Over the past few decades, the risk of occurrence of invasive fungal infections (IFIs) has risen (Reference Richardson1;Reference Abu-Elteen2). This alarming trend is explained by the increasing size of the population at risk (e.g., patients with compromised immunity), due to prolonged patient lifespan related with several factors, as improved patient management and the implementation of novel drugs (Reference Richardson1–Reference Lass-Florl, Griff and Mayr3). However, IFIs are still associated with high morbidity and mortality rates, as well as elevated health expenditure (Reference Richardson1;Reference Menzin, Meyers and Friedman4).
Amphotericin B deoxycholate (d-AmB) is an antifungal therapy used extensively to treat IFIs, but is commonly associated with the development of nephrotoxicity (Reference Berdichevski, Luis, Crestana and Manfro5;Reference Deray6). As a result, lipid-associated formulations of d-AmB have been developed as novel technologies with equivalent efficacy compared with conventional d-AmB, but with a superior safety profile (Reference Wingard, White and Anaissie7;Reference Fleming, Kantarjian and Husni8). The best known lipid formulations of d-AmB include amphotericin B lipid complex (ABLC) and liposomal amphotericin B (L-AmB) (Reference Lemke, Kiderlen and Kayser9). The additional lipid components act as drug delivery systems by permeating the target fungal cell wall, while remaining closely associated with the liposomes in the circulation, thereby reducing the potential for nephrotoxicity and infusion-related toxicity associated with conventional d-AmB (Reference Moen, Lyseng-Williamson and Scott10).
Both aforementioned lipid formulations are active against clinically relevant yeasts and molds (including Candida spp. and Aspergillus spp.), and are approved for the treatment of IFI in many countries worldwide (Reference Hamill11). L-AmB and ABLC also present similar rates of treatment responses and safety outcomes, as reported by two systematic reviews, including no statistically significant differences regarding associated-nephrotoxicity (Reference Safdar, Ma and Saliba12;Reference Tonin, Steimbach and Borba13).
Despite the clinical advantages of lipid formulations, many countries faced with budgetary constraints have restricted the use of high-cost antifungal therapies like L-AmB and ABLC to certain situations (Reference Stoll, Cola, Splitt and Moreira14). This includes circumstances when d-AmB is not indicated, such as patients with refractory infection after the use of d-AmB, patients presenting renal impairment before antifungal treatment, or patients presenting unacceptable d-AmB toxicity. Additionally, there is a significant acquisition cost difference between formulations, with the cost of L-AmB exceeding that of ABLC (Reference Yang, Chaudhari and Zhou15;16). However, the optimal choice between the two lipid formulations is still not well established for this subgroup of patients, nor were they the focus of any reviews available in the literature.
This systematic review, therefore, aims to compare effectiveness and safety outcomes between ABLC and L-AmB in patients with d-AmB refractory infection, previous renal impairment, or unacceptable d-AmB toxicity.
METHODS
This systematic review was performed in the databases Medline (Ovid), Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE (Ovid), and LILACS (Virtual Health Library) in January, 2017. The search strategy combined terms related to “liposomal amphotericin B” and “amphotericin B lipid complex” and are further described in supplementary Appendix 1. Because LILACS is a Latin American database, search terms were also translated into Spanish and Portuguese. No filters regarding publication period or language were applied. A manual search of all references was also performed. Principal authors were contacted to obtain missing information and any additional published or unpublished trials.
Eligibility Criteria
The study population was defined as patients with IFI who were not eligible for d-AmB treatment due to d-AmB refractory infection, previous renal impairment, or unacceptable d-AmB toxicity. Studies must include both ABLC and L-AmB treatment, and evaluate at least one of the following outcomes: time to fever resolution, therapy response, length of hospital stay, new-onset dialysis, mortality, and incidence of adverse events, with particular emphasis on the incidence of nephrotoxicity. Only randomized clinical trials (RCT) and observational cohorts were considered for this review. Additionally, inclusion criteria encompassed any year of publication, length of follow-up, or language.
Studies were excluded if they presented other types of study design, did not include patients with IFI, did not include the population of interest, did not include both types of lipid formulation, or did not contain any of the above mentioned outcomes.
Data Extraction and Analysis
Titles and abstracts were independently screened by two investigators and any disagreement was resolved by consensus. Articles listed for full manuscript review were once again evaluated for inclusion independently. Articles included were then summarized in an Excel spreadsheet in duplicate by the two investigators, listing the authors, year of publication, study design (observational or RCT), population, indication of use, definition of nephrotoxicity, number of patients per group, and the studies main results based on the outcomes of interest.
There was insufficient homogeneity between studies to allow a quantitative or meta-analytic approach. Therefore, data retrieved were presented using a critical and descriptive assessment.
Quality of Evidence
The quality of evidence was evaluated using Grading of Recommendations Assessment, Development and Evaluation (GRADE) by two reviewers, independently (Reference Balshem, Helfand and Schunemann17). The GRADE system provides guidance and decision criteria to be used when judging the certainty of evidence. The evidence of each outcome can be classified as high (high confidence that the true effect lies close to that of the estimate of the effect), moderate (moderate confidence in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different), low (limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the effect), or very low (very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect).
RESULTS
A total of 1,054 abstracts were retrieved from the search, of which eleven were selected for full-text reading (Figure 1). Six articles included a broad selection of patients (did not meet the population of interest criteria), and were excluded.

Figure 1. Flow diagram of included studies (adapted from PRISMA).
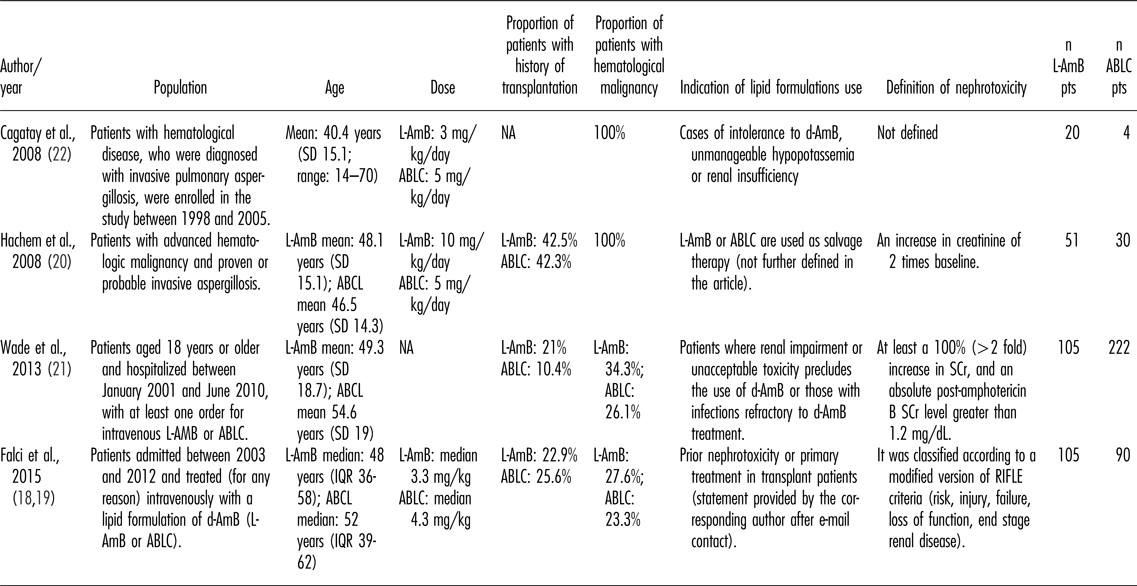
Finally, five articles matched the inclusion criteria, with two of them being different publications from the same patient cohort (Reference Falci, Da Rosa and Pasqualotto18;Reference Falci, Da Rosa and Pasqualotto19). All included articles were observational studies, published from 2008 to 2015, and were conducted in the United States of America (n = 2), Turkey (n = 1), and Brazil (n = 2). Two studies targeted only patients with hematologic malignancies, while the remained studies included a broader patient population and defined as inclusion criteria patients who were admitted to a hospital within a determined timeframe. Other main characteristics are described in Table 1.
Table 1. Descriptive Characteristics of the Included Studies

ABLC, amphotericin B lipid-complex; d-AmB, amphotericin B deoxycholate; L-AmB, liposomal amphotericin B; IQR, interquartile range; SCr, serum creatinine; SD, standard deviation.
Three studies evaluated differences in patient baseline characteristics among treatment groups that could lead to skewed results. Hachem et al. (Reference Hachem, Boktour and Hanna20) found that patients treated with ABLC and L-AmB presented similar age, gender, type of underlying malignancies, and neutropenia rate. The only difference between each group was the proportion of patients that used interferon during infection (p = .02). Conversely, Wade et al. (Reference Wade, Chaudhari and Natoli21) found that patients who received ABLC were significantly older (p = .02), had a higher proportion of African American participants (p = .02), had a higher proportion of urgent/emergent hospital admission (p < .01), had lower proportion of major solid organ transplantation (p < .01), had lower proportion of stem cell transplant (p = .03), and had lower proportion of nephrotoxic drug exposure before encounter (p = .02). Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) reported differences between the three treatment groups (d-AmB, L-AmB, and ABLC), but did not report each individual comparison between treatment groups. Therefore, it was not possible to evaluate whether the findings were determined only by differences between L-AmB and ABLC groups.
The main findings are summarized below and in Table 2.
Table 2. Summary of the Studies Main Outcomes per Treatment Group

ABLC, amphotericin B lipid-complex; CR: complete response; HD: high dose; L-AmB, liposomal amphotericin B; LD: low dose; LHS, length of hospital stay; NA, data not available; PAB, post amphotericin B; PR: partial response; SCr, serum creatinine.
*p-Values refer to any difference between d-AmB, L-AmB, or ABLC groups.
Time to Resolution of Fever
Only the study conducted Cagatay et al. (Reference Cagatay, Cosan and Karadeniz22) reported this outcome. The authors found that for L-AmB treatment, the time to resolution of fever (5.6 days; standard deviation [SD]: 5 days; n = 20) was slightly lower than ABLC treatment (8 days; SD 4.7 days; n = 4). However, this difference was not found to be statistically significant (p > .05).
Therapy Response
Cagatay et al. (Reference Cagatay, Cosan and Karadeniz22) and Hachem et al. (Reference Hachem, Boktour and Hanna20) evaluated the response to antifungal therapy. The complete response was broadly defined in the two studies as resolution or major improvements of signs and symptoms of infection, as well as radiologic changes of active infection. Whereas, partial response was defined as stability or partial improvement of radiologic changes.
Cagatay et al. (Reference Cagatay, Cosan and Karadeniz22) did not describe the proportion of complete remission per treatment group, the authors reported that it was achieved by fifty-four patients (58.1 percent). However, among those patients, thirty-nine died either during the therapy or in the 1-month period after the end of antifungal therapy.
In the study conducted by Hachem et al. (Reference Hachem, Boktour and Hanna20), the proportion of patients who achieved complete response in the L-AmB group (14 percent; n = 3/21) was higher than patients treated with ABLC (8 percent; n = 1/13) when high dose of lipid formulations was administered. However, an opposite trend was observed at lower doses (8 percent L-AmB group versus 20 percent ABLC group). Despite this finding, the study did not evaluate whether there was a statistically significant difference in the proportion of complete response between patients among treatments.
Length of Hospital Stay
Wade et al. (Reference Wade, Chaudhari and Natoli21) and Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) evaluated the length of hospital stay. Both studies showed a nonsignificant trend for patients treated with L-AmB to remain in the hospital for shorter periods in comparison with patients treated with ABLC (approximately 26 days for L-AmB patients and 30 and 35 days for ABLC). The same trend was observed by Wade et al. (Reference Wade, Chaudhari and Natoli21) when evaluating the length of stay post amphotericin B (time of the first amphotericin B order until hospital discharge).
Additionally, this variable was examined using a multivariate analysis, which showed that ABLC was not significantly associated with a different hospital length of stay when using L-AmB as reference (β-coefficient: 0.894; p = .27). The analysis included the following covariates: percentage of the treatment period during which nephrotoxic agent(s) was administered, medical diagnosis-relate group, current or prior heart failure, number of antibiotics classes, use of voriconazole before AmB, bacterial isolate present, and baseline serum creatinine (SCr).
Nephrotoxicity
Definition
The stated definition of nephrotoxicity varied among studies. Wade et al. (Reference Wade, Chaudhari and Natoli21) defined nephrotoxicity as at least a 100 percent (N2-fold) increase in SCr, and an absolute postamphotericin B SCr level greater than 1.2 mg/dl, as per Wingard et al. (Reference Wingard, White and Anaissie7). Hachem et al. (Reference Hachem, Boktour and Hanna20) defined it as an increase in SCr of 2 times baseline. Alternatively, renal impairment was classified by Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) according to the RIFLE criteria, which is divided in five categories: acute kidney risk (SCr increases 1.5–2 times the baseline value or glomerular filtration rate (GFR) decreases >25 percent); injury (SCr increases 2–3 times the baseline value or GFR decreases >50 percent); failure (SCr increases >3 times the baseline value or GFR decreases >75 percent or SCr >4 mg/dl); loss of function (persistent acute renal failure; complete loss of kidney function >4 weeks (requiring dialysis); and end-stage kidney disease (complete loss of kidney function >3 months (requiring dialysis). Cagatay et al. (Reference Cagatay, Cosan and Karadeniz22) did not present a clear definition.
Incidence of Nephrotoxicity
The incidence of nephrotoxicity varied largely in the two studies that evaluated only patients with hematologic malignancies (Reference Hachem, Boktour and Hanna20;Reference Cagatay, Cosan and Karadeniz22). The nephrotoxicity incidence ranged from 0 percent to 6 percent in patients treated with L-AmB, and from 0 percent to 10 percent in patients treated with ABLC (Reference Hachem, Boktour and Hanna20;Reference Cagatay, Cosan and Karadeniz22). However, SCr baseline levels were not reported in these studies and the different proportions between groups was not found to be statistically significant in an unadjusted analysis (p = .7)(Reference Hachem, Boktour and Hanna20).
Conversely, Wade et al. (Reference Wade, Chaudhari and Natoli21) found differences in the nephrotoxicity incidence between groups. The authors reported a nephrotoxicity incidence more than twice as common in patients treated with ABLC versus L-AmB (22.6 percent, n = 38/168 versus 10.6 percent, n = 9/85), a difference considered to be statistically significant in the unadjusted analysis (p = .02). Notably, the SCr levels were reported to be the same in both treatment groups at the pretreatment stage (1.5 mg/dl). Additionally, a multivariate analysis endorsed these findings and showed that the odds ratio (OR) of developing nephrotoxicity was 3.48 (95 percent confidence interval [CI], 1.05–11.52; p = .041) higher for ABLC patients in comparison with patients treated with L-AmB (23). The covariates included in the multivariate analysis were presence of lung disorders (e.g., alveolitis, pneumonitis), chemotherapy during index encounter, platelet count lower than 100 × 103/mm3 within 48 hours of admission, critical care admission, age, total exposure to AmB, and hypertension.
Although Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) described the proportion of patients treated with L-AmB and ABLC who develop nephrotoxicity, the authors did not directly analyze the difference between lipid formulations. The analysis informed any differences between groups, including patients treated with d-AmB. Therefore, it was not possible to draw conclusions regarding differences between formulations, despite their incidence being shown as similar. The authors also performed a multivariate analysis which found L-AmB treatment to be an independent protective factor for severe nephrotoxicity (OR, 0.18; 95 percent CI, 0.003–0.64; p = .006), but not ABLC (OR, 0.47; 95 percent CI, 0.15–1.25; p = .136). Both analyses used d-AmB as reference and, therefore, did not directly compare L-AmB with ABLC.
New-Onset Dialysis
No significant difference between L-AmB and ABLC treatment groups were found in the analysis presented by Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) and Wade et al. (Reference Wade, Chaudhari and Natoli21), which measured new-onset dialysis. Considering only patients with previous nephropathy, Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) found the proportion of patients who required dialysis to be larger than the overall cohort (21.7 percent L-AmB versus 26 percent ABLC), but also not statistically different between groups (p = .794). Moreover, the authors found evolution to dialysis to be a statistically significant factor associated to in-hospital mortality in a multivariate analysis (OR, 6.24; 95 percent CI, 2.93–14.42).
Other Safety Outcomes
Hepatotoxicity incidence was reported by two studies. Cagatay et al. (Reference Cagatay, Cosan and Karadeniz22) observed that only one patient treated with L-AmB developed hepatic failure (5 percent). Hachem et al. (Reference Hachem, Boktour and Hanna20) observed a higher proportion of cases (17.6 percent L-AmB, n = 9/51 versus 10 percent, n = 3/30 ABLC), although no significant differences existed between treatment groups (p = .52). Regarding infusion-related reactions, Wade et al. (Reference Wade, Chaudhari and Natoli21) reported, in an unadjusted analysis, that these events occurred with a significantly higher proportion in patients who received ABLC treatment (L-AmB 9.5 percent, n = 10 versus ABLC 23.9 percent, n = 53; p < .01).
Additionally, the incidence of hematological toxicity was assessed by Falci et al. (Reference Falci, Da Rosa and Pasqualotto19) in patients treated with d-AmB, L-AmB, and ABLC. Overall, the authors found that there was no significant difference in the occurrence of severe anemia, severe leukopenia, and severe thrombocytopenia between treatments.
Mortality
A considerable rate of mortality was observed among all studies, particularly in Hachem et al. (Reference Hachem, Boktour and Hanna20), that evaluated patients with hematological malignancies. Wade et al. (Reference Wade, Chaudhari and Natoli21) found no difference in mortality between the treatment groups in an unadjusted analysis (L-AMB 33.7 percent, n = 35/105; ABLC 31.5 percent, n = 70/222; p = .700). This result endorsed the findings of Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) of a nonsignificant difference in mortality (47.6 percent L-AmB, n = 50/105 versus 58.9 percent ABLC, n = 53/90; p = .248) in their unadjusted analysis. Conversely, L-AmB was found to be a protective factor (OR, 0.56; 95 percent CI, 0.32–0.99; p = .047) in a multivariate analysis, while ABLC was not (OR, 1.19; 95 percent CI, 0.65–2.15; p = 0.574). However, it is important to notice that the multivariate analysis used d-AmB as reference and did not compare directly the lipid formulations.
Quality Assessment
Each clinical relevant outcome observed by the included studies (time for fever resolution, hospital length of stay, nephrotoxicity, new-onset dialysis, and mortality) was rated using GRADE criteria and summarized in Table 3. The outcomes were classified as low and moderate quality of evidence due to study design (retrospective cohorts), heterogeneity of included population, heterogeneity of outcomes definition, and other methodological biases such as statistical analysis without adjusting for confounding (e.g., age, sex, baseline SCr, among others), and information based only on indirect comparison.
Table 3. GRADE Assessment of the Quality of Evidence

DISCUSSION
Only four cohort studies, reported in five publications, fulfilled the inclusion criteria in this systematic review. These studies showed low quality of evidence to conclude about differences in efficacy and nephrotoxicity between both lipid-associated formulations of d-AmB, in patients with previous nephrotoxicity or refractory IFI.
The lipid formulations of amphotericin B offer an advantageous toxicity profile without decreasing treatment efficacy in comparison with d-AmB (Reference Wingard, White and Anaissie7;Reference Fleming, Kantarjian and Husni8). The low nephrotoxicity rate and the economic burden cause the L-AmB and ABLC formulations to be restricted to patients who were not eligible to d-AmB therapy (Reference Stoll, Cola, Splitt and Moreira14;Reference Bates, Su and Yu23). However, the prescription profile regarding one or another lipid formulation is heterogeneous and requires the integration of best available evidence into the decision-making process (Reference McGregor and Brophy24).
The direct comparison between L-AmB and ABLC, reported in two RCTs, showed inconclusive results. Wingard et al. (Reference Wingard, White and Anaissie7) found that patients with normal renal function (SCr < 3 mg/dl) treated with L-AmB have significantly less nephrotoxicity when compared with ABLC (p = .01), while Fleming et al. (Reference Fleming, Kantarjian and Husni8) could not detect a statistical difference between groups. Overall, the published studies evaluating differences in nephrotoxicity between L-AmB and ABLC have not provided definitive evidence for the superiority of one lipid formulation over another. However, these trials included only patients with normal renal function that received lipid formulations as first line therapy.
The lack of standard clinical practices regarding the use of high cost drugs such as amphotericin B lipid formulations is an important gap in the current literature. To the best of our knowledge, this is the first systematic review that summarizes the published evidence to support decision making between the two amphotericin lipid formulations (liposomal or lipid complex) focused on patients with IFI who were not eligible for d-AmB treatment due to d-AmB refractory infection, previous renal impairment, or unacceptable d-AmB renal toxicity. The importance of systematic reviews based on questions raised by clinicians relies on the fact that the results will directly impact clinical practice decisions, and support the best resource allocation considering budgetary constraints.
No clinical trials comparing the efficacy and safety of both formulations in this population were found in this search. This systematic review retrieved and critically analyzed four observational studies that included the population of interest. It is important to notice that the included studies were mainly focused on safety outcomes. Hence, little or no evidence was found to enable further discussion on the difference in effectiveness outcomes among lipid formulations.
Among the four observational studies which met the inclusion criteria, two involved only patients with hematological malignancies. In this specific group of patients, the proportion of complete response to therapy was reported to be very low, while the mortality rate was high. Hachem et al. (Reference Hachem, Boktour and Hanna20) reported that less than 10 percent of patients receiving salvage therapy (either high or low doses of AmB lipid formulations) achieved complete response. Additionally, the mortality rate in this study exceeded 73 percent, whereas other studies observed rates between 30 percent and 59 percent. The authors attributed the worse outcomes to the high proportion of patients included in the study that were either critically ill or in advanced stage of hematologic malignancies. Similar findings were observed by Cagatay et al. (Reference Cagatay, Cosan and Karadeniz22), who reported a mortality rate of 72 percent in patients who achieved complete response.
The included studies provided diverse results of hypothesized differences between L-AmB and ABLC regarding incidence of nephrotoxicity. Hachem et al. (Reference Hachem, Boktour and Hanna20) found no difference in nephrotoxicity between L-AmB and ABLC. However, this analysis was not adjusted by any covariates, particularly baseline SCr levels. Alternatively, Falci et al. (Reference Falci, Da Rosa and Pasqualotto18) did not directly compare the two lipid formulations. Instead, the authors found in a multivariate analysis that L-AmB had a protective effect on nephrotoxicity using d-AmB as reference. The same conclusion was not extended to ABLC. Finally, the only study that compared both formulations directly using a multivariate analysis observed significantly higher chance to develop nephrotoxicity in the ABLC group in comparison to L-AmB (Reference Wade, Chaudhari and Natoli21). However, no statistically significant differences were found in the outcomes new on-set dialysis, length of hospital stay, or mortality between L-AmB and ABLC in any of the studies.
There are two noteworthy questions that remained unsolved after discussing the available evidence. The first one is regarding the new-onset dialysis outcome, which could ultimately been underreported in the prior studies. For instance, patients who developed a minor renal failure could have had the antifungal treatment changed before the need for dialysis. Due to the noncontrolled design of the studies, it would be possibly expected the physician would prematurely change antifungal therapy to avoid further renal function deterioration. In addition, a high overall rate of mortality was observed in the studied population, probably due to severity of underlying diseases. However, none of the studies distinguished mortality by cause of death. Thus, the fungal-related mortality could not be evaluated.
This systematic review has several limitations. First, a quantitative analysis of data was not conducted due to lack of homogeneity between study populations and definitions of outcomes. A pool data analysis in this case was found to be inappropriate, in addition to the likelihood of generating misleading results. The approach chosen in this review was to critically analyze study results, and to discuss their strengths and biases. Also, all four observational studies were designed retrospectively, which could present biases inherent to this study design, such as missing or incorrected inputted data in the selected databases, population selection bias, and confounding factors not well described or not included in the multivariate analysis. These biases could lead to inaccurate results.
In conclusion, the studies included in this systematic review pointed toward less nephrotoxicity events in the L-AmB group. However, when considering other significant clinical outcomes (length of hospital stay, new-onset dialysis, and mortality), the evidence showed no statistically significant differences regarding one lipid formulation or another in this population subgroup. Therefore, there is no definitive evidence of overall superiority in effectiveness or safety outcomes between each treatment option. While these findings appear to suggest that the choice of lipid formulations could be based solely on costs considerations, the poor quality of evidence suggests that additional research is necessary. Further studies, particularly an RCT, would provide information to better evaluate whether there is an actual difference between these formulations in this population subgroup.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S026646231800034X.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this study.