THE HELLENIC SYSTEM FOR THE PROVISION OF PHARMACEUTICAL CARE
The healthcare system in Greece is financed by the combination of state budget, social insurance contributions and private payments. As such, it cannot be classified as falling under either the predominantly Beveridge or Bismarck-type of financing system. Taxation contributes 29.1 percent of total health expenditure, while health insurance accounts for 31.2 percent. It should be noted that private expenditure accounts for a very high percentage of the mixed financial resources, and this public/private mixture is a significant feature of the system. In a little more detail, out-of-pocket payments account for 37.6 percent of total health expenditure, whereas private insurance accounts for 2.1 percent, calling the social character of the health system into question (Reference Polyzos, Karakolias and Dikeos1).
The 2011 health insurance reforms resulted in a unified Reimbursement Fund by including numerous long-existing social and health insurance funds of compulsory participation. Since June 2014, EOPYY (National Organization for Healthcare Services Provision) is responsible for the pharmaceutical care of 95 percent of the Hellenic population (10.816. 286 resident population) (2). Medicines are dispensed through private (local) pharmacies, hospital pharmacies, and twenty-eight pharmacies belonging to the EOPYY. It should be clarified that EOPYY's pharmacies are state-owned, nonprofit pharmacies that dispense high-cost medicinal products (mainly anti-cancer, biological, orphan medicinal products with high monthly cost of treatment or high monthly patients’ co-payment), as listed in a Ministerial Decree (3) or according to a decision made by the president of the EOPYY (Law 3816/2010 has introduced the Positive Reimbursement List and segregates medicinal products into two categories: one category includes medicinal products dispensed by private (local) pharmacies; the other includes high-cost medicinal products dispensed without any co-payment, mainly by public hospitals or EOPYY's pharmacies) (4).
Health technology assessment (HTA) has emerged as a measure in European Union (EU) countries to be compliant with the EU Transparency Directive (TD) 89/105/EEC (Reference Kolasa, Kalo, Zah and Dolezal5). The TD aims to ensure the transparency of pricing and reimbursement procedures for medicinal products for human use in EU member states. Additionally, HTA can enable policy makers to look beyond budget impact (Reference Lopert, Ruiz and Chalkidou6) and facilitate more rational decision making based on real-world data about the value (i.e., benefits, risks, and costs) of new and existing technologies (Reference Drummond, Schwartz and Jönsson7). Greece, however, although an EU member state, has not yet adequately addressed the issue of allocating financial and human resources by substantiating policy decisions with evidence (Reference Kaló, Gheorghe, Huic, Csanádi and Kristensen8;Reference Gulácsi, Rotar and Niewada9).
Additionally, Greece's economic crisis since 2011 and the consequent fiscal consolidation program has led to a drop in per capita income and several changes in health policy (Reference Zavras, Zavras and Kyriopoulos10). There has been a downward trend in self-rated health in Greece as a consequence of the recent financial crisis. Findings stress the need for urgent health policy responses to the recent economic collapse in Greece as the full impact of austerity measures unfolds in the coming years (Reference Vandoros, Hessel, Leone and Avendano11). The development and application of a formal HTA process seems to be the only way to achieve improved efficiency of available funds, which could otherwise be used in other interventions (Reference Vandoros and Stargardt12). Although there is growing consensus on the importance of international initiatives, such as the EUnetHTA Core Model®, specific tools for the assessment of level of care are rarely developed and implemented at national level (Reference Fuchs, Olberg, Panteli and Busse13).
OBJECTIVES
The aim of the present study is to describe the current regulatory environment in Greece to evaluate the potential introduction of health technology assessment (HTA) for medicinal products for human use at a national level.
METHODS
This study was conducted by descriptive method. Data sources consist of national legislation on pricing and reimbursement of health technologies. Furthermore, data are presented per pricing and reimbursement policies.
RESULTS
Current Pricing Policies
The National Organization for Medicines (EOF) is the competent authority for pricing in Greece, but the final decision lies with the Ministry of Health (MoH). (Table 1 shows the names of institutions, abbreviations, and their role in the present system.) In the case of Greece, the pricing policy follows the external reference pricing system based on all the prices of the rest of the twenty-seven EU countries. According to recent legislation (14), two general price revisions should be applied in November and May each year. Supplemental price bulletins should be produced every month for generic products and every three months for products containing on-patent active substances. The prerequisite for applying for pricing is that a medicinal product for human use has already been allocated a price within at least three EU countries and, in cases of medicinal products for human use authorized through the centralized procedure, blue box approval is also necessary at national level.
Table 1. Names of Institutions, Abbreviations, and Role in the System of Pricing and Reimbursement of Medicinal Products

More specifically, the maximum ex-factory price of the reference medicinal products under protection is defined as the average of the three lowest prices in the pool for the same medicinal products for human use (meaning the same active ingredient, dosage form, strength, and package size) in the EU member states that publish official data. (Data on prices are generally retrieved from EURIPID, the EU database that makes possible the comparison of prices of medicinal products for human use.) No price increases of existing medicinal products are permitted apart from cases where corrections of pre-existing errors take place (14).
The price of an off-patent medicinal product for human use is either set to 50 percent of the price of the same product during the on-patent period, or to the average of the three lowest EU prices for the same medicinal product for human use, whichever is lowest. The respective price cannot be less than the lowest EU price (14). The prices of generic medicinal products for human use are set at 65 percent of price of the off-patent medicinal product for human use (meaning at 32.5 percent of the price of on-patent medicinal product for human use).
The wholesale and pharmacy mark-ups for reimbursable medicines in the out-patient sector are statutorily regulated. The mark-up scheme for wholesalers is linear, depending on the ex-factory price. (For reimbursed medicines, the mark-up is 4.90 percent if the ex-factory price of the pharmaceutical is lower or equal to €200,00 or 1.50 percent if the ex-factory price of the pharmaceutical is higher than €200,01.) The mark-up scheme for pharmacies is degressive (varying from 30 percent to 2 percent if the pharmacy purchasing price of a reimbursed medicinal product is €0–€50,00 and above €3000,00, respectively).
For nonreimbursable and over-the-counter products, the pharmacy mark-up is set at 35 percent. In the case of high-cost medicinal products for human use with a retail price higher than €3000,00, where a prior authorization procedure or reference to a respective decision by the president of EOPYY is required, they are dispensed only through hospital pharmacies or EOPYY's pharmacies and purchased at hospital price minus 5 percent. (Hospital price is equal to the ex-factory price minus 8.73 percent.) Additionally, HIV medicinal products, growth hormones, and blood coagulation factors are dispensed only through public hospital pharmacies and purchased at hospital price minus 5 percent directly from pharmaceutical companies.
The value added tax (VAT) on medicines is currently 6 percent in general, and 13 percent for some categories of medicinal products for human use (compared with standard VAT rate, which is 24 percent).
Current Reimbursement Policies
The country applies a benefit package that includes imported pharmaceutical products for exceptional cases, special nutrition products, medical devices for personal use (such as glucose strips, dressings, etc.) and a Positive Reimbursement List for pharmaceuticals that are reimbursed by social health insurance (4). The procedure for pricing and reimbursement of pharmaceuticals is presented in Figure 1. There is also a negative list for medicinal products for human use excluded from the benefit package. The responsible authority for the final assessment and reimbursement recommendation of medicinal products for human use to the MoH is the Positive Reimbursement List Committee, an independent Committee of the MoH.

Figure 1. Pricing and reimbursement of medicinal products. EOPYY, Reimbursement Fund.
The Committee is appointed by ministerial decision and consists of nine members: one professor of Pharmacy, one professor of Medicine, one representative of the National Drug Organization (EOF), one representative of the Central Committee of Health Council (KESY, a consulting committee under the MoH, responsible for structural and operational proposals on the health system), one hospital pharmacist, and four members of EOPYY. (Table 1 shows the names of institutions, abbreviations and their role in the present system.) The compilation of the Positive Reimbursement List and the calculation of a reference price are based on an ATC4-therapeutic category (4th level, chemical subgroup) per grouping of pharmaceutical forms set by the EDQM (European Directorate for the Quality of Medicines); this system serves as the basis of the internal price referencing system.
Reference prices determine the maximum amount statutory health insurance providers will pay for a pharmaceutical of a certain substance group. The ATC4 classification refers to the clustering of similar medicines (ATC4 level) in the same group (reference group). In exceptional cases, when solid evidence of clinical and economic benefit is proven, identical medicines (ATC5 level) could also be used as a reference group. Reference prices are price limits on certain pharmaceutical substance groups which have been classified by the Positive List Committee as similar (therapeutic equivalent).
Additionally, according to current legislation, new active substances have to first be reimbursed in other EU countries, before being included in the Positive Reimbursement List (15). Although the development of HTA in Greece has not been implemented yet, the existing legislation for the inclusion criteria on the Positive Reimbursement List (Reference Kolasa, Kalo, Zah and Dolezal5) mentions that a Marketing Authorization Holder (MAH) could submit as supporting material, HTA decisions from other EU HTA bodies, such as NICE. The Positive Reimbursement List Committee has the jurisdiction to require the submission of additional data and to propose reimbursement on subgroups of patients or on sub-indications for a specific time period, or to define specific conditions for the reimbursement of medicinal products for human use. The submission of health-economic evidence is not a prerequisite for the decision-making process. However, the MAH may voluntarily include a health-economic evaluation in the submitted file to the Positive Reimbursement List Committee. However, there is neither an explicit cost-effectiveness threshold nor a methodological guideline for the conduct of health economic evaluations.
According to the national legislation, a Positive Reimbursement List should be issued 30 days after the publication of a Price Bulletin. In the Positive Reimbursement List, both retail and reimbursed prices are displayed. The reimbursed price corresponds to the number of daily dosages multiplied by the reference reimbursement price mentioned above.
The difference between the reimbursed and the retail price is generally covered by the patient. However, from 15 September 2014, a new legislation has been in force (16), defining that in cases where a product classified in an ATC4 group has no generic equivalents, the patient pays for 50 percent of the difference between the reimbursed and the retail price, while the remaining 50 percent is charged to the pharmaceutical company or the marketing authorization holder in the form of a rebate. Apart from the difference between the retail and the reimbursed price, a statutory co-payment of 0 percent (for medicinal products for human use for severe diseases and vulnerable social groups), 10 percent (for medicinal products for human use for specific chronic diseases), and 25 percent (the standard rate of co-payment) is paid by the patient on the reimbursed price, depending on the disease. Pharmacists have the obligation to inform patients of the generic equivalent with the lowest price (generic substitution) when an active substance without protection is prescribed (international non-proprietary name prescribing is obligatory); this aims to increase generic uptake (17). Pharmacists who do not follow the above measures may be subject to a fine or suspension of their licence to dispense medicinal products for EOPYY (18).
The second part of the Positive Reimbursement List contains high-cost medicinal products (referred to as high-cost products of Law 3816/2010). These medicinal products for human use have up to now been fully reimbursed without co-payment and without the application of reference price calculations. The high-cost medicinal products for human use list is divided into two parts: the first part contains medicinal products strictly for hospital use (as defined by the blue box), while the second part contains medicinal products whose distribution starts in hospital and their use can be continued at home. The high-cost medicines are dispensed through hospital or EOPYY pharmacies, and in some special cases also through private pharmacies.
Medicinal products for human use can be reimbursed on an exceptional basis before pricing/inclusion on the Positive Reimbursement List through named patient supply after prior authorization procedure from EOF and EOPYY. Additionally, compassionate use of medicinal products is granted by EOF before marketing authorization for individual patients either for cohort use or for named patient supply. Reimbursement is granted for each individual patient included in the compassionate use cohort or individual patient supply.
Off-label use for authorized and included on the Positive Reimbursement List medicinal products is allowed on a patient by patient basis for a specific time period upon approval of a Special Committee of the National Organization for Medicines (EOF). The reimbursement price is further reduced after the deduction of rebates paid mainly by pharmaceutical companies and, to a lesser degree, by private (local) pharmacies provided by law. More specifically, the following rebates are applied to pharmaceutical companies: (a) A 9 percent rebate and a volume rebate on ex-factory price are applied to products included on the Positive Reimbursement List and dispensed through private (local) pharmacies. (b) An additional 2 percent rebate is added when a product is classified as ATC5 level, and a 5 percent rebate is added in cases of new active substances for the first year of their inclusion on the Positive Reimbursement List. (c) For high-cost medicinal products for human use dispensed through EOPYY pharmacies, an additional 1.5 percent or 3 percent or 4.5 percent discount is applied to the hospital price, based on trimester sales of medicinal products having the same commercial name (16). (d) Additionally, extra rebates must be paid by pharmaceutical companies when high-cost medicinal products (as defined in the Positive Reimbursement List) are dispensed by retail pharmacies, so that the final cost for EOPYY is no greater than if the medicines had been dispensed through EOPYY pharmacies.
According to current legislation, a clawback mechanism for pharmaceutical expenditure for out-patients (19) is in force up to 2018, meaning that the monthly pharmaceutical expenditure of EOPYY (out-patient pharmaceutical expenditure) cannot exceed 1/12 of the outlay that is defined in the annual social budget and corresponds to pharmaceutical care. The monthly exceeding amount (clawback) is requested from the MAHs of the medicinal products for human use. The above amount is calculated on a 6-month basis, and every detail concerning the above-mentioned implementation, as well as the exact calculation algorithm of the amounts that each MAH should pay, are defined by a respective ministerial decision (20). A separate clawback mechanism is in force from 2016 to 2018 for the total amount of pharmaceutical expenditure of state hospitals and for hospital medicinal products dispensed from EOPYY pharmacies, which are not included in EOPYY's clawback.
The current procedure for the reimbursement of medical devices includes the application of the marketing authorization holder to EOPYY. After a positive recommendation by the Managing Board of EOPYY, new medical devices can be reimbursed after a subsequent ministerial decree. Diagnostics are reimbursed after a ministerial decree following a positive recommendation by KESY.
DISCUSSION
Currently, Greece does not have a formal process for evaluating health technologies in medical devices or medicinal products. The current situation has the advantages of easy access to medicinal products for human use on the market, long periods up to disinvestment, and gathering of big data on medicinal products consumption by a unique source. Disadvantages of the current system are the lack of strategy for alignment/data exchange between the various Organizations/Committees and the nonexistence of a concrete, systemic, and transparent reimbursement framework. More specifically, the inclusion of products on the Positive Reimbursement List with current procedure does not allow for the implementation of efficient horizon scanning which by default is performed to identify products eligible to undergo an HTA procedure.
Additionally, prospective budget planning, efficient planning for design and implementation for managed entry agreements (MEAs), and development of strategic procurement methods are not feasible without the existence of HTA. Furthermore, the delay in the usage of the HTA process leads to a high amount of clawback, which does not take innovation into account and lacks connection between the price and the value of medicinal products for human use.
To implement an HTA procedure, the following three main pillars should be established: (a) development of a legislative framework, (b) capacity building, and (c) financing research, process, and organizational structure for HTA (Table 2). Recent legislation (Reference Vandoros and Stargardt12) states that, up to December 2017, authorities should establish an HTA center, which will contribute to the process of inclusion of medicinal products for human use on the Positive Reimbursement List. A committee has been set up to provide recommendations for the HTA center. However, the progress of this committee has not been documented yet. Moreover, a negotiation committee has been established in EOPYY to negotiate reimbursed prices of medicinal products for human use among other health services (21).
Table 2. Main Barriers and Facilitators for Introducing HTA in Greece
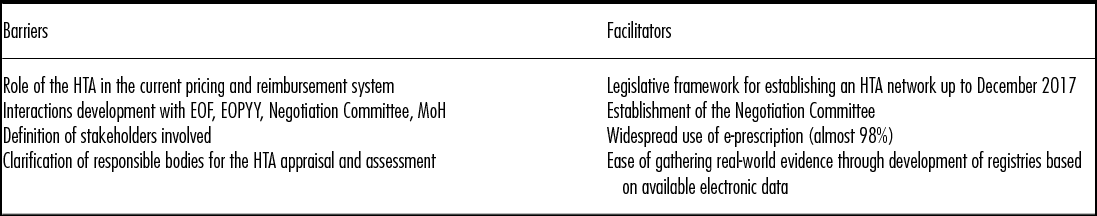
HTA, health technology assessment; EOF, National Drug Organization; EOPYY, Reimbursement Fund; MoH, Ministry of Health.
There is still need to define through legislation the following issues: The position of the HTA in the current regulatory framework and the establishment of the interactions with current existing jurisdictions like the Positive Reimbursement List, the Negotiation Committee, EOPYY, and the MoH. HTA should take place either during marketing authorization or during pricing, and considered when issuing the Positive Reimbursement List. Additionally, the MEAs should only be used when HTA identifies issues or concerns about key outcomes and/or costs and/or organizational/budget impacts that are subject to a reimbursement decision (Reference Klemp, Frønsdal and Facey22).
It is necessary to define the goals of the introduction of HTA, which in the case of Greece, could be the maximization of health benefits in a given budget by improving the quality of care. The decision-making system, according to current legislative framework, is set on insurance system level, but should probably be extended to a system level that includes all health services provided. First, the scope could include only new high-cost categories of medicinal products for human use, but potentially it could be expanded to include medical devices (for out-patients at first, and in-patients as a second phase) and nutrition for specific medical purposes (e.g., for patients with cancer, metabolic syndromes, etc.).
The mechanism of financing and delivery should clarify the extent to which doctors are eligible to prescribe and to that which patients could claim reimbursement against HTA recommendations. The context of the HTA (in terms of social principles) should be clarified, meaning how does Greek society weigh “public good” against the requests and needs of individual patients within a fixed budget. It should be clarified that there is universal health coverage in Greece (23). All stakeholders and their role in the HTA process should be defined. Therefore, the involvement of patients (Reference Gagnon, Candas and Desmartis24) and physicians in the HTA process is considered important to ensure acceptance of the HTA outcome.
As HTA is a two-step procedure including assessment and appraisal, legislation should define responsibilities and procedures. In the case of the Hellenic system, the assessment could be performed by a network of experts or local universities with relevant expertise. However, the appraisal and final recommendations should rely on the legal entity responsible for the budget and capable of assessing the budget impact (i.e. the EOPYY). It is suggested that methods used should focus, first, on the clinical-added value as assessed by the Transparent Value Framework (25) and on budget impact. Cost-utility analysis or the broader multiple criteria decision analysis (Reference Marsh, IJzerman and Thokala26) could be introduced, with the latter considered as more explicit and transparent regarding the inclusion of stakeholders’ judgments.
In that respect, in cases where data from randomized controlled trials are not available, the country could take advantage of the widely used e-prescription system (almost 98 percent penetration). This system contains info on unique patients, age, sex, ICD-10, active substances, brand names, quantities, and also includes a business intelligence system that has the ability to create standard and on-demand queries. Since September 2015, a patient registry for hepatitis C has been in place, collecting data from all EOPYY patients receiving the new direct-acting antivirals as a prerequisite for reimbursement. Recently, in March 2016 a patient registry for chronic myelogenous leukemia has also started to collect data.
Mapping of the available capacity in the country (government, health system, universities, professional and/or guidelines organizations, and patient bodies) to perform collection, analysis, appraisal, implementation of decisions, and development of respective clinical guidelines should be performed. At the same time, to maintain a sustainable system, incentives should be given to government personnel involved in the HTA procedure to remain in the public system rather than join the industry. A specific HTA budget should be allocated for the production of HTAs, as well as the respective training and technical resources (Reference de Labry Lima, Mochon, Martínez, Ruiz and Balbino27). Finally, by participating in EUnetHTA Joint Action 3, Greece could benefit, particularly from the transferability of the results in local settings from joint EU initiatives in HTA (Reference Heintz and Gerber-Grote28).
CONCLUSIONS
Greece has the opportunity to rapidly build capacity, implement, and take advantage of the HTA process by defining the goals, scope, systems, context, stakeholders, and methods that will be used in the local HTA process, taking into account the established e-prescription system and the recently adapted legislative framework.
CONFLICTS OF INTEREST
All of the authors are employed by a public health insurance fund. However, their views do not necessarily reflect those of the organization that employs them.





