertainty associated with this bias is limited by a large number of newborns included in the study (two-thirds of the French annual cohort), which provides a satisfying statistical power to the analysis. Our study was performed on French data, that is, a CF incidence of 1/5,809 births in 2012, and with French costs, which may threaten the generalization of our results.
Nevertheless, our results are consistent with published studies where IRT-PAP strategy was the reference (least costly) and IRT-DNA strategy was on the efficiency frontier (more expensive and more efficient than IRT-PAP strategy). Results on IRT-PAP-DNA were disparate (Reference Nshimyumukiza, Bois and Daigneault21–Reference Seror, Cao, Roussey and Giorgi23). Also, the analysis does not consider a DNA-based strategy in which only researched results of the screening are communicated to the parents. This strategy would probably raise other ethical questions requesting further analyses. Indeed, from an ethical point of view, not disclosing information obtained even though it was not sought is not similar to a situation in which this information is not known.
The evaluation compares different screening strategies but does not question the merits of routine screening itself. Indeed the screening program and specialized care centers were settled at the same time in France; no data are available to document the specific effect of the screening on the one hand and the effect of the specialized care on the other hand in the French context, inhibiting any robust evaluation taking into account the length of time spent in different health states. Also, survival data were lacking and could not be produced. This situation strongly affects the capacity to integrate the no screening strategy in a soundly documented economic model (Reference Grosse10). Only one study was found in the literature evaluating newborns screening of CF by providing a cost per life-year gained analysis (Reference van der Ploeg, van den Akker-van Marle and Vernooij-van Langen22). The estimation of saved years was based on an assumed reduction of 25 percent of the mortality of newborns identified through the screening compared with those newborns not identified. It is worth mentioning that the rank of strategies on cost-effectiveness may not have been changed by performing a cost-effectiveness analysis based on a hypothesis of mortality reduction.
The ethical evaluation, based on literature, must be interpreted in the context of the disease and the method applied. The assessment was based on identifiable ethical issues before the decision had been made. The emergence of new ethical issues at the time of the decision or its implementation could not be excluded. The literature search could not identify any qualitative study on parents’ understanding of the screening process or results. The implementation of the new strategy will allow a better estimation of the uncertain parameters, which could modify the ethical issues at stake.
In addition, in the ethical analysis, we refer to the method recommended by HAS, that does not balance values and thus cannot conclude when two values are supporting opposite decisions. Alternatively, such ethical dilemmas could be resolved by specifying norms as described by Richardson (Reference Richardson24).
In conclusion, this multi-attribute evaluation is based on a rigorous method in which the quantification of cost, effectiveness, and other relevant outcomes of different health strategies are interpreted through the analysis of ethical aspects. It illustrates an actual application of a method to document ethical aspects along with the cost-effectiveness evaluation, showing how the complementary analysis brings the evaluation further. This approach may be especially relevant when the opinions associated with the different outcomes of health interventions are not known. The consideration of ethical aspects along the assessment of health technologies does not claim to cover all of the difficulties inherent in decision making. It can, however, contribute to facilitating the decision acceptance and implementation in identifying potential barriers. It also contributes to revealing decision-maker motives.
SUPPLEMENTARY MATERIAL
Supplementary Figure 1: https://doi.org/10.1017/S0266462318000181
Supplementary Table 1: https://doi.org/10.1017/S0266462318000181
Supplementary Table 2: https://doi.org/10.1017/S0266462318000181
Supplementary Table 3: https://doi.org/10.1017/S0266462318000181
Supplementary Table 4: https://doi.org/10.1017/S0266462318000181
Supplementary Figure 2: https://doi.org/10.1017/S0266462318000181
Supplementary Table 5: https://doi.org/10.1017/S0266462318000181
Supplementary Table 6: https://doi.org/10.1017/S0266462318000181
CONFLICTS OF INTEREST
The authors have nothing to disclose.





