Introduction
The development of organic materials on planets can arise from two sources: endogenous production or exogenous delivery. There has been a long history of estimating the relative effectiveness of both methods for generating organic materials on young terrestrial planets (e.g. see Chyba & Sagan Reference Chyba and Sagan1992). A third possible source for organics was pointed out by Bar-Nun et al. (Reference Bar-Nun, Bar-Nun, Bauer and Sagan1970), and is the result of the shock-driven chemistry that arises when objects from space strike the Earth and its atmosphere at high speed. This latter possibility can also increase the chemical complexity of organics already present in the impactor. Impact-driven synthesis is thus of interest as regards to the possible inventory of complex organic materials on Solar System bodies.
As well as the Earth, there are other possible habitats for life in the Solar System. These include the putative sub-surface oceans on some of the ice covered larger satellites of the outer gas giant planets, e.g. Europa (see Kargel Reference Kargel1991, Reference Kargel1998; Kargel et al. Reference Kargel, Kaye, Head, Marion, Sassen, Crowley, Ballesteros, Grant and Hogenboom2000) and possibly the interior of Enceladus, where there is evidence for pre-biotic chemistry, i.e. the hydrocarbon containing water rich plumes from the poles of Enceladus (Matson et al. Reference Matson, Castillo, Lunine and Johnson2007). These satellites are heated by tidal forces exerted on their interiors which can produce enough energy as waste heat to melt the lower levels of the ice mantle surrounding the rocky core. There is spectroscopic evidence that not all the ice on the surface of such bodies is pure H2O (e.g. Cruikshank et al. Reference Cruikshank, Owen, Ore, Geballe, Roush, de Bergh, Sandford, Poulet, Benedix and Emery2005) and other elements such as carbon and nitrogen are reported. The resulting rich chemistry can even include silicates from the rocky interior (e.g. for evidence of silicate rich salts on the surface of Europa see McCord et al. (Reference McCord1998, Reference McCord1999)). In such cases, impacts can again drive increasing organic complexity, in this case in the target ice (as suggested by, amongst others, Borucki et al. (Reference Borucki, Khare and Cruishank2002)). Recently, Nna-Mvondo et al. (Reference Nna-Mvondo, Khare, Ishihara and McKay2008) have reported on increasing organic content of ices using pulsed laser illumination to simulate impacts. Here we go a step further than such work, and use actual high-speed impacts of macroscopic projectiles into ice targets to study changes in the organic content of ices as a result of the impact.
On Earth, organic geochemists studying naturally occurring processes that effect biologically derived organic matter are typically concerned with two factors: (1) the degree to which the biological origin of a compound can be proven; and (2) the chemical stability of compounds and how they have been altered by subsequent geological processes. Highly labile compounds are typically not preserved in the fossil record, unless shallow surfaces occurring at the Earth's surface can convert them into a more stable form, although there are exceptions (such as the conversion of β,β-carotene to β,β-carotane; see Hebting et al. (Reference Hebting, Schaeffer, Behrens, Adam, Schmitt, Schneckenburger, Bernasconi and Albrecht2006)). To survive for longer periods compounds need to be chemically stable in the thermal regime encountered. Previous studies have shown that fossil fuel biomarkers survive in rocks that have experienced hypervelocity impacts (Bowden et al. Reference Bowden, Court, Milner, Baldwin, Lindgren, Crawford, Parnell and Burchell2008) – but in these instances the organic matter has already been processed by naturally occurring processes that remove unstable compounds. Additionally, rock matrices provide both mechanical and physical protection to their organic component, in a way that icy matrices do not. Thus there are two knowledge gaps: (1) the fate of non-fossil fuel biomarkers during the short duration high-temperature environment generated during an impact; and (2) the fate of biomarker compounds within icy matrices during hypervelocity impacts (HVI).
Accordingly, here we report on HVI experiments using ice targets. The ice targets used were doped with three different organics (β,β carotene, stearic acid and anthracene) to represent a range of materials with different possible origins and chemical stabilities (see Table 1). β,β-carotene is a carotenoid, a type of biological pigment ubiquitous to many organisms. The compound assists in light-harvesting by broadening the spectral-range over which energy can be adsorbed, but also plays a crucial role by reacting with free radicals, and photosynthesis in the absence of carotenoids has been shown to be fatal for a range of organisms. Its origin is unambiguously biological, although its reactive nature means that it does not persist for geologically long periods of time. Fatty acids (such as stearic acid) are found in the lipids that form the cell membranes in two of the three domains of life (eukaryotes and prokaryotes); their chemical stability is reflected in their role as chemical and physical barriers that protect internal cell processes. Fatty acids are found in fossil fuels and fossil organic matter and represent chemical fossils in this context, but they can also form via Fischer Tropsch synthesis; hence they can have a biological or abiological origin. Anthracene, a polycyclic aromatic hydrocarbon (PAH), is a cosmically abundant compound that can form from both pyrolysis and combustion of biomass, but is also present in organic matter in extraterrestrial materials of non-biological origin (e.g. interplanetary dust particles (IDPs) and carbonaceous chondrites). Relative to the other compound types studied here, PAHs are the most geologically stable.
Table 1. Organic compounds used as targets (frozen in ice) in the HVI experiments
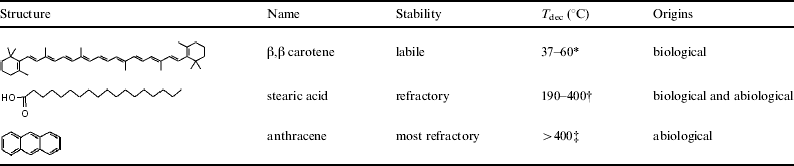
T dec=approximate temperature for the onset of thermal degradation at standard conditions.
* From Mordi et al. (Reference Mordi, Walton, Burton, Hughes, Ingold, Lindsay and Moffatt1993).
† From Santos et al. (Reference Santos, Dos Santos, De Souza, Prasad and Dos Santos2002).
‡ Although anthracene is stable at relatively high temperatures it has a boiling point of 340°C at standard conditions.
Method
The experimental setup is shown in Fig. 1. The impacts were obtained using a two-stage light gas gun at the University of Kent (Burchell et al. Reference Burchell, Cole, McDonnell and Zarnecki1999). The gun fired projectiles (in this work, stainless steel spheres of 1 and 1.5 mm diameter) in a discardable sabot (only the projectiles strike the target). The projectile speed was measured in flight (to better than ±1%) by passage through two laser light curtains. During the shots the target chamber was lowered to a pressure of approximately 5 mbar to assist in the firing of the gun. This pressure (a partial atmospheric pressure containing oxygen), whilst low, is still greater than that expected on the surfaces of icy satellites. The effect of this might be to facilitate degradation pathways that depend on oxygen but such an effect would be extremely slight and short lived.

Fig. 1. Experimental setup showing position of ejecta collection trays, the target and the ice layer doped with organic compounds and gun chamber. The projectiles enter horizontally from the left.
The ice targets comprised a greater than 100 mm basal layer of water ice, covered by a thin ~10 mm layer of water ice doped with a mixture of all three organic compounds and a less than 5 mm layer of water ice deposited to prevent sublimation of the layer containing organic compounds. The ice forming the basal layer of the targets was frozen from reverse osmosis purified water held in square containers (20 cm×20 cm). Freezing was performed in a CO2 freezer at 160 K.
All the organic compounds used for this study were poorly soluble in water. Thus to increase the concentration of these compounds in the target layer, DMSO (dimethylsulfoxide) was used to help solvate these compounds. DMSO solutions containing the target compounds were mixed with water to produce a 1:1 v/v solution of DMSO/water that yielded a material with approximately 75 mg/g of each compound in the final ice layer. Adhering to a zigzag pattern, and taking care to maintain an even coverage, the 1:1 solution of DMSO/water was poured onto the surface of the ice to a depth of ~10 mm and frozen in place. The resultant layer was not as hard as the basal pure water ice layer (as determined qualitatively by its resistance to having a probe pushed into it), presumably because the crystalline structure of the water ice was disrupted by non-water components.
After application of the uppermost thin layer of water ice, the ice target was directly placed in the light gas gun. Of particular interest to this study were the ejecta from an impact. To collect the target material thrown off as ejecta, an ejecta capture system was placed in front of the target. This was the same system used in early work to capture ejecta from ice targets at the same light gas gun (Burchell et al. Reference Burchell, Galloway, Bunch and Brandao2003). Each tray in the capture system samples a different angle of ejection, with those closest to the target sampling shallow angles and those furthest sampling higher angles of ejection. Four trays were used, sampling angles of ejection in the ranges 0–50, 50–65, 65–75 and 75–85 degrees from the target surface. There was a gap in coverage at normal incidence to permit entrance of the projectile. These trays were cleaned with DMSO prior to each shot.
After the shot, ejecta samples, in the form of a liquid phase, were recovered from the capture trays. Note that these ejecta may have initially been in the ice state, but melted during the removal from the capture system. Samples were also taken from the crater aureole and at various depths from beneath the crater (these samples are not discussed further here and will be the subject of a later paper). During analysis, the samples were primarily characterized by UV-VIS adsorption spectroscopy and then by gas chromatography mass spectrometry (GC-MS) analysis to detect lower concentrations of some compounds.
Single-beam UV-VIS adsorption spectroscopy was performed using a USB4000 ocean optics spectrometer (200 to 800 nm) and a DT-MINI-2-GS tungsten-halogen light source (200 to 800 nm). Integration times were selected to yield comparable absolute adsorption values between different analyses. While UV-VIS adsorption spectroscopy is not sensitive to low concentrations of analyte, it is capable of detecting all of the compounds used during this experiment and spectrometers covering relevant parts of the spectrum are a payload common to many remote sensing platforms, whereas a GC-MS is not.
GC-MS analysis was performed using an Agilent 6890N GC, fitted with a J&W DB-5 phase 50 m length column (0.25 mm id, 0.25 μm film thickness), connected to a 5975 MSD and a quadruple mass spectrometer (ionization energy 70 eV; operating in selected ion monitoring mode). Samples were injected manually using a split/splitless injector operating in splitless mode (purge 40 ml min−1 for 2 min). The temperature program for the GC oven was 80–300°C, holding at 80°C for 2 min, rising at 10°C min−1 for 8 min and then 3°C min−1, and finally holding for 10 min. Compounds were identified by comparing retention times to well-characterized materials and commercial standards. Stearic acid was analysed without derivatization. GC-MS is capable of detecting much lower concentrations than UV-VIS adsorption spectroscopy and was used to prove the presence of stearic acid when it was not prominent in UV-VIS adsorption spectra.
Results
Two shots were carried out in this work. Shot 1 was at 4.90 km s−1 with a 1 mm diameter projectile. Shot 2 was at 4.85 km s−1 with a 1.5 mm projectile. Both shots were successful and images of the targets can be seen in Fig. 2; in both cases the impact crater has penetrated the doped surface layer and exposed pure water ice in the base of the crater. The crater in Shot 1 had a diameter of 65 mm. The crater in Shot 2 was slightly more irregular in shape with a mean diameter crater diameter of 72 mm. The ejecta were captured at the various angles of ejection and samples from walls of the craters in the ice targets were also taken. The ejecta masses collected for each tray are given in Fig. 3.

Fig. 2. (a) Photograph of a target mounted in the door of the gun before a shot. (b, c) Images of targets post shots 1 and 2, respectively. (d) An oblique view showing the crater and the layering in the ice target in Shot 2. Note the yellowish colour of the DMSO still visible through the relatively thin uppermost water ice layer in (a) to (c). The whiter appearance of the surface layer in (d) is due to frosting which occurred after the shot when the target was exposed to air. The scale in (a)–(c) is established by the 20 cm width of the ice target container. In (d) the crater is approximately 72 mm across.

Fig. 3. Data for the mass ejected from each shot (as captured in trays 1–4) are shown on the left. The results for Shot 1 are given in dark red (projectile diameter d=1 mm) and Shot 2 (d=1.5 mm) in blue. On the right the UV-VIS adsorption data is shown for Shot 2. Reference spectra are sketched for: Fa=stearic acid, An=anthracene, βC=β,β-carotene. Baseline spectra are given in red for the DMSO: water mix solvating the organic compounds. Note that for convenience this diagram is arranged vertically although the shots were done horizontally.
The ejecta samples from Shot 1 were weighed and protocols for handling tested. The larger masses of ejecta material from Shot 2 were then analysed for chemical composition by UV-VIS spectrometry and GC-MS (Fig. 3). All of the compounds originally present in the organic ice layer were detected in the ejected material, although the relative proportions of these compounds changed and the degree of difference varied for ejecta collected from different trays (i.e. at different ejection angles).
The UV-VIS spectra of the ejecta from tray 1 (shallowest angle of ejection) were dominated by the spectra of the fatty acid with a small spectral feature in the correct region for β,β carotene. The presence of anthracene was confirmed by GC-MS, although it is not evidenced in the UV-VIS adsorption spectra. The UV-VIS spectra of the second capture tray (angles 50 to 65°) were dominated by spectral features of anthracene, with a much reduced fatty acid signal. (The contents of tray 3 were discarded due to a handling error.) The UV-VIS spectra of ejecta from the fourth capture tray (angles 75 to 85°) contained only spectral features for anthracene; GC-MS was necessary to confirm the presence of a small proportion of stearic acid (Fig. 4).

Fig. 4. GC-MS ion chromatograms showing that a smaller amount of stearic acid is present in the ejecta with steepest angle of ejection. The peak for stearic acid is labelled, an asterisk is used to denote peaks observed in both the ejecta and the procedural blank (the latter is a sample of the Water/DMSO concentrate not used in a shot), and a U has been used to identify peaks that may be unidentified decomposition products.
Only a small proportion of beta-carotene was present, and only in the ejecta at the shallowest angles (tray 1). Stearic acid was present both in the shallow and steep angle ejecta, although there were notably lower abundances in the steep angle ejecta. Anthracene increased in proportion as ejecta angle increased, although the current data do not constrain whether or not this is an absolute rise in concentration or if additional anthracene was being created.
The variation of concentrations of the various compounds with angle of ejection can be interpreted in terms of varying chemical alteration. In this respect we note the thermal stability of the compounds, e.g. β,β carotene has a thermal degradation temperature (T dec) at standard conditions of 30–60°C (Mordi et al. Reference Mordi, Walton, Burton, Hughes, Ingold, Lindsay and Moffatt1993), while T dec for anthracene is greater than 400°C. This suggests that the shallow angle ejecta were (shock) heated less during the impact than that at higher angles.
Thermal decomposition in the presence of oxygen is distinct from the pyrolysis reactions that might be expected within the oxygen-limited environment of the evacuated chamber used during the experiments. Thus, potentially, the absence of oxygen might be expected to inhibit chemical degradation. However, the ice was frozen at standard laboratory conditions and must contain some oxygen; furthermore, the ionization of water and formation of hydroxyl radicals might also be important considerations.
At this stage degradation products have not been unambiguously identified, quantified or traced back to the starting compounds. Despite this many new peaks appear in the GC-MS traces – a few peaks not present in the procedural blank are labelled in Fig. 4. The ion chromatograms for other mass fragments contain many peaks not present in the procedural blank and the combined area for these peaks is greater than that attributable to the two starting compounds. It is possible that some anthracene may be a combustion product, although its very high abundance relative to other PAHs suggests that this is not the case for the anthracene detected at the largest angles of ejection. The lower proportion of anthracene closer to the target could reflect the greater volatility of anthracene relative to the other compounds, e.g. it has been volatilized and removed from the low-angle ejecta. Its concentration in the high-angle ejecta is remarkable, greatly above what would be expected by a relative enhancement caused by the destruction of other compounds. Thus mass transfer of anthracene within the ejecta plume appears to have occurred. This cannot be explained by physically driven processes alone, e.g. using the different rates of thermal degradation for different compounds and applying them to variably heated ejecta. One possible explanation may be the nature of ejected material immediately after the impact; immediately following the impact, a single high-temperature water phase may be present. High-temperature water phases under high pressures have good solvating potential for a range of hydrocarbon compounds (Hawthorne et al. Reference Hawthorne, Yang and Miller1994); in this situation anthracene would be preferentially solvated in the high-temperature (most shocked) ejecta phase. To conclusively prove this, the anthracene within the ejecta would need to be mass balanced, and shown to be concentrated in the high-angle ejecta significantly above a background threshold.
Thus, within the ejecta plume, two thermally driven processes are important for governing the distribution of organic compounds in the C14 to C40 carbon number range. Our preliminary evidence suggests thermally driven degradation reactions limit labile compounds to the low (shallow) angle part of the ejecta, whilst volatilization causes partitioning of volatile compounds to the high-angle parts of an ejecta plume.
Discussion
Figure 5 seeks to explain our observations in the context of geological observations of impact crater ejecta (French Reference French1998). Ballistic ejecta are the ejecta deposits found furthest from an impact crater; typically they contain the most shocked material, e.g. tektites are dehydrated glass ejecta formed at the highest shock pressures. Proximal ejecta deposits emplaced by ground surges typically contain the least shocked material, such as intact lithic fragments and clasts of intact rock. Our experimental results for organic compounds in water ice fit this general pattern, except for the possible caveat regarding the mass transfer of anthracene to the highest angle ejecta. The most proximal ejecta (low-angle ejecta) contain the highest concentrations of the least thermally stable compounds, whereas the most distal eject (ejected at the highest angles) contain only the most thermally stable and volatile compounds.

Fig. 5. Conceptual model of the thermal alteration of organic compounds during a hypervelocity impact with ice. Based on extrapolation of laboratory experiments (a) to geological scale (b). Also shown is the zonation of shock metamorphic effects observed for ballistic and layered ejecta (c). The arrow (d) refers to the possible mass transfer of volatile organic compounds to the high-angle ballistic ejecta.
Our results, in addition to indicating that complex organics can be found in ejecta after HVI, have relevance to Solar System exploration. A variety of missions (planned and active) combine HVI impactors with analysis instruments carried on a parent spacecraft, which then flies through the plume or attempts to analyse the plume from nearby. The NASA Deep Impact mission to comet 9P/Tempel 1 successfully created a HVI using a projectile dropped by its parent craft, which then analysed the resulting eject plume using cameras (A'Hearn et al. Reference A'Hearn2005). More relevant to the work reported here is perhaps the NASA LCROSS mission (see http://lcross.arc.nasa.gov for a discussion of the mission, accessed November 2008). This mission to the Moon (planned for 2009) will deliberately impact the lunar surface in a permanently shadowed region where there may be icy deposits. A shepherding spacecraft will then fly through the plume (and itself impact the surface), sampling the plume for water (ice or vapour state) and evidence for hydrocarbons (see Shuvalov & Trubetskaya (Reference Shuvalov and Trubetskaya2008) for a prediction of the size of the ejecta plume).
Moving out further in the Solar System, a variety of missions to the icy satellites of the outer planets is regularly proposed. Given the difficulty (cost, mass and complexity) of a lander with surface sampling capability, such missions often propose HVI impactors with the parent craft sampling the ejecta plume during a related fly by. It is thus useful for planning such purposes to better understand the evolution of organic materials during ejection after a HVI event.
The observation that small amounts of a compound with a purely biological origin (β,β-carotene) survived is significant in this above context; however, it is critical to note that β,β-carotene was only detected in the ejecta for a limited range of angles of ejection. Thus the results from space missions such as those discussed above will potentially represent an opportunity for detecting organic compounds, but in a highly biased fashion. To obtain a full picture of the contents of the target (particularly of any biological products) may require sampling the lower angle ejector, potentially ejected later in the impact, having suffered less shock processing, although this too may be biased in the case of an HVI impact with a water ice target.
Further work in this programme of experiments will look at samples from the impact crater itself, as well as testing survival of a wider range of organic compounds of varying complexity.
Conclusions
This study has demonstrated that ice can be doped with organic compounds, impacted at hypervelocities, and organic compounds detected in the ejecta. Our initial results indicate that the organic contents of ice targets are present in HVI ejecta. A variety of organics in the C14–C40 range survive and, significantly, an organic compound with a purely biological origin (β,β-carotene) survived in the HVI ejecta, albeit with a low survival rate and only at shallow angles of ejection. By contrast, anthracene (with an abiological origin) survived better and at a wider range of angles of ejection, and may even have been preferentially concentrated in the highest shock heated ejecta. It is thus critical where an ejection plume is sampled, as this will influence what compounds can be detected.
Acknowledgement
We thank M. Cole for firing the light gas gun used in this work.








