Down syndrome (DS), also known as trisomy 21 (T21), is the most common genetic cause of significant intellectual disability (Reference Patterson1). It occurred in approximately 1.7 per 1,000 babies born in 2006 in the world, according to the International Clearinghouse for Birth Defects Surveillance and Research (2). Many countries monitor the incidence of DS using birth defect surveillance systems. China is a populous country with a birth rate of 12.1 per 1,000 people in 2012. A report indicates that the incidence of DS was approximately 1.47 per 1,000 babies born in 2012, which was equivalent to 23,000 to 25,000 new cases every year in China (3). According to a 2003 study, the average life-time economic burden of a new DS case, from the family perspective and the societal perspective, amounted to US dollar (USD) 47,000 and 55,000, respectively, in China (Reference Chen, Qian, Zhang, Li, Chu and Schweitzer4).
Currently, there is no cure for DS, but it can be detected during pregnancy by prenatal screening technology, followed by invasive diagnostic testing if the result of the screening is positive. Conventional maternal serum screening (CMSS) using several blood markers, such as α-fetoprotein (AFP), unconjugated estriol (uE3), human choionic gonadotophin (hCG), etc., can be used to estimate or predict the risk of DS during the first or second trimester and are usually combined with ultrasound technology by the indictor of nuchal translucency (NT) in the first trimester. CMSS in China typically incorporates measurements of serum AFP, hCG and uE3 in the second trimester, which is also called triple screening, and has a detection rate of approximately 69 percent with a 5 percent false positive rate (FPR) in the general pregnancy population (Reference Wald, Kennard, Hackshaw and McGuire5;Reference Alldred, Deeks, Guo, Neilson and Alfirevic6). CMSS could also assist to detect non-DS aneuploidies like neural tube defects (Reference Wortelboer, Koster, Stoutenbeek, Loeber, Visser and Schielen7).
The recent introduction of non-invasive prenatal testing (NIPT), which analyzes cell-free DNA (cfDNA) in maternal plasma and uses next-generation sequencing technology, has improved the detection rate of DS and has been shown to have a very low FPR (Reference Palomaki, Deciu and Kloza8;Reference Norton, Brar and Weiss9). Furthermore, DS could be identified as early as 10 weeks of gestation, and it can avoid miscarriages induced by invasive diagnostic testing.
NIPT has been implemented in the United States and some other countries since late 2011, and several guidelines have been established since 2012 such as those by the International Society for Prenatal Diagnosis, American College of Medical Genetics and Genomics, and Italian College of Fetal Maternal Medicine (Reference Benn, Borell and Chiu10–Reference Gregg, Gross and Best12). These guidelines have several common recommendations: First, NIPT is just a prenatal screening technology rather than a diagnostic method, so a more invasive diagnostic test such as amniocentesis is still required to confirm the positive results of NIPT. Second, informed consent and genetic counseling to pregnant women is highly recommended to ensure the quality of screening. Further studies are still needed to evaluate the effectiveness and safety of NIPT.
In China, the use of NIPT has been controversial. NIPT was once provided by genetic companies, sometimes without involving health care providers, and the “business to customer model” was hailed as an innovative business approach (Reference Zhang and Li13). But the provision of NIPT service without prior genetic counseling might give rise to some clinical and ethical issues like sex selection, paternity testing, and consent issues. Regulations governing NIPT in China were generally absent before 2014, with many blood samples tested without oversight and without necessary genetic counseling, leading to overuse or misuse. Therefore, the clinical use of NIPT was “called off” by the central government in 2014. Afterward, the National Health and Family Planning Commission (NHFPC) initiated a “pilot project” (14) in 2015, in which 108 hospitals were allowed to conduct NIPT on a trial basis. During that time period, there was little information regarding NIPT's effectiveness, safety, and cost-effectiveness for policy and decision making in China.
This study is intended to assess the cost-effectiveness of NIPT for DS in China from a societal perspective to provide evidence-based information to decision makers for promoting rational use of NIPT and improving population health and quality of life.
Methods
Literature Review and Field Survey
A systematic review of the literature was carried out to gain an understanding of the cost-effectiveness of NIPT for DS. The literature search focused on collecting effectiveness data in relation to NIPT and CMSS.
Three provinces and one centrally administered municipality (Hunan Province, Zhejiang Province, Shandong Province, and City of Shanghai) were selected in eastern and central regions of China as study sites for the field survey, based on the number of medical institutions hosting a NIPT pilot project and the level of economic development of the location. Twenty-five pilot hospitals in the four locations were surveyed in 2015. Relevant data were collected by means of a hospital questionnaire which included questions regarding volume of health services and prices of NIPT and CMSS administered during the second trimester. Interviews with physicians and experts were also conducted during the field survey to provide additional information about the current status of NIPT use in their hospitals.
Economic Evaluation
A cost-effectiveness analysis was designed to evaluate NIPT and CMSS from a societal perspective in a simulated cohort of 10,000 pregnant women.
Decision Tree Model
A decision-tree-analytic model was developed to compare three different strategies applied to a simulated cohort of 10,000 pregnant women: (i) “CMSS strategy”: use triple screening for pregnant women in the second trimester, which is the current clinical practice in China, followed by amniocentesis only for women tested positive after triple screening. (ii) “Contingent screening strategy”: implement NIPT as an optional secondary screening for women tested positive by CMSS, and then with amniocentesis for those tested positive by NIPT. (iii) “Universal screening strategy”: use NIPT as the primary screening test, replacing CMSS.
Five health outcomes were included in the model: DS averted (DS diagnosed by amniocentesis and the pregnancy terminated following counseling and with informed consent), DS live birth, fetal loss due to amniocentesis, spontaneous miscarriage, and non-DS normal live birth.
Assumptions
Costs and outcomes were estimated based on several assumptions: (i) All women consented to undergo NIPT when women tested positive by CMSS for contingent screening strategy. (ii) All CMSS-“positive” or NIPT-“positive” women had an additional genetic counseling session with their doctors. (iii) All NIPT-“positive” women consented to undergo amniocentesis. (iv) All women identified as CMSS-“negative”, NIPT-“negative” or without amniocentesis abnormalities continued with their pregnancy and had no further prenatal screening.
Determination of Costs and Effectiveness
Cost measurements and valuation
From the societal perspective, the price of health services obtained at medical institutions was calculated based on the charges set by the local government. This study treated the price of health service as cost because it was paid by the medical insurance authority and the pregnant woman. Direct medical costs included the costs of NIPT, CMSS, genetic counseling, amniocentesis, and termination of pregnancy if DS was diagnosed and requested by woman. Direct nonmedical costs and nondirect costs were not included in this study. All costs were measured within a 1-year period and, therefore, discounting was not needed. Costs in Chinese yuan were converted into USD at the 2015 exchange rate of 6.50 yuan = USD 1.00.
Effectiveness and Safety Index
The indicator of effectiveness of these strategies was the number of DS averted. The safety of the strategy, also called safety index, was measured by the number of fetal losses due to amniocentesis per DS averted. The smaller the safety index, the safer was the strategy (Reference Wald, Kennard, Hackshaw and McGuire15).
Cost-Effectiveness Analysis
The cost-effectiveness ratios of these strategies were compared in terms of the cost per DS averted. Incremental cost-effectiveness ratio (ICER) was calculated to demonstrate additional effectiveness resulting from marginal additional costs. Because ICER thresholds were unavailable in China, there could be difficulties interpreting the study results.
Sensitivity Analysis
One-way sensitivity analysis was conducted to evaluate the robustness of the results. The key factors that influenced the results were identified and allowed to vary to see how sensitive the results were to each of these variables.
Results
Model Parameters
Supplementary Figure 1 showed the decision tree model for the CMSS strategy, contingent screening strategy and universal screening strategy. The model variables were mainly derived from the literature review and field survey (Table 1).
Table 1. Main Model Parameters and Sources
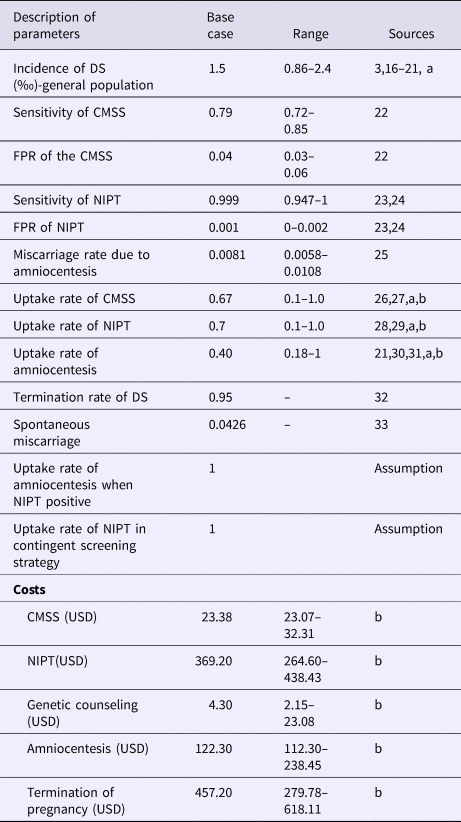
a, expert opinion/consultation; b, data from field survey; CMSS, conventional maternal serum screening; DS, Down syndrome; FPR, false-positive rate; NIPT, non-invasive prenatal testing.
Simulated Effectiveness
In a cohort of 10,000 pregnant women, the decision tree model showed that the CMSS strategy, contingent screening strategy and universal screening strategy could prevent 3.02, 7.53, and 9.97 DS births, respectively. In terms of reducing DS births, the universal screening strategy was the most effective. The CMSS strategy, contingent screening strategy and universal screening strategy could lead to 0.89, 0.07, and 0.14 fetal losses due to amniocentesis, and the safety indices for these strategies were 0.296, 0.009, and 0.014, respectively. Therefore, from the perspective of safety, the contingent screening strategy was the best (Table 2).
Table 2. Simulated Effectiveness of Three Strategies in a Cohort of 10 000 Pregnant Women

CMSS, conventional maternal serum screening; DS, Down syndrome; safety index, is measured by the number of fetal losses due to amniocentesis per DS averted.
One-way sensitivity analysis demonstrated that the incidence rate of DS, the uptake rate of CMSS and the uptake rate of NIPT could affect the effectiveness of these strategies. If the uptake rate of CMSS was over 88.6 percent, with other factors held constant, the contingent screening strategy would detect more DS than the universal screening strategy. If the uptake rate of NIPT was over 52.9 percent, the universal screening strategy can detect more DS than the contingent screening strategy. Other parameters had no impact on effectiveness, including the sensitivity of CMSS or NIPT, the FPR of CMSS or NIPT, miscarriage rate due to amniocentesis, etc. (Supplementary Table 1).
Cost-Effectiveness Analysis of the Strategies
The cost-effectiveness ratios (cost per DS averted) of the CMSS strategy, contingent screening strategy, and universal screening strategy were USD 57,385, 35,075, and 263,073, respectively. The cost-effectiveness ratio of the contingent screening strategy was the lowest. Compared with the CMSS strategy, the incremental cost-effectiveness ratio of the contingent screening strategy was USD 20,160, while the incremental cost-effectiveness ratio of the universal screening strategy was USD 352,388 (Table 3).
Table 3. Cost-Efectiveness Analysis of These Strategies

CMSS, conventional maternal serum screening; ICER: incremental cost-effectiveness ratio, ICER = (Costs1-Costs2)/(Effectiveness1-Effectiveness2).
The one-way sensitivity analysis showed that there were many factors influencing the economics of these strategies, including the cost of NIPT, the uptake rate of amniocentesis and the incidence of DS. On the other hand, other parameters were shown to have little impact on cost-effectiveness, including the uptake rate of CMSS, the FPR of CMSS or NIPT, the sensitivity of CMSS or NIPT, etc. One-way sensitivity analysis showed that if the cost of NIPT could be decreased to USD 76.92, the cost-effectiveness ratio of the universal screening strategy would be lower than the CMSS strategy in China (Supplementary Table 2).
Discussion
Cost-Effectiveness of NIPT in China and International Experiences
This study compared different strategies in relation to DS, including CMSS and the new NIPT in China. The universal screening strategy was the most effective for it could detect more DS cases and the contingent screening strategy was the safest and most cost-effective, compared with CMSS.
Although there is limited evidence-based information about the cost-effectiveness of NIPT in China to date, there are several economic evaluations of NIPT implementation in the United States, Australia, and some European countries. Literature reviews have revealed the following findings.
First, a comparison of the universal NIPT screening strategy with CMSS in some studies have indicated that NIPT might not totally replace CMSS because of the former's higher price (Reference Xu, Li and Ming34). But in some circumstances, it was estimated that NIPT could be a cost-effective replacement for CMSS when the lifetime costs of DS live births were taken into consideration (Reference Walker, Jackson, La Grave, Ashwood and Schmidt35). Thus, from a societal perspective instead of a narrower perspective of a payer, universal NIPT could be a cost-effective alternative to CMSS (Reference Walker, Nelson, Jackson, Grenache, Ashwood and Schmidt29). However, NIPT as a primary screening strategy would not be recommended at its current price level (Reference Fairbrother, Burigo, Sharon and Song36).
Second, using NIPT as an optional secondary screening has been recommended, but should not be used as the sole diagnostic tool. Several studies (Reference Cuckle, Benn and Pergament20;Reference Walker, Nelson, Jackson, Grenache, Ashwood and Schmidt29;Reference Neyt, Hulstaert and Gyselaers37–Reference O'Leary, Maxwell, Murch and Hendrie42) showed that implementing NIPT as an optional secondary screening for pregnancies at high risk of DS would be more cost-effective than CMSS. Beulen et al. (Reference Beulen, Grutters, Faas, Feenstra, van Vugt and Bekker43) indicated that none of these prenatal screening strategies was clearly dominant. Although CMSS was the least costly, NIPT could increase T21 detection rate and simultaneously decrease the risk of invasive procedure-related miscarriages. When viewed from a government or payer perspective, contingent NIPT provides a cost-effective alternative to CMSS (Reference Walker, Nelson, Jackson, Grenache, Ashwood and Schmidt29). NIPT as an optional secondary screening could be more cost effective and lead to far fewer losses of normal fetus but should not be used as a prenatal diagnostic technology to detect DS (Reference Ohno and Caughey28). The Society of Obstetricians and Gynecologists of Canada issued a policy statement in 2013, which supported the use of NIPT as an option only among “high risk” women (Reference Langlois and Brock44).
Costs of NIPT in China and Its Influences
Although many people have mentioned the advantages of NIPT, this prenatal test is still expensive, particularly for people in lower-income countries such as China. One-way sensitivity analysis showed that if the cost of NIPT could be decreased to USD 76.92, the cost-effectiveness ratio of the universal screening strategy was lower than the CMSS strategy. However, there was no uniform pricing of NIPT in China because pricing appeared to be market-based. After nearly 1 year of NIPT pilot testing in 2015, several provincial governments separately set prices for NIPT, such as USD 369.23 per case in Sichuan Province, USD 340 in Jiangsu Province, USD 262.31 in Guangdong Province, etc. In December 2016, the price of NIPT was set at USD 200 per case in Zhejiang Province, which is the lowest price in China to date. But there are indications that the price of NIPT will decrease further. If this turns out to be true, the cost-effectiveness of NIPT will become more evident when it is used as a contingent screening strategy and its cost-effectiveness will be even stronger if used as a primary screening strategy.
We did not include the costs of DS live birth and non-DS normal live birth in our study design and the decision tree model. There is also an argument if we do not consider the cost of live birth and DS live birth, then the cost of pregnancy termination for DS detected should also be excluded. We have fully considered this and did an alternative analysis by excluding the cost of pregnancy termination for DS detected. The results showed that the CER of the contingent screening strategy was still the lowest and, thus, did not contradict our original findings. As well, according to our knowledge, we would like to defend that pregnancy termination following DS detection is one component of screening intervention. Because the women could not have a healthy baby or baby with DS due to termination procedure, we should include the costs of termination in the analysis. Therefore, details of the alternative analysis and its results are not presented here.
Based on literature review, we used 40 percent as the amniocentesis uptake rate after CMSS-positive detection and hypothesized a 100 percent uptake rate of amniocentesis after NIPT-positive detection. This was based on the fact that compared with the gold standard of amniocentesis (100 percent accuracy rate of DS diagnosis), the accuracy of NIPT and CMSS are 99.9 percent and 79 percent, respectively. Accordingly, we assumed that pregnant women were more likely to undergo amniocentesis after NIPT-positive. In addition, relative to CMSS, NIPT is less likely to produce false-positives and is, therefore, more reliable. Because the accuracy of CMSS is only 79 percent, when pregnant women were screened positive, false positive rate and safety challenges made amniocentesis uptake rate not very high in China (Reference Jin, Wu and Jiang45). Further research on such assumptions are needed.
Policy Implications
First, the price and the uptake rate of NIPT could influence the cost-effectiveness ratio of NIPT in this study. A study (Reference Verweij, Oepkes, Vries, van den Akker, van den Akker and de Boer46) showed that more than half of the pregnant women who had rejected prenatal screening had a positive attitude toward NIPT for T21 and would undergo NIPT if it were available. In addition, the unaffordability of NIPT also could adversely affect its uptake rate. Thus, if the wider use of NIPT in DS detection is an adopted national strategy, setting an appropriate price level for NIPT is a necessary first step.
Second, the wider use of NIPT needs to be discussed in conjunction with ethical considerations, such as consequences of incorrect results (e.g., false positives), sex selection, paternity testing, and consent issues. A study (Reference Ming, Zhou and Xu47) reviewing the ethical considerations of NIPT for DS raised several issues such as information overload and sensitive information, elective abortion and welfare of the unborn, and lack of informed consent. So, the ability of physicians to carry out NIPT should be assessed and opportunities for physicians, as well as other health workers, to learn more about NIPT and to enhance their genetic counseling skills should be made available. There is also a need to inform pregnant women about the use and limitations of NIPT and to obtain their informed consent.
Limitations
This study tried to use real world data from China as much as possible, but the analysis was limited by the current unavailability of some evidence. Most base variables were retrieved from the literature, and some base parameters like women's preferences and NIPT uptake rates are extrapolated from studies conducted in other countries. Nevertheless, our sensitivity analysis showed that the model was robust over a wide range of values for most variables.
In conclusion, at the present time, contingent screening using NIPT may be an appropriate DS-prevention strategy for pregnant women in China, and possibly in other developing nations, as a way to balance the effectiveness and cost factors of this new genetic testing technology. Although NIPT could not replace CMSS because of its higher cost, better sensitivity of CMSS and, with the assistance of NIPT, higher uptake rate of amniocentesis when CMSS screened positive would help reduce the cost per DS averted. Further economic evaluation studies need to be conducted to confirm our findings. Additionally, further investigations are necessary when NIPT is more widely used as a first-line screening strategy for DS among pregnant women.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266462319000308.
Conflicts of interest
This study has no conflict of interest.





