Introduction
There is an increasing body of observational evidence for the existence of large molecules in the interstellar medium and in interplanetary space. Observations reflect a very interesting chemistry, in which of particular interest are large partly hydrogen-saturated molecules (Snyder Reference Snyder, Lovas, Hollis, Friedel, Jewell, Remijan, Ilyushin, Alekseev and Dyubko2006), many of them being of prebiotic nature (e.g. the isomers glycolaldehyde, methyl formate and acetic acid). Such molecules share several chemical properties with amino acids. Except for the controversial detection of glycine (Kuan et al. Reference Kuan, Charnley, Huang, Tseng and Kisiel2003; Snyder et al. Reference Snyder2005), amino acids have not been detected in interstellar space. However, amino acids are commonly found in comets and incorporated in minor bodies of the Solar System (Chyba & Hand Reference Chyba and Hand2005). This is consistent with laboratory studies (e.g. Ehrenfreund et al. Reference Ehrenfreund, Bernstein, Dworkin, Sandford and Allamandola2001) suggesting that amino acid in the gas phase would be easily destroyed by ultraviolet (UV) radiation. Thus, amino acid must have been shielded from UV radiation prior to its delivery to early planets. If efficiently formed (e.g. Bernstein et al. Reference Bernstein, Dworkin, Sandford, Cooper and Allamandola2002; Muñoz Caro et al. Reference Muñoz Caro, Meierhenrich, Schutte, Barbier, Arcones Segovia, Rosenbauer, Thiemann, Brack and Greenberg2002), amino acids may induce the synthesis of larger, more complex biomolecules in space (Wincel et al. Reference Wincel, Fokkens and Nibbering2000).
During the very early stages of life of a solar-type star, spectral components different from the UV contribute significantly to the stellar flux. From the study of hundreds of young solar-type stars, it appears that the Sun must have been much more active than today and it emitted copious amounts of energetic radiation, both in the X-ray and extreme UV ranges, especially in the form of intense daily or weekly flares (Feigelson & Montmerle Reference Feigelson and Montmerle1999; Favata et al. Reference Favata, Flaccomio, Reale, Micela, Sciortino, Shang, Stassun and Feigelson2005; Feigelson et al. Reference Feigelson, Getman, Townsley, Garmire, Preibisch, Grosso, Montmerle, Muench and McCaughrean2005). According to observations of solar-type stars in the Pleiades cluster, it can be inferred that when the Sun was only 100 Myr old X-ray emission was more than three orders of magnitude higher than today (Micela Reference Micela, Montesinos, Gimenez and Guinan2002). Only recently has the importance been recognized of such energetic emission in the formation and evolution of stars and planetary systems, as well as in life emerging and its initial evolution on our planet (Bergin et al. Reference Bergin, Aikawa, Blake, van Dishoeck, Reipurth, Jewitt and Keil2007). Synthesis and evolution of complex organic molecules must thus have proceeded under conditions that are quite different from today, and might have been accelerated or even made possible by the high-energy radiation ubiquitous in solar-type protostars.
The subject of the effects of ionizing radiation on amino acids has also received renewed attention in relation to the astrobiological search for biomarkers, such as evidence of past or present life on Mars (Parro et al. Reference Parro2005). The exposure of amino acids and other organic compounds on Mars, or in protoplanetary environments, to ionizing radiation from space and to the decay of radionuclides might induce a severe reduction of their concentrations, as well as an increase in the degree of local complexity (e.g. it might induce the formation of oligopeptides; Simakov et al. (Reference Simakov, Kuzicheva, Dodonova and Antropov1997)). The aim of the present study is the investigation of photostability of amino acids and their derivatives, in a radiation scenario characteristic of the early evolutionary stages of a solar-type star, as a part of a more comprehensive project devoted to the study of the effects of such radiation on the synthesis and evolution of complex organics at the dawn of prebiotic chemistry.
In this work we present the results of soft X-ray irradiation of tryptophan in aqueous solutions with water (H2O) and deuterated water (D2O) at room temperature (planetary conditions). Meteoritic materials show the significant presence of β-and γ-amino acids (e.g. β-alanine; Sephton Reference Sephton2002). Nevertheless, tryptophan is a reasonable choice in light of the existing large body of literature, and because aromatic amino acids play a major role in the photochemistry of proteins. Since the experiments of Stein & Weiss (Reference Stein and Weiss1948) on deamination of amino acids in aqueous solutions by X-rays, which followed the theory proposed by Weiss (Reference Weiss1944), several works on the subject have been put forward, most of them generally aimed at amino acid irradiation by very high energetic photons (e.g. 50 keV; Moan Reference Moan1974; Moan & Kaalhus Reference Moan and Kaalhus1974), and/or exploiting very powerful sources (e.g. Zubavichus et al. Reference Zubavichus, Fuchs, Weinhardt, Heske, Umbach, Denlingerc and Grunzea2004). Moreover, in those experiments amino acids are frequently in the solid phase, in the form of thin films or pressed powders.
Energy and flux are selected to somehow ‘resemble’ the emission of a young active flaring solar-like star. In addition, the irradiation experiments have been performed with a ‘gentle’ energy deposition in order to reproduce more realistic energy injection. Due to the resulting low irradiation doses, we detected moderate effects. Although difficult to analyse, the products highlight major reaction channels.
In the following section we present the experiments and in the third section the results of the analysis of the samples. The fourth section contains a discussion of the results. In the final section we summarize our results. High-performance liquid chromatography–mass spectrometry (HPLC/MS) and nuclear magnetic resonance (NMR) techniques are briefly described in Appendix.
X-ray irradiation
Experimental set-up
X-ray experiments were performed at the X-ray Astronomy Calibration and Testing (XACT) facility of the INAF–Osservatorio Astronomico di Palermo ‘G.S. Vaiana’. The facility, designed for development and calibration of X-ray astronomical instruments, includes several sources, monochromators and detectors covering the wavelength range 0.6–7000 Å (see Collura et al. Reference Collura, Barbera, Inzerillo, Mirabello, Sciortino and Serio1994 and Barbera et al. Reference Barbera, Candia, Collura, Dicicca, Pelliciari, Sciortino and Varisco2006 for a detailed description). The samples were irradiated using the emission from an electron impact X-ray source, with a high-purity Cu anode. A filter of 20 μm of Cu was used to cut the bremsstrahlung continuum above the absorption edge. The X-ray spectra emitted by the source was measured using a solid-state Amptek XR100-CR detector before and after the irradiation to monitor the stability of the source. The X-ray spectrum reported in Fig. 1 includes three components, the Cu Kα 8047 eV and Kβ 8905 eV lines, and the continuum. Among the possible choices of soft X-ray energies available we selected the Cu spectrum because, while it is entirely absorbed within the 2.5 mm thick sample, it was also a reasonable choice to simulate the X-ray emission of a young solar-like star dominated by frequent very energetic flaring activity (Favata et al. Reference Favata, Flaccomio, Reale, Micela, Sciortino, Shang, Stassun and Feigelson2005). The flux at the sample (~1 erg s−1 cm−2) used in the experiments is about one order of magnitude larger than that hitting the Earth atmosphere today, in the band 1.5–12.4 keV, during large Solar flares.

Fig. 1. X-ray spectrum (crosses) obtained in 1000 s. The spectrum is the result of three components: the continuum (solid line), the Cu Kα 8047 eV line and the Kβ 8905 eV spectral line (dashed lines).
The irradiation with soft X-ray requires the sample to be placed in a vacuum. Thus, a stainless steel vacuum tight container with an X-ray transparent entrance window (of 25 mm diameter) was used. The container had a cylindrical inner cell for the sample of 25 mm diameter and 2.5 mm height. To prevent sedimentation and/or not uniform irradiation of samples, the container was assembled on a rotating device that provided a slow and continuous mixing of the solution during the entire irradiation time. The temperature inside the irradiation chamber was also monitored during the experiments.
Tryptophan irradiation
Of the 20 biogenic amino acids, tryptophan (C11H12N2O2) is among the biggest and it contains both aspects of the molecular structure of the amino acids, i.e. aromatic moiety (indole) and aliphatic alanine-like chain; see Fig. 2. Thus, tryptophan provides the possibility of investigating the radiation effects on the aliphatic chain as well as on the more stable aromatic ring. Furthermore, studies of the effects of hard X-rays on tryptophan molecules present in the literature provide the opportunity for a comparison. The irradiation was performed at room temperature in solution with unbuffered D2O and for comparison in H2O as well. L-(−)-tryptophan from Alpha-Aesar (Italy) of 99% purity and D2O and H2O from Sigma/Aldrich (Italy) of 99.9% purity were used. We used solutions with high tryptophan concentrations of 9 mg ml−1 corresponding to 4.41×10−2 M at 20°C, in order to achieve the highest signal in the NMR diagnostic analysis. Moreover samples in solution with D2O can directly be analysed by NMR technique reducing the possible contamination effects in the process of the sample preparation. The concentration used implies a number of water molecules approximately 1300 times larger than the number of tryptophan molecules, while the number of atoms of the solvent is 150 times higher than the number of tryptophan molecules. The high penetration of X-ray photons and the continuous mixing of the sample during irradiation prevent screening effects due to high concentrations. Total X-ray energies of 1.3 and 7.8×106 erg, equivalent to doses of about 11 and 60 kRad, were deposited on both samples in the D2O and H2O solutions.
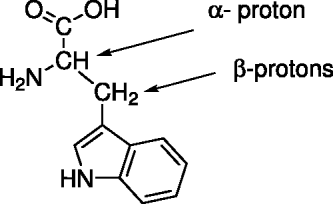
Fig. 2. Tryptophan molecular structure.
Sample analysis and results
We analysed the irradiated samples by means of HPLC/MS, which is the most sensitive and capable technique able to give information on trace amounts of compounds. In the attempt to corroborate the mass spectroscopy analysis, the structural modifications were also investigated by NMR. For this purpose we used a 300 MHz spectrometer.
Mass spectroscopy
A brief description of the HPLC/MS techniques and the specific procedure used in the analysis is given in Appendix. A background subtraction of the total ion chromatogram (TIC) of tryptophan from the TIC traces of the irradiated samples enhanced the differences, showing the chemical modifications of the tryptophan under X-ray irradiation. Figure 3 shows TICs for the non-irradiated (top panel, hereafter called the reference sample) and the irradiated samples in D2O (middle and bottom panels). The deposited X-ray energies were 1.3×106 and 7.8×106 erg for the samples shown in the middle and bottom panels, respectively. The effects of the exposure to X-rays of tryptophan in H2O is shown in Fig. 4. The total X-ray energy deposited is 1.3×106 erg, as in the middle panel of Fig. 3.

Fig. 3. HPLC/MS chromatograms of tryptophan in D2O solution: non-irradiated (top panel), irradiated with a total X-ray energy of 1.3×106 erg (middle panel); irradiated with 7.8×106 erg (bottom panel). The chromatogram of the non-irradiated sample has been subtracted from the irradiated ones.

Fig. 4. HPLC/MS chromatogram of irradiated tryptophan in H2O. The deposited X-ray energy is 1.3×106 erg.
TIC analysis can be summarized as follows:
• the chromatograms of the irradiated samples (see Figs 3 and 4) showed several peaks over the entire range of retention time (Rt) that did not exist or were negligible in the reference sample;
• the profiles of the chromatograms at Rt greater than 14 min suggested on-going radiation-induced formation of complex molecular structures;
• transient species formed: peaks arising with low irradiation times disappeared as deposited X-ray energy increased, the most evident example being the peak at R t=8.97 min (the middle panel of Fig. 3), which was missing in the chromatogram of the sample irradiated with a dose an order of magnitude larger (see the bottom panel of Fig. 3);
• X-ray irradiation effects were different for tryptophan in D2O and H2O;
• the most intense peaks in Figs 3 and 4 were at R t=8.97 and 2.36 min obtained in the D2O and in H2O solutions, respectively.
In Table 1 we list Rt and the corresponding protoned molecular masses MH+ of the peaks in the chromatograms of Figs 3 and 4, where the intensities of the extracted ions were higher than 105 counts s−1, i.e. higher than 1% of the peak of the unaffected tryptophan molecule. The chromatograms of the reference samples were checked to ensure that the peaks of Table 1 were not present or are at least one order of magnitude less intense than in the irradiated samples. As an example, we report in Fig. 5 the chromatograms of the extracted ions for the peaks of the irradiated sample in H2O related to the molecular structures in Fig. 6 (see the Discussion Section). Many of the new derivatives present in the sample irradiated in D2O are often present in their not deuterated form in the sample irradiated in H2O. Table 1 lists several peaks corresponding to a mass higher than that of tryptophan indicating an increase in the molecular complexity of the solution, such as polymerization in response to the irradiation. Several reactions involving polymerizations seem indeed to take place in our experiments, for example ions at m/z=448,526 and 552 (see Table 1). The peak at R t=16.07 min, corresponding to m/z=391, suggests the possible formation of the tryptophan dipeptide molecules. The most probable molecular structures for the ions marked with ‘†’ in Table 1 are reported in Fig. 6. These structures are hypothesized on the basis of peroxyl radical oxidation reactions, which have previously been reported to involve both side chain and aromatic moieties of aminoacids (Domingues et al. Reference Domingues, Domingues, Reis, Fonseca, Amado and Ferre-Correia2003). Moreover, as shown in Table 1, several cationated species are present that further confirm the identified quasi-molecular ([M+H]+) ion masses.

Fig. 5. HPLC/MS chromatograms of some extracted ions for the sample irradiated in H2O (Fig. 4). The deposited X-ray energy is 1.3×106 erg.

Fig. 6. Suggested structures for the ions marked with † in Table 1. The values of m/z of the deuterated species are shown in parentheses. Note that the protonation site in all of the indole structures has been arbitrarily attributed to the indole nitrogen.
Table 1. Retention times and corresponding protonated molecular masses

The number of deuterium atoms in the deuterated species is shown in parentheses.
† Masses with proposed molecular structures, see Fig. 6.
* Adducts with Na species are also detected.
NMR
Although the effects detected in the NMR spectra are often negligible when small amounts of chemical changes are involved, we obtain from such a diagnostic technique additional information which is consistent with the HPLC/MS analysis and the literature. The 1H NMR spectra of the sample in D2O solution irradiated with a total energy of 1.3×106 (middle panels) and 7.8×106 erg (bottom panels) along with the reference (top panels) are shown in Fig. 7. The sample irradiated with 1.3×106 erg shows very small variations as compared to the reference spectrum (top panels), testifying to the slight extent of the changes in the chemical composition. Under these experimental conditions, the spectrum shows only a well detectable signal appearing as a doublet at δH=1.19 ppm (peak b) consistent with the presence of CH3– or R–CH2– protons both coupling with an adjacent –C–H–. Such a peak could account for an alanine-like chain, probably detached from the aromatic indole ring by reaction with species such as D, OD or OOD radicals originating from the D2O interaction with X-ray photons, as mentioned in Beenakker et al. (Reference Beenakker, De Heer, Krop and Möhlmann1974) and Ajello (Reference Ajello1984). Given the high concentration of the tryptophan in the sample, the direct formation of radical fragments from the solute could also contribute to this effect. In other experiments dealing with radical reactions, a chemical shift range similar to that observed in our samples has been attributed to paraldehyde species (Matsushita et al. Reference Matsushita, Tran, Nosaka and Nosaka2007).

Fig. 7. 1H NMR spectra of tryptophan in D2O solution: the reference (top row); sample irradiated with 1.3×106 erg (middle row); with 7.8×106 erg (bottom row). The peak of the solvent is calibrated at 4.80 ppm. The chemical shift is expressed in ppm. The spectra intensities are amplified differently in order to highlight weaker peaks. The first two columns from the left represent the portion of the aromatic structure while the others refer to the aliphatic chain.
In addition, a weak multiplet signal is detectable at δH=4.05 ppm (peak a in Figure 7), which is slightly shifted upfield of 0.2 ppm with respect to the α-parent proton peak. This moderate shielding effect suggests that some modifications occurred onto the β-hydrogens. Probably, the former (the less hindered and randomly more favored) could be attacked and substituted by D radicals producing an alanine-like coupling system. The analysis of the peak area of the proton signals highlights a moderate increase of the doublet at δH=1.19 ppm at the expense of those at δH=3.25–3.55 ppm. Such a pattern could be consistent with substitutions occurred onto β-protons by radical species or with a total detachment of an alanine-type chain.
At higher irradiation dose (the bottom panel in Fig. 7), a number of significant changes are evident in the spectrum, both in the chemical shifts and in the intensities of the resonances. Noteworthy among these there are the progressive changes observed mainly in the aliphatic regions, i.e. the peaks in the range δH=1.14–3.50 ppm. In this range, we can observe, other than the unmodified peaks such as for the α and β protons, signals indicating the formation of further products of degradation of the supposed detached alanine-like chain. Consistently, the peak a disappears, while the doublet (peak b) splits into a multiplet at δH=1.14–1.19 ppm (peak g, bottom panels in Figure 7). Taking into account their related deuterated forms which influence the chemical shift, this signal falls in the range of small molecules such as alkyl amines, 2-propanol and other species. Analogously, the weak singlet at δH=2.07 ppm (peak f) is compatible with other products of degradation such as acetic acid, acetaldehyde and acetonitrile (Gottlieb et al. Reference Gottlieb, Kotlyar and Nudelman1997). Some of these molecules have also been detected in harsher irradiative conditions (Matsushita et al. 2006). The peak at δH=3.35 ppm (peak e) is attributable to the methanol signal, which is a typical product of degradation of the aliphatic chain. At δH=3.50 ppm (peak d), a new multiplet signal, slightly shifted downfield, may suggest modifications of the α-hydrogen, but remains difficult to attribute. Thus, as the energy furnished to the sample increases, the alanine-like compounds behave as transient species. Another main characteristic feature of the high irradiation dose is the appearance of new signals at high δH values (10.2 ppm, peak c), the typical range of aldehyde protons. Under such conditions (100% D2O), and with reference to standard chemical shifts, the resonance originating at δH=10.2 ppm can be reliably assigned to an aldehyde proton. The high exchange rate of the labile indole ring NH proton with D2O does not permit the appearance of mobile protons in the same field range. In support of the aldehyde-containing molecule, in the HPLC/MS analysis we detected a peak at m/z=147 associated to a formyl-tryptophan-derived compound (see Fig. 6 for related proposed structures). Finally, given the low sensitivity of the NMR diagnostic technique, the very weak signals observed in the aromatic range are difficult to interpret reliably.
The NMR data presented above mainly provide some support for the degradation products of tryptophan in D2O solution. However, the formation of aldehyde species associated with radicalic tryptophan fragments provides favourable conditions for the formation of more complex molecules as detected in the HPLC/MS analysis.
Discussion
The irradiation of tryptophan molecules in solution with D2O and H2O at room temperature with X-rays of 8 keV leads to the formation of light species indicative of fragmentation of tryptophan, as well as to large molecular structures. Given their high energy, X-ray photons interact with single atoms in a molecule rather than directly affecting the molecular bonds. The subsequent ionization injects high-energy photo-electrons within the solution, which in turn loose their energies by ionizing, exciting and dissociating molecular species. The amount of energy deposited onto the samples was 1.3 and 7.8×106 erg, corresponding to a total of ≈1–6×1014 X-ray photons. The ratio of atoms in the samples to the total number of X-ray photons deposited into the samples is greater than 108. Furthermore, the number of molecules and atoms in the solvent was much larger than in tryptophan, i.e. 1300 and 150 times, respectively. Therefore, direct photo-destruction channels are unlikely, and chemical effects must arise mainly through exchange with the solvent. X-ray irradiation of water leads to the production of H (D) and O atoms, OH (OD) radicals (as recognized more than 50 years ago by Weiss (Reference Weiss1944)) as well as ions producing secondary emission over a broad range of energies, dominated by H I Ly-α and Ly-β lines (Beenakker et al. Reference Beenakker, De Heer, Krop and Möhlmann1974; Ajello Reference Ajello1984). Among all the secondary products caused by the X-ray radiation, both H (or D) atoms and OH (or OD) radicals are very reactive, the first being a reducing agent while the latter a strong oxidizing reactant. Thus, the main destruction channels of tryptophan are most likely radical reactions, as suggested by the HPLC/MS and NMR analysis, although some contribution from photodestruction by secondary UV radiation, in the range 200–300 nm cannot be excluded. H I Ly-α radiation contributes additional OH (OD) radicals from water dissociation. Therefore, solvents play a major role in determining the final products (see Figs 3 and 4), as D and OD, and H and OH forming in D2O and H2O solutions have different reactive effects.
Evidence for polymerization is suggested by several high mass derivatives listed in Table 1. The chemistry leading to the polymerization products is not easily attributable, being formed through aspecific radical mechanisms. The peak at 16.07 min corresponding to m/z=391 that is present in both D2O and H2O samples is in agreement with the formation of a dipeptidic bound; see Fig. 6. The presence of tryptophan dipetide is more evident in H2O than in D2O, probably due to H and D isotopic effects, and it appears to be dependent on the irradiation dose, decreasing with the increase of deposited energy.
We also observed ions at m/z=345 and 577, which along with m/z=391 could arise from condensation reactions of tryptophan. In particular, m/z=577 is reasonably attributable to tryptophan tripeptide, while ions at m/z=345 could be due to the de-carboxy de-hydro tryptophan dimer.
Summary and conclusions
In this work we have presented experiments in which tryptophan molecules in water solutions, at room temperatures, are irradiated with soft X-ray radiation of 8 keV and flux one order of magnitude larger than the flux reaching the Earth atmosphere today, in the band 1.5–12.4 keV, during large solar flares. The irradiation was carried out at a low energy rate, in order to reproduce a realistic radiation environment. Analysis of the irradiated sample showed that X-rays lead to both fragmentation of the tryptophan molecules and to the formation of larger species of increasing complexity. Among the largest molecular structures tryptophan dipetide and tripeptide species were detected.
Our experimental conditions imply that X-rays cannot be directly responsible for the effects observed on tryptophan molecules. We detected second generation products mainly induced by radicals originating from the interaction of the X-rays with solvents. The results of this study strengthen the role of ‘primordial’ radiation field conditions in the understanding of pre-biotic chemical evolution, and open out to the more general problem of chemical evolution in space. Since amino acids possess a limited photostability to UV light (Ehrenfreund et al. Reference Ehrenfreund, Bernstein, Dworkin, Sandford and Allamandola2001) the evolution to the next level of complexity in peptidic chains might occur either in protected environments or under substantially different radiative conditions. Soft X-ray induced chemistry may be a promising candidate. This suggestion is also supported, for example, by the work of Kaneko et al. (Reference Kaneko, Tanaka, Narita, Kitada, Matsui, Nakagawa, Aguid, Fujiid and Yokoya2005). who demonstrated glycine dimer formation, via soft X-ray irradiation of thin films of glycine, and found evidence for the activation of heterogeneous pathways on the surface of solid amino acids.
In this work liquid solutions have been used. In space conditions most material is frozen into water icy mantles accreted onto the surface of dust grains (e.g. Gibb et al. Reference Gibb, Whittet, Boogert and Tielens2004). The present investigation bears out our interest in exploring a more extensive set of experimental conditions. It would be of interest to compare energy yields in the solid and liquid phases. The recent discovery of a mixture of water ice and organic materials, directly detected on the surface of an asteroid (Campins et al. Reference Campins2010; Rivkin & Emery Reference Rivkin and Emery2010), supports the importance of such studies.
Diagnostic methods
As mentioned in the results section, the HPLC/MS experiment is among other analytical techniques one of the most sensitive and is capable of giving information on trace amounts of compounds. The approach used implied the analysis of the same amount of pure and irradiated tryptophan in the same experimental conditions. In particular, through HPLC/MS analysis, a separation among tryptophan and its derivatives was achieved, which were shown in the chromatograms as peaks at certain Rt. In the case of HPLC/MS, the signal is constituted by mass spectra that are recorded during the run at a rate of one spectrum every second. Ion intensities are generally summed to give a TIC trace (as in Fig. 3). The mass spectrum itself is a bi-dimensional plot, where the m/z ratio of each ion is reported versus its intensity (in counts s−1). This implies that a HPLC/MS trace is thus a three-dimensional array, where, for each specific Rt, information on the mass of the species can also be deduced from the corresponding mass spectrum. On the other hand, given the m/z ratio of a selected ion its R t can be easily identified. This allows a direct detection of trace components that cannot be easily distinguished on the basis of the TIC alone (Fig. 5). It must also be pointed out that the HPLC/MS technique, once the experimental conditions are definitely established, is very reproducible providing the opportunity to compare the chromatograms of different samples. This also renders the background subtraction an available option in order to evidence minor or trace amounts of compounds, as in the middle and bottom panels of Fig. 3 and in Fig. 4. In our case just a simple background subtraction of the TIC of tryptophan from the TIC traces of the irradiated samples shows peculiar changes that give evidence for the fact that chemical modification of the starting substance has taken place as in Figs 3 and 4.
HPLC/MS measures were performed on an Thermo TSQ Quantum Access triple Quadrupole instrument coupled with a Thermo Accela HPLC/MS system and equipped with an Heated Electrospray (H-ESI) source. The chromatographic conditions were as follows: Column Thermo Hypersyl Gold, 2.1 mm inner diameter, length 10 cm; the volume injected was 10 μl. The analysis was carried out using a gradient combining a mobile phase (A, water 0.1% formic acid) and a mobile phase (B, methanol) as reported in Table 2. The mass spectrometry experiments were performed under the following experimental conditions: spray voltage 4100 V, skimmer offset −10 V, vapourizer temperature 80°C, sheath gas pressure 20 (a.u.), ion sweep gas pressure 2.0 (a.u.), aux gas pressure 35 (a.u.), capillary temperature 270°C, capillary offset 35 (a.u.).
Table 2. Parameters of the HPLC/MS analysis

1H NMR spectra were recorded at 298 K with a Bruker Avance 300 MHz spectrometer. Routinely, one-dimensional spectra of tryptophan in unbuffered 100% D2O were acquired with spectral widths of 14 ppm at 298 K. Chemical shifts (ppm) are calibrated with respect to the sharp peak of D2O at δH=3.80 ppm.
Acknowledgments
We are grateful to Professor L. Ceraulo and Dr G. Micela for their very helpful comments and suggestions. We also thank R. Candia and S. Varisco for the technical support to the experiments. LC/MS experimental data were provided by Centro Grandi Apparecchiature, UNINETLAB–Università di Palermo.
This work was supported by PRIN-INAF 2006, ‘Survival of prebiotic compounds in the very intense X and EUV radiation field of the Young Sun’.











