A new framework for collaborative production and sharing of HTA information, the HTA Core Model®, hereafter referred to as the “Model”, was developed within the EUnetHTA project in 2006–08 (Reference Lampe, Mäkelä and Garrido1). The aim was to reduce redundant duplication of assessments in different countries through enabling the production of robust evidence base of high quality that could be easily used and tailored for local needs and updated whenever needed. This goal was sought primarily by standardizing the information contents of HTA and summarizing key research methodologies. A comprehensive set of HTA information was defined by analyzing relevant literature and using practical experience from HTA projects. This large set was divided into standardized units which were designated as assessment elements indicating their nature as fundamental parts of an HTA. The elements were divided across the following nine domains of HTA identified earlier in the EUR-ASSESS project (Reference Liberati, Sheldon and Banta2):
-
• Health problem and current use of technology
-
• Description and technical characteristics of technology
-
• Safety
-
• Clinical effectiveness
-
• Costs and economic evaluation
-
• Ethical analysis
-
• Organizational aspects
-
• Social aspects
-
• Legal aspects
The original, mostly paper-based development and testing of the Model have been reported elsewhere (Reference Lampe, Mäkelä and Garrido1;Reference Pasternack, Anttila, Mäkelä, Ikonen, Räsänen and Lampe3–8). In short, the Model was originally constructed by several international expert groups each focusing either on the overall design or one of the nine domains. Several workshops, piloting and formal validation were used in the process. From the beginning, however, future employment of the Model was meant to take place through computerized networks, because using these for the production and publication of information would be needed for effective international collaboration.
We are not aware of similar frameworks in the field of health research. Most relevant systems take a quite different approach, focusing primarily on research reporting or appraisal of information (Reference Liberati, Altman and Tetzlaff9–13). They do not define the contents of the research in similar detail as the HTA Core Model does. The MAST (Model for Assessment of Telemedicine) is to our knowledge the only more closely related work, applying some of the basic ideas of the HTA Core Model in the field of telemedicine (Reference Kidholm, Ekeland and Jensen14).
The HTA Core Model Online, available at www.corehta.info, referred to also as “the online tool”, provides a web-based interface for using the Model for HTA information production. According to the Policy for the HTA Core Model and core HTA information, EUnetHTA Partners and Associate organizations, listed at www.eunethta.eu, can use the site for producing and publishing HTA information (15). Information produced and published within the HTA Core Model Online was designated in the policy as core HTA information. The Model can assist also other parties (beyond EUnetHTA members) in designing or reporting health-related research. Any noncommercial party can use the online tool for producing information, but organizations that are not EUnetHTA members must publish their products elsewhere, for example, on the user's own web site. Commercial organizations can access the core HTA information—like anyone else—free of charge, but they are not allowed to access the information production functions.
In this study, we describe the further development within EUnetHTA Joint Action (JA) in 2010–12 of moving the Model from paper to the online environment. The intention was to capture a generic project flow that would allow effective usage of the Model online and sharing of structured HTA information amongst agencies, while at the same time allowing for local, research-related internal processes within HTA agencies.
METHODS
The HTA Core Model Online was developed in EUnetHTA Joint Action Work Package 4 Strand A (WP4/A) through an iterative process on the basis of the paper-based version of the Model.
The work was led by a team within the Finnish National Institute for Health and Welfare, supported by an international expert group consisting of twenty-three individuals representing HTA agencies in nine countries. Challenges, options, and solutions were discussed and developed through email communication, e-meetings, and a total of ten face-to-face meetings. Decisions were mostly made by consensus or, if needed, by majority. The broad experience of these agencies in HTA allowed us to better understand the needs of HTA professionals and consequently balance between the theoretical ideal and the practically feasible.
Within the technical development, we aimed at ensuring good usability, sustainable database structures, features for group work and work flow management, effective search functions, as well as standardized interfaces, reusability, and publishing. The online service was developed using the Microsoft ASP.NET environment and C# programming language. The database is managed by Microsoft SQL Server. Jointly agreed-on guidelines for EUnetHTA tools’ design were applied whenever feasible (16).
When implementing the Model in the online environment, some concepts behind the original ideas required further clarification, reconsideration, and consolidation so that the resulting information system would be based on a clear conceptual basis. This formed a large part of the development work.
The practical development of the HTA Core Model Online was divided into two phases, first focusing on the basic functionalities needed to support core HTA production and then considering advanced functionalities, such as publishing, information search and retrieval and adaptation of information (17). Development was supported by continuously collecting users’ feedback and carrying out two specific pilot testing phases during autumn 2010 and spring 2011. Most of the feedback came from WP4 member agencies participating in the pilots. Improvements to the system were made after both pilot tests. The piloting focused on the tool's ability to support users in developing a project protocol for assessments. Subsequently, WP4 Strand B (WP4/B) piloted the complete production process, by producing two core HTAs between 2011 and 2012 using the online tool. This experience of WP4/B is reported elsewhere in this issue (18) and was used for further developing the tool.
WP4 also developed a policy (15) to guide use of the HTA Core Model and information produced by using the Model. This was done by surveying the views of EUnetHTA member agencies. Preferences of the majority were typically chosen to be included in the policy, but feedback from stakeholders and public consultation was taken into account as well. The policy crafting process also required us to further clarify some concepts. The policy was used when preparing a more concise document, the Terms of Use for the Model (available at www.corehta.info).
The EUnetHTA's Stakeholder Forum contains a broad range of stakeholders who participate in the activities of the network (19). The Forum nominated representatives to the WP4 Stakeholder Advisory Group (SAG). The SAG was consulted during various development processes. The HTA Core Model Online was also subject to public consultation and formal validation by HTA agencies during 2012 (17).
RESULTS
Basic Concepts
The Model and its Use
Three separate, but strongly inter-connected components of the Model were identified. First, the assessment elements delineate the contents of an HTA and hence constitute an ontology of HTA, that is, a formal representation of knowledge within HTA. The second component of the Model consists of all the methodological guidance included in various parts of the content. Finally, the Model also contains a common reporting structure that aims at a standardized, flexible and informative presentation of information.
The three components all aim at producing and reporting a set of structured HTA information. The ontology defines the potentially relevant research questions for an HTA, the methodological guidance assists in finding answers to the questions and the reporting structure defines how to present the answers. Users can employ either some or all of the three components in their HTA work, as long as the Terms of Use are followed. The terms allow a versatile use of the Model, but certain requirements are set both for noncommercial and commercial use. For example, when using the noncommercial license, the produced information must be made publicly available. The commercial license, in turn, sets for instance some conditions for advertising nearby materials produced using the Model (Figure 1).
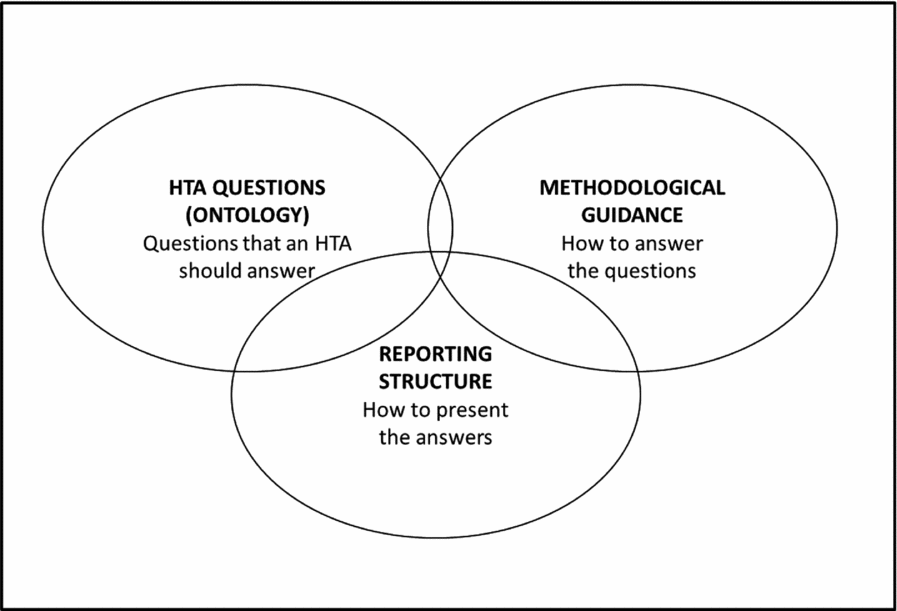
Figure 1. The three components of the HTA Core Model.
The online environment was taken into account for the first time while preparing an application of the Model for screening technologies (20). The interest for such an application had been identified in discussions when setting up the JA as a project. The texts were shortened and structured under consistent subtitles similar for all domains; relations between assessment elements were identified and coded to enable cross-referencing in the online tool. Furthermore, methodological guidance applicable to several domains was placed in appendices and marked with hyperlinks in the domain chapters (Table 1).
Table 1. Terms Used in the HTA Core Model.
HTA Ontology
The HTA ontology defines a generic set of questions about health technologies. It is a formal representation of knowledge within the field of HTA, providing a standardized structure for any set of HTA information.
The original list of assessment elements was created when developing the HTA Core Model for medical and surgical interventions. Another list was subsequently created during the development of the Model for diagnostic technologies. Further modifications to the elements were made after completion of the Model application for screening technologies and rapid relative effectiveness assessment of pharmaceuticals. It was decided that these application-specific lists would draw from a common pool of assessment elements. In other words, the HTA Core Model contains an extensive number of assessment elements, flexible for future developments. Each model application uses only part of this whole pool. The most extensive application (for diagnostic technologies) makes use of 157 elements while the most concise application (for medical and surgical interventions) uses only 135 elements.
The generic questions, referred to as “issues” in the Model are organized within the nine domains using an intermediate layer of “topics”. Hence each element is defined by a domain, topic, and issue.
The following are examples of assessment elements:
-
• Safety (domain) / Occupational Safety (topic) / What kind of occupational harms can occur when using the technology? (issue)
-
• Clinical Effectiveness / Function / How does use of the technology affect activities of daily living?
-
• Ethical analysis / Autonomy / Is the technology used for patients/people that are especially vulnerable?
The assessment elements each define a “generic question” that could be answered in an HTA. Each element is defined in further detail in an element card that is application-specific. For example, a question that considers “mortality related to using the technology” may be defined differently depending on whether it is applied to a therapeutic intervention or to a screening test.
We maintained the earlier division of elements into core elements and non-core elements, based on their importance and transferability (Reference Lampe, Mäkelä and Garrido1). The core elements are those whose importance and transferability are defined as higher than those of non-core elements. Consequently they are likely to be more useful in the international context. The importance and transferability of each element can be different across model applications.
Methodological Guidance
Different levels of methodological guidance are a major constituent of the Model. International working groups consisting primarily of HTA experts have produced the guidance using relevant literature and their own expertise. The guidance is provided on the level of individual elements, domains, and on whole HTA projects. Assessment element-level guidance provides practical assistance to researchers for tackling a specific research question. Such guidance is available in the element cards. Domain-level guidance is provided for each of the nine domains, consisting of an overview of recommended scientific methodologies within different domains. The project-level guidance is currently still scarce, but for example specific ethical requirements have been set for projects using the Model.
We maintained the original (Reference Lampe, Mäkelä and Garrido1;5;6) principle that all guidance—across the above mentioned three levels—can be either in the form of hints/tips, somewhat stronger recommendations or strict requirements or standards. It has been the task of the expert groups and WP4 to decide on the level of guidance.
Common Reporting Structure
The Model provides a standardized structure for presenting and reporting the HTA information produced using the Model. The information is organized into collections of result cards. The fundamental difference in the reporting structure compared with the reporting of traditional HTA reports is that the results are presented as standardized question-answer pairs, organized under the nine domains. The information is located in a standard, easily identifiable and searchable location in the reporting, instead of being embedded within the narrative text flow of traditional reports.
According to the experience gained in the two pilot core HTA projects (18), most assessment elements translate into a single research question. It is, however, possible to divide the generic question in an assessment element into more than one question. The answer(s) are presented in result cards, each containing the answer(s) to question(s) defined by one assessment element. The result cards are then organized into collections, each consisting of a set of result cards and collection-level content, for example, an introduction and a summary. Each result card should contain the answer in a relatively concise format. Although no definite character limit was set at this point, an overall recommendation of 1–2 pages per answer was given to pilot projects. This length was considered appropriate when designing the Model within the EUnetHTA Project 2006–08, as it would allow the text fit on a single page and hence resemble a “card”. More extensive materials, such as large evidence tables or detailed results can be added as appendices to the collection.
The earlier paper-based reporting has evolved to a structured set of information that can be used, shared and updated in computerized environments. The standardized information structure allows flexible searching and viewing of information. Only relevant parts of the information can be used for each purpose. From the technical viewpoint, the predetermined structure also enables interoperability with other information systems, such as the EUnetHTA POP Database or any systems used, for example, for guideline development or decision-support.
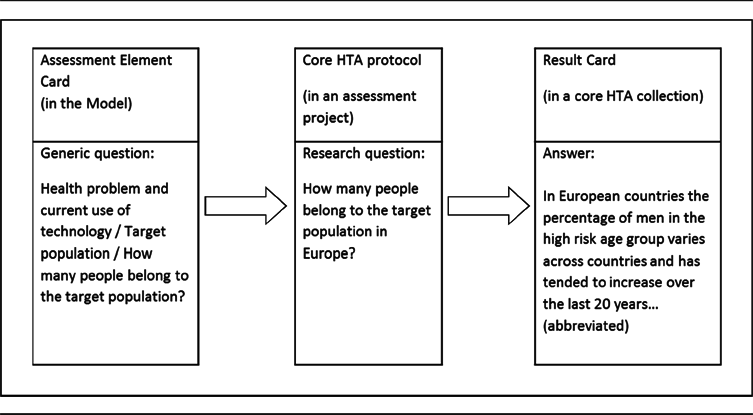
A practical example of how a generic question in the ontology is translated into a project-specific research question and answered within a core HTA project is available in Table 2.
Table 2. From Generic Question to Result Card
The Core HTA Structure
The interrelation between core HTA information and local HTA information was originally suggested in Lampe et al. (Reference Lampe, Mäkelä and Garrido1) and designated as the core HTA structure. It consisted of a pool of structured HTA information, a core HTA as an intermediary information product and local HTAs as end products. The current new structure illustrated in Figure 2 allows different types of core HTA information collections. The features of EUnetHTA Collections, such as “core HTAs” are defined by EUnetHTA, while Other Collections may be formed based on users’ own preferences. It was again agreed on—now in the formal policy—that core HTA information is published in English language. The process between the collections and underlying information pool (containing result cards) is automated and hence the user interacts mostly with collections. The utilization process from core HTA information to local products is voluntary and the format, language and content of end products are defined mostly by local users. Local reports can also form a collection in the database.
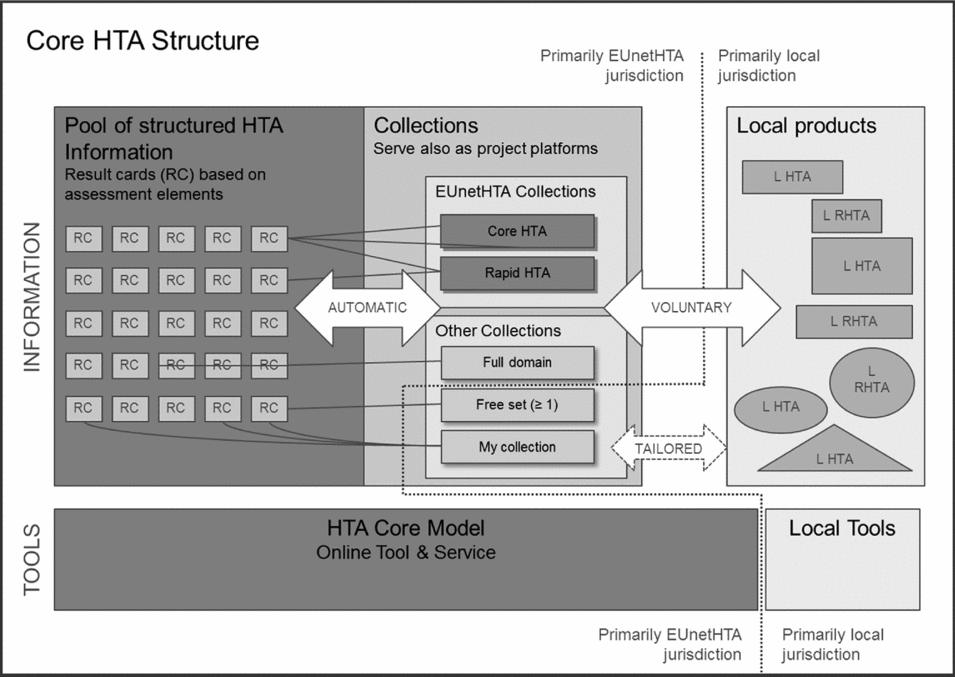
Figure 2. The core HTA structure defined in EUnetHTA Joint Action. (L HTA = Local HTA; R HTA = Rapid HTA)
The HTA Core Model Online
A service for using the HTA Core Model in the online environment was delivered at www.corehta.info. It allows production and publication of core HTA information according to concepts outlined above. Some key functions are discussed below.
Phases of Information Production
The online tool organises the workflow of information production into five phases. Proceeding from one phase to the next requires locking of the phase, after which no further changes are allowed in the preceding phases. Unlocking a phase is possible, but as any changes to the earlier phases may have an impact on the subsequent phases, it should be done consciously and after consideration.
1. Project definition and scope
In the first phase the project is created as a new entity within the database, including its name, scope and metadata.
The project scope is organized in a manner that (a) allows a robust scope for each project from the research perspective, as well as (b) enables automated functionalities within the subsequent phases. The authors are requested to name the technology, the indications and comparators they want to assess. The ‘indication’ includes information of the disease in question, including its level of severity; the patients, with possible age and gender limits and risk level; and the intended use of the technology, for example, for screening or diagnosing the disease, or for first or second line treatment. The most important outcomes assessed in the project are outlined in the scoping phase, but they are further expanded later during the project within each domain separately.
2. Protocol design
During the second phase, a set of generic questions defined by the ontology and divided within the nine domains of the Model are presented to the user, who must consider their relevance in the context of the technology under assessment. If a question is relevant, it should be translated into a topic-specific research question that should be answered in the subsequent phases. The system assists the researcher in this phase by suggesting questions based on the structured scope.
Each domain uses by default the project scope as its research frame. The user has the option of applying a wider frame, if that suits the analysis better. For example the project scope may define the technology as “multi-slice Computed Tomography (CT)”, but in the safety domain it may be more useful to consider the safety of “CT” in general. The project scope should always be embedded within the frame, keeping the overall analysis focused on the same technology, while at the same allowing flexibility in various domains’ analysis.
The project protocol defines the research questions that should be answered in the project and should be used to formulate a further detailed research protocol including plans about methodology used. The research protocol is not yet included in the tool, allowing variation in local practices.
3. Research (that is, finding the answers)
In this phase the answers to the questions included in the protocol are sought and formulated. In addition to the methodological guidance provided by the Model, the researchers can use any tools and practices they normally apply in their work to find answers. The nature of the questions and the answers varies to some extent across domains. In some cases the answer may be a compilation of scientific evidence, while in other cases the answer may consist of data from a single database or reporting of moral deliberations. Independent of these differences, the results of this phase are always reported in a standardized format using an MS Word document template available for each domain. The template is based on the common reporting structure and contains all topic-specific research questions of a domain selected in the previous phase, and all other required domain-specific fields.
The only mandatory information is included in the “Results” field, which should contain the answer in a concise format. Optionally, researchers can add a description of question-specific methodology (if it deviates from domain methodology) and a comment text. The methods should be disclosed as detailed as in any scientific work. The comment can address, for example, research methods used, reliability of results or needs for further research.
On the domain-level researchers should include text chapters providing an introduction and a description of methodology used in the analysis, as well as a general discussion related to the findings for the specific domain. The discussion section allows for bringing together and interpreting the findings that are presented in the result cards of a domain, as well as for a discussion on methodological challenges, indications for further research and any other domain-level considerations.
4. Results
In this phase all results are uploaded to the system and consequently another entity, the collection, is created in the database. The HTA Core Model Online presents the user with a standard collection structure that contains all relevant parts. The researchers should upload all the domain-specific results (including the result cards) and add the rest of the collection-level information, such as a summary.
The summary provides an overview of the facts found in the assessment, but it must not contain any recommendations for or against the use of the assessed technology. Control of this principle takes currently place at the JA work packages producing EUnetHTA collections, but more detailed processes will be developed in the future. Such recommendations can be added to the local reports based on the collection. Collection-level information also contains an introduction and a methodology chapter.
Currently the upload process from the MS Word templates to the HTA Core Model Online is a manual process.
5. View and Submit
The final phase allows viewing and submitting the collection for publication. Viewing is available at any point of time, but (before publication) restricted to those who participate in the project. If the project is owned by one of the EUnetHTA Partners or Associates, it can be published within the pool of core HTA information. If the project is owned by others, publication within the system is currently not allowed. In such cases the researchers can save/download the collection and publish it elsewhere. Allowing a more free publication platform would have required additional editorial control mechanisms as well as administrative effort, which were not prioritized at this phase.
Presentation of Collections
Each collection contains a cover page that displays key information: the collection's name, producers, publishing agency and various data on the Model version used, the scope and summary. All other contents of the collection are available through selecting the desired parts (or all) of the content to be viewed. This allows flexible viewing that easily adapts to various users’ needs.
The key contents of each result card are included in the collection view as flowing text one after another. It is also possible to open each card in a separate window to access all detailed contents of each card.
Some key features of the Adaptation Toolkit, developed by WP5 of the EUnetHTA project 2006–08, were included in the HTA Core Model Online to assist local use of core HTA information (Reference Chase, Rosten, Turner, Hicks and Milne21).
DISCUSSION
We succeeded in clarifying the conceptual basis of the HTA Core Model and a pragmatic information production process needed for the online implementation of the Model. Some themes that required more attention during the development process or that perhaps necessitate further development in the future are discussed next.
Compared with formal ontology development and some existing ontologies (Reference Vickery22;Reference Lumsden, Hall and Cruickshank23) the HTA ontology is still in a crude format and lacks certain features (for example identification of some important relations between the content). This turns into some practical disadvantages, for example, in the form of overlapping content within different assessment elements and even across domains. Further development work is needed to address these issues by identifying relevant relations and by removing redundant overlaps.
The aim of the HTA Core Model has been, from the beginning, to provide overviews of worthwhile research methods within various HTA domains, as well as practical advice on different assessment elements. A considerable amount of guidance is embedded in the Model itself, but an equally important aspect is the possibility to link to existing guidance elsewhere—whether produced by the EUnetHTA or other parties. Practical examples include the SureINFO service (http://vortal.htai.org/?q=sure-info) and EUnetHTA guidelines.
After the Joint Action the Model supports the assessment of medical and surgical interventions, diagnostic and screening technologies, and rapid relative effectiveness assessment of pharmaceuticals. Due to the inherently extensible design, further categories of technologies can be included in the Model through amending the ontology and methodological guidance.
The rationale for organizing information as collections of result cards is twofold. First, organizing information in result cards available through an electronic database allows for their usage at an elementary level not embedded within targeted reporting for a particular context, such as country-specific setting. Second, representatives of some countries wished or required that the end result should not be viewed as a “European HTA report”, because the local information needs and requirements may be different and such a report might bear consequences—beyond those intended—to national health systems. Consequently, the developers aimed at a system that provides a robust evidence base that should be interpreted by local HTA operators. How this process will take place is beyond the influence of EUnetHTA, but it could be done for example by producing a local HTA report that is partly, largely or only based on a collection of core HTA information (24).
The choice of presenting information as “collections of cards” is likely to challenge researchers who are more used to reading reports and articles. We assume, however, that after reaching a specific point on the learning curve, the standardized structure will facilitate easy access to and sharing of relevant information. This will be further tested in EUnetHTA Joint Action 2 (2012–15).
Currently the pool of core HTA information is limited to the HTA Core Model Online. This allows concentration of still relatively scarce pilot materials into a single knowledge base that can be further developed, piloted and used. In the future, it may be useful to expand the concept of core HTA information to cover also information published in other sources. It could mean inclusion of any information produced using the Model or—for example—a select assortment of web sites of trusted partners. The common information structure would allow shared production, publication, and usage of structured HTA information even if it would be distributed to more than one information repositories. The political and practical implications of such decisions need to be carefully considered in the future.
The relation between the project protocol and a (much-needed) more detailed research protocol requires further consideration. The current solution allows many different and locally familiar practices in developing research protocols. On the other hand, requests for better functionalities for this purpose have also been expressed during the piloting. Incorporating into the HTA Core Model Online an optional feature of designing a detailed research protocol could be considered in the future.
The manual work required within the research phase at the moment has raised criticism by several participants of the pilot projects. The choices are based on relevant decisions within the original EUnetHTA project by 2006–08, where the primary focus was standardization of the contents and methodology of HTA to allow easier sharing of information. Automated uploading of results is now on the subsequent development list. The overall process of using external text editing program versus building web-based text tools should be further considered.
There were repeated requests from researchers to include evidence table templates in the Model. The Model does not yet provide such templates. A slightly different—and more advanced—approach has been considered for the Model. It involves the idea of extracting information from original studies into study cards, each containing information from one study. Such cards could then be used in a flexible way to produce different kinds of evidence tables, reducing double work within single projects and across projects. A pilot version of such a study card was drafted to encourage piloting, but the work was not fully completed within EUnetHTA JA. It forms a basis for further development, taking also into account the work of the Evidence Tables Working Group of the Guidelines International Network (http://www.g-i-n.net/activities/etwg).
The possibility of allowing users to add comments to existing result cards was considered, but not yet implemented.
The policy (15) requires that all those who use core HTA information must provide an English language summary of the final conclusions of their local report. This summary will be included in the HTA Core Model Online. This feature has not been implemented yet, but it will be included in the future.
An important challenge in the production and usage of core HTA information has been to address all the different requirements and expectations of various countries’ HTA agencies. This has been addressed by promoting the idea of core HTA information as a robust information source that is used and possibly further processed locally. When users of core HTA information start adding summaries of their conclusions into the database, a new important information base is formed. By scrutinizing these local reports and associated comments on the core HTA information, users can possibly gain important insights into how specific collections should or could be interpreted in the subsequent users’ own context.
Overall, we managed to reach good consensus in the key aspects of the development and proceeded implementing them online for further piloting. The pilot projects of WP4/B provided a valuable testing experience through which some important benefits and hurdles of the system were identified (18). The more challenging themes included for example the appropriate granularity of the ontology (so that each assessment element would contain a proper amount of information), the overlaps between assessment elements, and the method we used to divide assessment elements into core elements and non-core elements. These themes require further consideration and development.
The HTA Core Model is a registered trademark, but it should not be seen as reserved for a limited group of HTA agencies only. The information production features of the HTA Core Model Online are available to any noncommercial users. The Model in its PDF format can be used by anyone. Licenses exist for both noncommercial and commercial use (available at www.corehta.info).
It remains to be seen how useful the Model is in practice. Therefore it is important that HTA researchers start or continue using the online tool, to build up experience and identify strengths and remaining weaknesses, which can then be remedied to improve the practicality and added-value of the Model and the online tool. The development of the HTA Core Model Online continues within EUnetHTA Joint Action 2. Main challenges include updating and harmonizing the contents of the Model and adding features to enable production of rapid HTAs and local reports.
In conclusion, we have developed through an international collaborative network an online tool for facilitating the usage of the HTA Core Model by many researchers and organizations. The resulting pool of core HTA information is likely to develop into an important information source that can be used as a service for local HTA information production. Links to resulting local reports are also likely to be very useful for users.
Existing core HTA information has an important potential in reducing overlapping work within HTA agencies and promoting efficient use of HTA resources. By using special expertise available through international collaboration and use of jointly developed or agreed-on scientific methodologies, the quality of HTA information is likely to increase as well.
CONTACT INFORMATION
Kristian Lampe, MD, Senior Medical Officer, Finnish Office for Health Techology Assessment (FINOHTA), National Institute for Health and Welfare (THL), POB 30, 00271 Helsinki, Finland
Iris Pasternack, MD, HTA professional, Summaryx Ltd, Untuvaisentie 2 A 19, 00820 Helsinki, Finland
Oskari Saarekas, MSc, Software Developer, Finnish Office for Health Techology Assessment (FINOHTA), National Institute for Health and Welfare (THL), POB 30, 00271 Helsinki, Finland
Leena Raustia, MSc, Senior Planning Officer, Finnish Office for Health Techology Assessment (FINOHTA), National Institute for Health and Welfare (THL), POB 30, 00271 Helsinki, Finland
Irina Cleemput, Professor Health Economics, Hasselt University Senior Health Economist, Belgian Health Care Knowledge Centre (KCE), Kruidtuinlaan 55, 1000 Brussels, Belgium
Mirella Corio, PhD, MSc, Health economist - researcher, Innovation Research and Development Department, National Agency for Regional Healthcare Services (Agenas), Via Puglie 23, 00187 Rome, Italy
Gottfried Endel, MD, Team HTA, Department for Evidence Based Economic Health Care, Main association of Austrian Social Insurance Institutions (HVB), Kundmanngasse 21, Vienna, Austria
Katrine Frønsdal, BASc, MSc, PhD, Senior Researcher, Norwegian Knowledge Centre for Health Services (NOKC), PO Box 7004 St. Olavs plass, 0130 Oslo, Norway
Iñaki Imaz, MD, PhD, MPH, Senior Researcher, Agencia de Evaluación de Tecnologías Sanitarias (AETS), Instituto de Salud “Carlos III”, 5 Monforte de Lemos, 28029 Madrid, Spain
Sarah Kleijnen, MSc, Advisor, Dutch National Health Care Institute (ZIN), Postbus 320, 1110 AH Diemen, The Netherlands
Finn Kristensen, MD, PhD, Adjunct Professor, University of Southern Denmark, Campusvej 55, DK 5230 Odense M; Director, EUnetHTA Secretariat, Danish Health and Medicines Authority (DHMA), Axel Heides gade 1, 2300 Copenhagen S, Denmark
Alric Rüther, MD, PhD, Head of Department, Department of Health Care Quality, Institute for Quality and Efficiency in Health Care (IQWiG), Im Mediapark 8, 50670 Cologne, Germany
Sophie Werkö, PhD, International Relations Manager, Project Director, Swedish Council on Health Technology Assessment, SBU, Box 3657, 103 59 Stockholm, Sweden
Marina Cerbo, DSc, Director, Innovation Research and Development Department, National Agency for Regional Healthcare Services (Agenas), Via Puglie 23, 00187 Rome, Italy
CONFLICTS OF INTEREST
Kristian Lampe reports grants to his institution from the European Commission; he presented results of this work in a workshop organised by CIRS (Centre for Innovation in Regulatory Science), which covered his travel costs. Iris Pasternack has received consultancy money from Summaryx Ltd, a company she owns; has been employed by THL (National Institute for Health and Welfare); her institution has received co-funding through the grant for the work described in this paper; and has had travel expenses covered by ISPOR. The other authors report grants to their institutions from the European Commission.






