Published online by Cambridge University Press: 02 March 2005
Objectives: The purpose of this health technology assessment (HTA), commissioned by the German Federal Ministry of Health and Social Security, was to systematically review the evidence for the effectiveness and cost-effectiveness of antiviral treatment (AVT) with interferon (INF) or peginterferon (PegIFN) in combination with ribavirin (RBV) in treatment-naïve patients with chronic hepatitis C (CHC) and to apply these data in the context of the German health-care system.
Methods: We performed a systematic literature search on effectiveness and cost-effectiveness of AVT and summarized results using meta-analysis and evidence tables. We applied the German Hepatitis C Model (GEHMO), a decision-analytic Markov model, to determine long-term clinical effectiveness, costs, and incremental cost-effectiveness ratios (ICER) of the examined treatment strategies. Model parameters were derived from German databases, published international randomized clinical trials (RCT), and a Cochrane Review.
Results: Overall, nine RCTs, two HTA reports, one Cochrane review, two meta-analyses, and seven economic evaluations met the inclusion criteria. These studies indicate that PegIFN+RBV achieved the highest sustained virological response rates (SVR) (54–61 percent), followed by IFN+RBV (38–54 percent) and IFN monotherapy (11–21 percent). Based on our meta-analysis, PegIFN+RBV reduced cases without SVR by 17 percent compared with INF+RBV. International cost-effectiveness studies indicate that INF+RBV is cost-effective when compared with INF monotherapy. For PegIFN+RBV, our decision analysis yielded an ICER of €9,800 per quality-adjusted life-year gained.
Conclusions: This HTA suggests that initial combination therapy prolongs life, improves quality of life, and is cost-effective in patients with CHC. Peginterferon plus ribavirin is the most effective and efficient treatment among the examined options. However, because not all chronic hepatitis C patients will develop progressive liver disease, a thorough assessment of the eligibility and appropriateness of treatment with combination therapy must be performed in each individual patient.
Chronic hepatitis C (CHC) is an emerging problem in public health. Hepatitis C virus (HCV) infects an estimated 170 million persons world-wide with 5 million in Western Europe (55). In Germany HCV prevalence has been estimated to be 0.5 percent and incidence to be 5,000 newly infected persons per year resulting in more than 400,000 prevalent cases of CHC (40;42).
The virus imposes significant personal and social burdens on infected individuals, as well as substantial costs to society. Progression to chronic disease occurs in the majority of HCV-infected persons (1). Approximately 20 percent of patients with CHC develop compensated liver cirrhosis within 20–30 years (4;13;14;17;23;27;34;52), which is associated with high mortality risk due to liver failure.
Antiviral combination therapy with interferon alpha and ribavirin has been considered the standard of care for treatment naïve patients with CHC infection and elevated alanine amino transferase (ALT) levels (4;35;38), but recent multinational randomized controlled clinical trials (18;32) showed that combination therapy with peginterferon alpha and ribavirin yielded higher sustained virological response rates (SVR).
However, antiviral combination therapy is relatively expensive, raising the question of whether its clinical benefit supports the costs. With rising medical costs and limited health-care budgets, attention is increasingly being focused not only on the clinical benefits of new drugs but also on their economic impact.
Therefore, the German Agency for Health Technology Assessment at the German Institute for Medical Documentation and Information (DIMDI)/German Federal Ministry of Health and Social Security commissioned this health technology assessment (HTA). Its objectives were (i) to systematically review the evidence on effectiveness and cost-effectiveness of initial antiviral combination therapy in patients with CHC, (ii) to develop a decision-analytic Markov model for treatment naïve patients with CHC for the context of the German health-care system, and (iii) to analyze the effectiveness and cost-effectiveness of initial antiviral therapy in patients with CHC and elevated ALTs in Germany.
All research questions of this study were based on a population of treatment naïve patients with CHC and elevated ALT levels. The following specific research questions were examined: (i) How does the effectiveness regarding SVR compare between the evaluated antiviral treatment strategies? (ii) How does the effectiveness compare between the evaluated antiviral treatment strategies regarding the following long-term outcomes: compensated liver cirrhosis, decompensated liver cirrhosis, hepatocellular carcinoma, liver transplantation, CHC-related mortality, total mortality, and quality-adjusted life expectancy? (iii) What is the incremental cost-effectiveness of each antiviral therapy in comparison to the next best strategy? (iv) Which antiviral therapy can be recommended as standard therapy for the German health-care context according to effectiveness and cost-effecti- veness?
Electronic databases, HTA-information networks, and bibliographic sources were systematically searched to identify randomized controlled trials (RCT), meta-analyses, or HTA reports that evaluated initial antiviral combination therapy in patients with CHC. The time horizon of the literature search was limited from 1990 to 2002. Study quality and transferability to the German context were assessed with instruments developed by the German Scientific Working Group Technology Assessment for Health Care (30;45;54). The information was summarized in evidence tables (47). Results are reported in country-specific currencies. To facilitate comparison across countries, all results were additionally converted to US dollars (US$) of the index year of each study. As currency conversion methods in the individual studies were poorly described, we used exchange rates expressed as national currency units per US$ instead of applying purchasing power parities (37).
A meta-analysis of randomized clinical trials on the medical effectiveness of antiviral combination therapy with peginterferon plus ribavirin was performed using random and fixed effects models. Based on the standards of the Cochrane Collaboration (11), the pooled relative risk (RR) for the outcome “No SVR” with its 95 percent confidence interval (CI) was reported. Results were presented as a forest plot.
During this HTA, we collaborated with several institutions and established the interdisciplinary HTA Expert Panel on Hepatitis C, which included members of the German Hepatitis C Model (GEHMO) Group and further experts from different areas who consulted information concerning actual unpublished data and methodological issues. Names, affiliations, and assigned areas of the expert panel members are listed in the Acknowledgments footnote.
A modified version of the German Hepatitis C Model (GEHMO) was used for the decision analysis (see Figure 1). This decision model was designed to include pooled effectiveness data from meta-analyses, as well as benefits and costs, for using different antiviral treatment strategies for patients with CHC. Pooled effectiveness data were derived from meta-analyses performed by the Hepato-Biliary Cochrane Group (24;25) and additional meta-analyses were performed by the authors. GEHMO is a decision-analytic Markov model based on a previously published and validated Markov model for the natural history of disease (8;57;58) and modified for the German health-care system and German hepatitis C–specific practice patterns.

Simplified schematic of the German Hepatitis C Model (GEHMO). Each circle represents a health state of a patient with chronic hepatitis C. Each arrow represents possible transitions between health states, which may occur each year. Dotted arrows represent lower transition probabilities, reflecting the viral negative state. The two boxes represent the target population defined by histological states and separated by viral-positive and viral-negative status. Histology-specific rates for no response, relapse after response, and sustained response after antiviral treatment are applied to the three viral-positive health states: mild hepatitis, moderate hepatitis, and compensated cirrhosis. In the model, separated states were considered for liver diseases from decompensated cirrhosis, as variceal hemorrhage, hepatic encephalopathy, and liver transplant, separated into the first year and subsequent years, and ascites, separated in diuretic-sensitive and diuretic-refractory ascites. Individuals in any health state may die from causes related to their age and gender as occurs in the general population of Germany, and individuals with decompensated cirrhosis, hepatocellular carcinoma, or liver transplantation may die from liver-related causes.
The model was used to determine long-term morbidity, life expectancy, quality-adjusted life expectancy, lifetime costs, and discounted incremental cost-utility ratios (ICUR) of the following strategies: (i) no antiviral therapy, (ii) interferon monotherapy (3×3 MU/week) for 48 weeks, (iii) combination therapy with interferon (3×3 MU/week) and ribavirin (1,000–1,200 mg/day) for 48 weeks, and (iv) combination therapy with peginterferon (180 μg/week for peginterferon alpha-2a; 1.5 μg/kg for peginterferon alpha-2b) plus ribavirin (800–1,200 mg/day) for 48 weeks. According to the European guidelines (4), interferon monotherapy was stopped after 12 weeks and combination therapies were stopped after 24 weeks if no virological response was observed at this time. Dosing was based on European recommendations for patients with CHC and European drug approved labeling (4).
Natural history data were estimated from several published studies and have been described elsewhere (8;57;58). Histological classification as mild or moderate chronic hepatitis or compensated cirrhosis was defined by the modified histology activity index of Knodell (12;26). For the German context, demographic and clinical parameters as well as utilities were based on original data from a quality-of-life survey in CHC patients (n=428) (46;48). Utility data included empirically estimated relative reductions in short-term quality-of-life due to positive HCV status (2 percent) and adverse events during antiviral treatment (5 percent for interferon plus ribavirin and 10 percent for peginterferon plus ribavirin).
Our model included pooled short-term outcomes (overall SVRs and respective relative risks) from recently published RCTs and meta-analyses. Relative virological response rates of interferon monotherapy and combination therapy with interferon alpha plus ribavirin were based on a recently published meta-analysis (24) and a Cochrane Review (25). We performed a meta-analysis to derive pooled virological response rates of combination therapy with peginterferon plus ribavirin published in two randomized clinical multicenter trials (18;32).
Direct annual costs were calculated based on frequencies of inpatient and outpatient visits, diagnostic and laboratory testing, medication, and procedures related to the specific health states (Table 1). Health resource utilization frequencies were derived from a German expert panel (n=10) and an economic survey in CHC patients (n=196). Costs were derived from health-care databases and currently applicable pharmaceutical prices (5) of interferon alpha, peginterferon alpha, and ribavirin in Germany (Table 2).

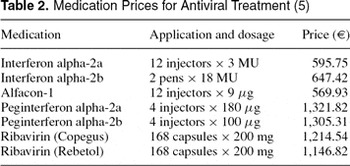
A 5 percent deduction from pharmaceutical prices for the proportion of persons insured by the social health insurance in Germany was performed. For modeling the costs of liver transplantation, a study (21) based on German patient data for the year 1993 was used. All costs were converted to year 2002 Euros (1 Euro=1.95583 German mark) by using the medical care component of the Consumer Price Index for Germany (50). An annual discount rate of 3 percent was applied to costs and effectiveness based on international recommendations (16;20) and varied in sensitivity analyses between 0 percent and 10 percent regarding German recommendations (3). For detailed model parameter tables, see the online HTA, the full text of which is available at www.dimdi.de (49).
The decision model was validated on three levels: (i) technical validation (i.e., “clean up” of the software program from potential programming bugs), (ii) internal validation (i.e., comparison of model predictions with epidemiological and clinical data used in the model), and (iii) external validation (i.e., comparison of model predictions with published epidemiological data not used in the model).
The technical validation using different routine tests (e.g., setting SVR equal for all strategies, eliminating antiviral treatment costs, eliminating CHC-related mortality, and so on) yielded the expected results. In the internal validation, all data values used were reproduced exactly by the decision model (e.g., SVR rates, progression incidences, background mortality).
In the external validation, the incidence of developing compensated liver cirrhosis in patients with mild CHC was adjusted for the spontaneous HCV remission rate of 31 percent in patients with acute HCV infection (2). The model predicted a 20-year incidence of developing compensated liver cirrhosis of 19 percent in patients with initial HCV infection. This result is consistent with published data from prospective studies (1;29).
As the spontaneous HCV remission rate and the incidence of liver cirrhosis have been extracted from different sources, using different values for spontaneous HCV remission could lead to a proportional deviation from the cirrhosis incidence used in the validation. However, different rates for spontaneous HCV remission would not influence results from this model, because the target cohort of our analysis are patients with CHC.
To assess the robustness of base-case results, univariate sensitivity analyses were performed for all model parameters based either on 95 percent confidence intervals or on ranges used in the literature. Costs were halved and doubled to obtain lower and upper limits. In addition to univariate sensitivity analyses, multivariate sensitivity analyses were performed on the entire set of disease progression rates. As it has been shown that the progression of hepatitis C observed in epidemiologic studies varies and strongly depends on the study design (17), we performed conservative sensitivity analyses with extremely low progression rates. Furthermore, we analyzed a worst-case scenario using extremely conservative estimates for benefits and costs for the combination therapy with peginterferon and ribavirin.
Decision analytic calculations were performed with DATA Pro for Health Care (TreeAge Software, Inc., Williamstown, MA). SAS 8.1 (SAS Institute, Inc., Cary, NC) was used for statistical analyses of primary data.
Twelve studies regarding the medical efficacy of combination therapy with interferon plus ribavirin compared with interferon monotherapy were included herein: two HTA reports (19;44), one systematic Cochrane review (25), two meta-analyses (24;36), and seven controlled randomized clinical trials (7;10;28;31;35;38;39). Two controlled randomized multicenter studies (18;32) regarding medical efficacy of combination therapy with peginterferon and ribavirin compared with interferon plus ribavirin were identified and included.
All included studies reported significantly higher SVR for combination therapy with interferon and ribavirin compared with interferon monotherapy (38–54 percent versus 11–21 percent). Meta-analyses reported SVRs of 32–41 percent for interferon in combination with ribavirin compared with 8–16 percent for interferon monotherapy.
For peginterferon combined with ribavirin, multicenter clinical trials reported SVRs of 54 percent vs. 47 percent (p=.01) (32) and 56 percent versus 44 percent (p<.001) (18) compared with standard combination therapy, respectively. In a subgroup data analysis, one multicenter study (32) showed that patients treated with a dose of 10.6 mg or more ribavirin per kg body weight had higher SVRs. Sixty-one percent of patients treated with peginterferon plus ribavirin compared with 48 percent of patients treated with interferon plus ribavirin achieved an SVR. The pooled relative risk for the outcome “no SVR” for the combination therapy with peginterferon plus ribavirin versus interferon plus ribavirin was 0.83 (95 percent CI, 0.76–0.91 for fixed effects model and 0.75–0.91 for random effects model).
Seven studies, including one HTA report (9;41;43;44; 51;58;60) assessing cost-effectiveness of antiviral combination therapy with interferon and ribavirin in patients with CHC, were identified in the search period of this HTA (January 1990–December 2002), but no publications were found examining cost-effectiveness of antiviral combination therapy with peginterferon and ribavirin.
Incremental cost-effectiveness ratios varied over a wide range, depending on discount rate, treatment duration, and population characteristics (Table 3). In all these studies, antiviral therapy with interferon plus ribavirin seemed to be reasonably cost-effective. For full evidence tables and results on study quality and transferability, see the online HTA, the full text of which is available at www.dimdi.de (49).

Based on our decision analysis, initial antiviral therapy in patients with CHC had the potential to prevent a substantial fraction of clinical events such as progression to cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplantation, and death due to liver failure. For each of these outcomes, the number needed to treat to prevent one clinical event during 20 years was approximately 8 for interferon monotherapy, 3 for interferon plus ribavirin, and 2 peginterferon plus ribavirin, when compared with no antiviral therapy. Compared with no antiviral therapy, interferon monotherapy saved 1.1 life years, interferon plus ribavirin saved 2.9 life years, and peginterferon plus ribavirin saved 4.6 life years. Compared with no antiviral therapy, interferon monotherapy saved 1.2 quality-adjusted life years (QALY), whereas combination therapy with interferon and ribavirin saved 3.0 QALYs, and peginterferon plus ribavirin saved 4.8 QALYs.
Table 4 shows undiscounted treatment costs and lifetime costs. After discounting for future benefits, interferon monotherapy gained 0.53 QALYs with additional costs of €2,800 resulting in an ICUR of €5,300 per QALY compared with no antiviral therapy (Table 5). Moving from interferon monotherapy to interferon plus ribavirin was associated with 0.78 QALYs gained, additional costs of €9,000, and an ICUR of 11,600 €/QALY. Compared with interferon monotherapy (as the next best nondominated strategy), peginterferon plus ribavirin gained 1.53 QALYs and increased costs by €14,900 yielding an ICUR of 9,800 €/QALY. Therefore, combination therapy with peginterferon plus ribavirin was the most effective treatment strategy and was more efficient than interferon plus ribavirin. To facilitate comparison with ICURs presented in Table 3, we converted the model-based ICURs for Germany to 2002 U.S. dollars using the currency exchange rate (US$1 equals €1.07) (37). When compared with interferon monotherapy, the ICURs of interferon plus ribavirin and peginterferon plus ribavirin were 10,800 US$/QALY and 9,200 US$/QALY, respectively.


In sensitivity analyses, results were robust when varying most relevant model parameters. Even when reducing SVR to 50 percent, combination therapy with peginterferon plus ribavirin was still the most effective strategy. Peginterferon plus ribavirin remained the most effective and cost-effective strategy when varying the proportion of patients with compensated cirrhosis from 0 percent to 52 percent, the proportion of male CHC patients from 20 percent to 100 percent, or when body weight was increased by 20 percent.
Several sensitivity analyses were performed on progression rates. In a conservative scenario, the 20-year incidence of compensated cirrhosis was set to 7 percent as reported in a meta-analysis of results from community-based studies (17). This scenario is conservative, because the study population of community-based studies included 38 percent of patients with normal ALT levels, which is associated with a reduced risk of developing liver cirrhosis compared with our target population of CHC-patients with elevated ALT levels (33). In this scenario, peginterferon plus ribavirin was the most effective therapy and, with an ICUR of 21,150 €/QALY, was still reasonably cost-effective. When we further removed the 2 percent quality-of-life reduction due to HCV infection in viral-positive CHC patients in this conservative scenario, the ICUR increased to 26,200 €/QALY.
In the worst-case scenario, which was performed to obtain extremely conservative estimates for benefits (e.g., SVR) and costs for the combination therapy with peginterferon and ribavirin, peginterferon plus ribavirin was the most effective treatment strategy and resulted in an ICUR of 27,300 €/QALY compared with the next best nondominated strategy (i.e., interferon monotherapy).
In this health technology assessment, a systematic evaluation of the medical efficacy and cost-effectiveness of antiviral combination therapy as an initial treatment for patients with CHC was performed. In addition, a modified version of the GEHMO was applied to predict the 20-year risks of CHC-related liver diseases, life expectancy, quality-adjusted life expectancy, lifetime costs, and incremental cost-utility ratio for different antiviral treatment strategies and for the German health-care context.
Several randomized trials (7;10;28;31;35;38;39) and meta-analyses (24;25;36) reported combination therapy with interferon plus ribavirin to be more efficient than interferon monotherapy (SVR: 32–54 percent versus 8–21 percent). Two randomized multicenter studies (18;32) reported a higher SVR for peginterferon plus ribavirin compared with combination therapy with interferon plus ribavirin (54–56 percent versus 44–47 percent). In terms of life expectancy and quality-adjusted life expectancy, combination therapy with interferon plus ribavirin was more effective and also reasonably cost-effective when compared with interferon monotherapy, based on international cost-effectiveness studies (9;41;43;44;51;58;60).
In our decision analysis for the German health-care system, initial antiviral therapy with interferon and ribavirin compared with interferon monotherapy had a discounted ICUR of 11,600 €/QALY. Compared with interferon monotherapy, peginterferon plus ribavirin cost €14,900 and gained 1.53 QALYs, resulting in an ICUR of 9,800 €/QALY. Therefore, peginterferon plus ribavirin was the most effective and cost-effective treatment strategy. Compared with other well-accepted medical interventions, for example, hemodialysis (53) or coronary artery bypass grafts (59) with ICURs of 50,000 to 60,000 €/QALY, combination therapy with peginterferon and ribavirin can be considered as cost-effective.
As is the case with all model-based cost-effectiveness analyses, ours has several limitations due to the availability of data on the natural history of CHC. The risk of progression to cirrhosis is especially controversially. In a recently published review of fifty-seven studies on the natural history of hepatitis C (17), the authors classified the identified studies into four categories of study design and used regression analysis to derive pooled progression estimates for each category. The estimated 20-year risk of cirrhosis was 24 percent for posttransfusion cohorts, 22 percent for liver clinic series, 7 percent for community-based cohorts, and 4 percent for blood donors. Adjusting for demographic and clinical characteristics explained only a small part of the heterogeneity. It has been argued that biases such as referral bias and selection bias may explain the high cirrhosis risks in liver series and posttransfusion cohorts as well as the low estimates in blood donors (14). The fraction of patients with elevated ALT levels varied between these different settings and was as low as 62 percent in the community-based studies (17).
The target population of our study was a patient cohort with elevated ALT values and a mix of different histological stages as observed in clinical trials and routine clinical practice in the absence of systematic screening. Posttransfusion studies were the only category that required the presence of clinical or biochemical hepatitis and, thus, may be the category that best represents the advanced disease stage of the population we studied. However, transfusion may be associated with underlying chronic disease, which itself may influence the progression of hepatitis (14). In community-based studies, most patients had normal ALT values, and some studies included patients with acute hepatitis C. Thus, these studies do not reflect the decision context and the population we studied. However, even after reducing the progression rates of our model to the extent that 20-year cirrhosis risk was only 7 percent (i.e., reflecting the community-based estimate), the ICUR for combination therapy with peginterferon plus ribavirin remained below 22,000 €/QALY. This finding indicated the robustness regarding the optimal choice among the evaluated strategies even under very conservative assumptions.
However, the results may be different for patients with normal ALT levels, with acute hepatitis C, or in populations in which a systematic screening for HCV was performed (leading to a detection in a very early stage of the disease). For an evaluation in these populations, the decision model and its data must be adapted to the specific context. In particular, this means that, even if future studies yield good SVRs in screened patients or patients with normal ALT values, long-term effectiveness or cost-effectiveness cannot be automatically inferred from these results without additional decision analyses.
Severe adverse events may occur more frequently in patients treated with peginterferon plus ribavirin than in patients treated with interferon plus ribavirin (18;32). As the absolute number of adverse events was small and no utility data were available for each type of adverse event, we were unable to develop a micromodel for severe adverse events. Instead, we empirically estimated the overall relative reduction in quality of life due to different antiviral treatment regimens from the German CHC quality-of-life survey.
Our economic analysis likely underestimates disease-related costs for several reasons and, therefore, likely underestimates treatment-related savings due to prevented future complications. First, we used variable costs and did not consider fixed costs nor costs due to productivity loss. Second, our model does not include the cost of future liver biopsies and further therapy for nonresponders. Third, we did not consider histology normalization in responders, nor reduced incidence of hepatocellular carcinoma in nonresponders.
Country to country differences in sociodemographic structure, distribution of patient's clinical characteristics, utility profiles, resource utilization, and prices make it difficult, if not impossible, to transfer the results of our qualitative review of economic evaluations to other health-care systems and countries (6;15;22;54;56). However, the cost-effectiveness patterns for interferon and ribavirin in other industrialized countries were similar to the results derived from the German decision model. As none of the included economic evaluations examined the cost-utility ratio of peginterferon plus ribavirin, the German model results are currently the only data for this new treatment.
Future studies should examine the efficacy and the need for antiviral therapy in patients with normal ALT levels, with histological mild hepatitis C, and with certain risk and comorbidity profiles (e.g., HIV infection, intravenous drug users, hemophilia). All clinical trials used the SVR as a surrogate marker for the clinical efficacy. Further epidemiological studies evaluating long-term clinical outcomes (e.g., incidence of cirrhosis, mortality) should be performed to provide more evidence on the long-term benefit of antiviral therapy.
Furthermore, further research is needed regarding the natural progression of the disease considering different prognostic factors. More observational long-term studies on the natural history of hepatitis C and the medical effectiveness of different therapeutic strategies should be performed, as well as prospective studies assessing actual cost for treatment and side effects in the routine health care setting.
This HTA suggests that initial combination therapy for CHC prolongs life, improves quality-adjusted life expectancy, and is cost-effective. The combination of pegylated interferon and ribavirin is currently the most effective and efficient antiviral treatment regimen for treatment-naïve patients with chronic hepatitis C. For these reasons, it should be the preferred antiviral treatment strategy for this patient population. However, because not all chronic hepatitis C patients will develop progressive liver disease, a thorough assessment of the eligibility and appropriateness of antiviral treatment with combination therapy requires a careful discussion between patients and physicians. This discussion must consider the demographic and clinical characteristics of the patient and the trade-offs between the expected prognosis, side effects, and the willingness to consider antiviral treatment to prevent potential future liver complications.
Further interdisciplinary research is needed to tailor drug dosage and treatment duration based on HCV genotype and individual patient characteristics as well as to assess clinical effectiveness, severe adverse events, and economic outcomes of antiviral treatment in specific subgroups such as children, intravenous drug users, patients with HIV/HCV coinfection, patients with normal ALT levels, and relapsers or nonresponders to prior antiviral therapy. The results of this research should provide useful information to aid in updating guidelines for hepatitis C.
Uwe Siebert, MD, MPH, MSc, Program Director (usiebert@hsph.harvard.edu), Institute for Technology Assessment, Massachusetts General Hospital, Harvard Medical School, 101 Merrimac Street, 10th Floor, Boston, Massachusetts 02114-4724, USA; Director, Program on Health Technology Assessment and Decision Sciences, Bavarian Public Health Research and Coordinating Center, Institute of Medical Informatics, Biometry, and Epidemiology, Ludwig-Maximilians-University, Marchioninistrasse 15, D-81377 Munich, Germany
Gaby Sroczynski, MPH, Research Scientist (gaby@mgh-ita.org), Institute for Technology Assessment, Massachusetts General Hospital, Harvard Medical School, 101 Merrimac Street, 10th Floor, Boston, Massachusetts 02114-4724, USA; Instructor, Program on Health Technology Assessment and Decision Sciences, Bavarian Public Health Research and Coordinating Center, Institute of Medical Informatics, Biometry, and Epidemiology, Ludwig-Maximilians-University, Marchioninistrasse 15, D-81377 Munich, Germany
This work was commissioned and funded by the German Agency for Health Technology Assessment at the German Institute for Medical Documentation and Information, German Federal Ministry of Health and Social Security [Grant No. 05/01.2.] Further members of the German Hepatitis C Model (GEHMO) Group and the HTA Expert Panel on Hepatitis C participated in this study. Epidemiology/Health Reporting: B. M. Kurth and H. K. Neuhauser (Dept. for Epidemiology and Health Reporting, Robert Koch-Institut, Berlin). Infectious Diseases: K. Stark (Dept. for Infectious Disease Epidemiology, Robert Koch-Institut, Berlin), N. Mühlberger (Dept. of Infectious Diseases and Tropical Medicine, University of Munich). Gastroenterology/Hepatology: S. Rossol (Dept. of Internal Medicine/Gastroenterology, General Hospital Ruesselsheim, University of Mainz), M. P. Manns (Dept. of Gastroenterology and Hepatology, Medical School of Hanover), S. Zeuzem (Dept. of Internal Medicine II, University of the Saarland, Homburg/Saar), J. K. Rockstroh (Dept. of Medicine, University of Bonn), M. Sagmeister (Dept. of Internal Medicine, Hospital Feldkirch, Austria), M. Corzillius (Dept. of Internal Medicine, University of Kiel). Systematic Reviews, Cochrane Collaboration: L. L. Gluud and C. Gluud (Cochrane Hepato-Biliary Group, Centre for Clinical Intervention Research, University Hospital Copenhagen). Health Economics: J. Wasem, P. Aidelsburger, F. Hessel, and F. Buchner (Alfried Krupp von Bohlen and Halbach Foundation, Institute for Medical Management, University of Duisburg-Essen). Pharmacoeconomics: E.-S. Dietrich (Dept. Pharmaceuticals, KBV—National Association of Statutory Health Insurance Physicians, Cologne). Quality of Life, Utility Measurement: U. Ravens-Sieberer and M. Erhard (Health Outcomes Research Group, Robert Koch-Institut, Berlin), M. Bullinger (Dept. of Medical Psychology, Clinics Eppendorf Hamburg, University of Hamburg), W. Greiner and J.-M. Graf von der Schulenburg (Centre for Health Economics and Health System Research, University of Hanover). HTA Methods: M. Perleth (AOK-Bundesverband, Stabsbereich Medizin, Dependance Berlin), D. Husereau (Canadian Coordinating Office for Health Technology Assessment, Ottawa). Public Health: M. Wildner (Bavarian Health and Food Safety Authority), A. Manstetten (Bavarian Public Health Research and Coordinating Center, Munich). Decision-analytic Modelling: J. B. Wong (Division of Clinical Decision Making, Informatics and Telemedicine, Tufts-New England Medical Center, Tufts University School of Medicine, Boston). Biostatistics: A. Koch (BfArM—Federal Institute for Drugs and Medical Devices, Bonn), R. Holle (Institute of Health Economics and Health Care Management, GSF—National Research Centre for Environment and Health, Neuherberg). Medical Informatics: A. Conrads-Frank (Institute for Technology Assessment, Massachusetts General Hospital, Harvard Medical School, Boston). Medical Ethics: G. Marckmann (Institute of Medical Ethics, University of Tübingen). Health Policy and Management, Decision Makers: B. Gibis (Dept. of Quality Assurance, National Association of Statutory Health Insurance Physicians, Berlin).

Simplified schematic of the German Hepatitis C Model (GEHMO). Each circle represents a health state of a patient with chronic hepatitis C. Each arrow represents possible transitions between health states, which may occur each year. Dotted arrows represent lower transition probabilities, reflecting the viral negative state. The two boxes represent the target population defined by histological states and separated by viral-positive and viral-negative status. Histology-specific rates for no response, relapse after response, and sustained response after antiviral treatment are applied to the three viral-positive health states: mild hepatitis, moderate hepatitis, and compensated cirrhosis. In the model, separated states were considered for liver diseases from decompensated cirrhosis, as variceal hemorrhage, hepatic encephalopathy, and liver transplant, separated into the first year and subsequent years, and ascites, separated in diuretic-sensitive and diuretic-refractory ascites. Individuals in any health state may die from causes related to their age and gender as occurs in the general population of Germany, and individuals with decompensated cirrhosis, hepatocellular carcinoma, or liver transplantation may die from liver-related causes.

Annual Treatment Costs for Hepatitis C Virus–Related Health States

Medication Prices for Antiviral Treatment (5)

Discounted Incremental Cost-Effectiveness Ratios (ICER) and Discounted Incremental Cost-Utility Ratios (ICUR) for Combination Therapy with Interferon Plus Ribavirin (24 or 48 Weeks) vs. Interferon Monotherapy (48 Weeks) in Treatment-Naive Patients with Chronic Hepatitis C

Undiscounted Therapy Costs and Lifetime Costs in € (Base-Case Analysis

Base-Case Analysis: Absolute and Incremental Discounted Costs and Efficacy, Discounted Incremental Cost-Effectiveness Ratio and Discounted Incremental Cost-Utility Ratio for Different Treatment Strategies at Annual 3% Discount Rate