Introduction
Formaldehyde is a prebiotic molecule fairly well distributed in the universe (Chang, Reference Chang, Greenberg, Mendoza-Gomez and Pirronello1993; Cleaves, Reference Cleaves2008). Two input sources of formaldehyde in the primitive Earth have been suggested: the photoreduction of atmospheric CO2 and H2O (Cleaves, Reference Cleaves2008) and delivery by comets (Chyba et al., Reference Chyba, Thomas, Brookshaw and Sagan1990; Greenberg et al., Reference Greenberg, Mendoza-Gomez and Pirronello1993). This molecule has been synthesized in prebiotic simulation (Bossard et al., Reference Bossard, Raulin, Mourey and Toupance1982; Stribling and Miller, Reference Stribling and Miller1987), and after formation in the atmosphere, it may be transported to the ocean and other water bodies by the rain and undergo further chemical changes (Chang, Reference Chang, Greenberg, Mendoza-Gomez and Pirronello1993).
The oxidation state of the prebiotic atmosphere and the conditions of the primitive ocean are still under debate. The ocean might provide a more productive environment for prebiotic synthesis, but plausible prebiotic conditions are difficult to achieve (Chang, Reference Chang, Greenberg, Mendoza-Gomez and Pirronello1993). The atmosphere/ocean interface with several physical and chemical processes presented via recycling of chemical compounds such as formaldehyde to the sea surface, shallow waters, tile pools bordering the sea and marine hydrothermal vents. Formaldehyde concentration in the ocean and other water bodies was dependent on several factors, such as production/rainout rate/decomposition. Several authors have estimated that the prebiotic oceanic concentration of formaldehyde was 4 × 10−4–1 × 10−3 M (Cleaves, Reference Cleaves2008 and the references therein). It has also been proposed that formaldehyde can be produced in hydrothermal vents (Chang, Reference Chang, Greenberg, Mendoza-Gomez and Pirronello1993; Ferris, Reference Ferris1994). The possible prebiotic relevance of formaldehyde has been pointed out because of its chemical reactivity mainly by condensation and redox reactions; for example, formaldehyde is a starting raw material for the synthesis of carbohydrates. Ribose has been produced from formaldehyde by the formose reaction (Gabel and Ponnamperuma, Reference Gabel and Ponnamperuma1967; Weber and Pizzarello, Reference Weber and Pizzarello2006). Carbohydrates act as construction materials and energy sources (Weber, Reference Weber2001; Benner et al., Reference Benner, Kim, Kim and Ricardo2010). Together with proteins and nucleic acids, these materials play an essential role in biological systems (Cleaves, Reference Cleaves2008).
It has been proposed that diverse radiation sources are able to initiate chemical changes in prebiotic compounds. Among them are UV radiation, electric discharges, radionuclide decay and cosmic rays (Ferris and Chen, Reference Ferris and Chen1975; Chyba and Sagan, Reference Chyba and Sagan1992; Draganić et al., Reference Draganić, Draganić and Adloff1993; Bassez, Reference Bassez2015).
Energy, in the form of ionizing radiation, was probably of great importance in the chemical reactions that occurred in the primitive Earth and extraterrestrial environments (Draganić et al., Reference Draganić, Draganić and Adloff1993; Mosqueira et al., Reference Mosqueira, Albarrán and Negrón-Mendoza1996; Draganić and Draganić, Reference Draganić and Draganić1998, Reference Garzon and Garzon2001). The current knowledge in nuclear sciences and the growing interest in ionizing radiation have contributed to showing the importance of this energy source in prebiotic chemistry (Draganić et al., Reference Draganić, Draganić and Borovipanin1976; Negrón-Mendoza and Ponnamperuma, Reference Negrón-Mendoza and Ponnamperuma1976; Brack and Raulin, Reference Brack and Raulin1991; Akaboshi et al., Reference Akaboshi, Fujii and Navarro-Gonzalez2000; Draganić, Reference Draganić2005; Negrón-Mendoza et al., Reference Negrón-Mendoza, Ramos-Bernal, Colín-García and Heredia-Barbero2016). Calculations indicate that in primitive Earth, 40K decay reached up to around 0.29 mGy per year (Ernst, Reference Ernst1983), which emitted γ and β radiation (Dymek and Klein, Reference Dymek and Klein1988; Draganić, Reference Draganić2005). On the other hand, it has been estimated that the energy deposited by the decay of radionuclides in comets, asteroids and meteorites waste about 14 × 109 mGy over 4.6 × 109 years (Cataldo et al., Reference Cataldo, Ursini, Angelini, Iglesias-Groth and Manchado2011; Cherubini and Ursini, Reference Cherubini and Ursini2015). The search related to the interactions between ionizing radiation and organic substances, such as formaldehyde, is essential for understanding certain steps in chemical evolution and the origin of life.
The present work is devoted to evaluating the stability of formaldehyde exposed to high doses of ionizing radiation simulating prebiotic environments, as well as to studying the formation of other molecules of prebiotic importance.
Materials and methods
Preparation of the glassware
The glassware was cleaned according to the techniques used in radiation chemistry. This cleaning involves treatment with a hot mixture of nitric and sulfuric acids (O'Donnell and Sangster, Reference O'Donnell and Sangster1970).
Preparation of the formaldehyde solutions
For all the experiments, the water used was triple distilled. Additionally, a 37% formaldehyde solution (Sigma-Aldrich, Saint Louis, Missouri) was used to prepare solutions with a concentration of 1.1% (v/v).
Irradiation
Aliquots (5–10 ml) of formaldehyde at room temperature, oxygen-free, at pH 5 (the pH of aqueous solution formaldehyde), were exposed to several doses (8–505 kGy) of 60Co-γ radiation (Gammabeam 651 PT source) at Instituto de Ciencias Nucleares, UNAM. The dose rate (266 Gy min−1) was measured using a ferrous sulphate–cupric sulphate dosimeter (O'Donnell and Sangster, Reference O'Donnell and Sangster1970).
Analysis of the samples
pH determination
The pH of the samples was determined using Whatman Panpeha indicator strips (Sigma-Aldrich) covering an interval of 0–14, before and after being irradiated.
Analysis of formaldehyde by gas solid chromatography
The gas solid chromatography technique was used to evaluate the decomposition of formaldehyde by γ radiation. These analyses were carried out in a Varian Aerograph 2400 chromatograph, equipped with a flame ionization detector. A stainless-steel column (1.22 m × 0.32 mm) packed with Chromosorb 102 (100/80 mesh) was used with nitrogen as a carrier gas (flow rate 30 ml min−1). The programming temperature was from 60 to 230°C with a rate of 6 °C min−1. The volume of injection was 3 µl.
Analysis by gas chromatography–mass detector
Gas chromatography–mass detector (GC-MS) analyses were performed to identify the decomposition products of formaldehyde in a range of molecular weights from 30 to 90 g mol−1. Irradiated samples were analysed in an Agilent Technologies 6850 Network Gas Chromatography system coupled with a mass spectrometer Agilent Technologies MSD 5975C quadrupole detector. A capillary column HP-5MS (Agilent Technologies 19091-433E), 30 m × 0.25 mm I.D. and film thickness 0.25 µm was used. The carrier gas was helium at a flow rate of 1 ml min−1. The column temperature was programmed from 150 to 210°C at 6°C min−1. The volume of injection was 3 µl.
Analysis of formic acid
The irradiated samples were evaluated by titration with a normalized base to determine the concentrations of formic acid produced from formaldehyde.
The titrant solution was sodium hydroxide 0.1 N, and the indicator was phenolphthalein 0.5% (Sigma-Aldrich). The assay was performed at least three times. Formic acid (Sigma-Aldrich) 1 × 10−3 M was used as a control.
Search for aldehydes and ketones using 2,4-dinitrophenylhydrazine
2,4-Dinitrophenylhydrazine (DNPH) derivatives were prepared following the formation of aldehyde and ketones. They were prepared according to the procedure of Shriner et al. (Reference Shriner, Fuson and Curtin1966). The irradiated solution of formaldehyde was derivatized with 0.15 M of DNPH. The derivatives were filtered, recovered and dried at room temperature. Later, 0.003 g of this powder was dissolved in 50 mL of acetonitrile (Merck) and then analysed by HPLC (Thermo-Scientific Dionex Ultimate 3000) coupled with a UV detector. A Cortecs C-18 column was used, 2.7 µm, 4.6 mm × 150 mm (Waters). The eluent phase was a mixture of acetonitrile (Meyer) and water (70;30), with a flow rate of 0.5 ml min−1 and a pressure of 1100 psi. The volume of injection was 20 µl. The products were detected with a UV-vis detector at a wavelength of 360 nm.
Results
pH variation
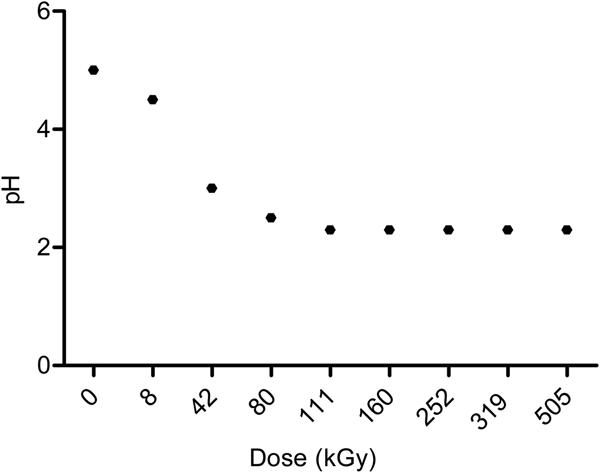
The decomposition of formaldehyde was accompanied by pH variations. The pH of the solution changed abruptly, from 5 in a non-irradiated solution to 2.5 at 111 kGy; after that, it stays constant (Fig. 1). Two of the decomposition products of formaldehyde are methanol and formic acid, the last one causes a change in the pH of the irradiated solution until it reaches 2.3 (Navarro-González et al., Reference Navarro-González, Castillo-Rojas and Negrón-Mendoza1990).
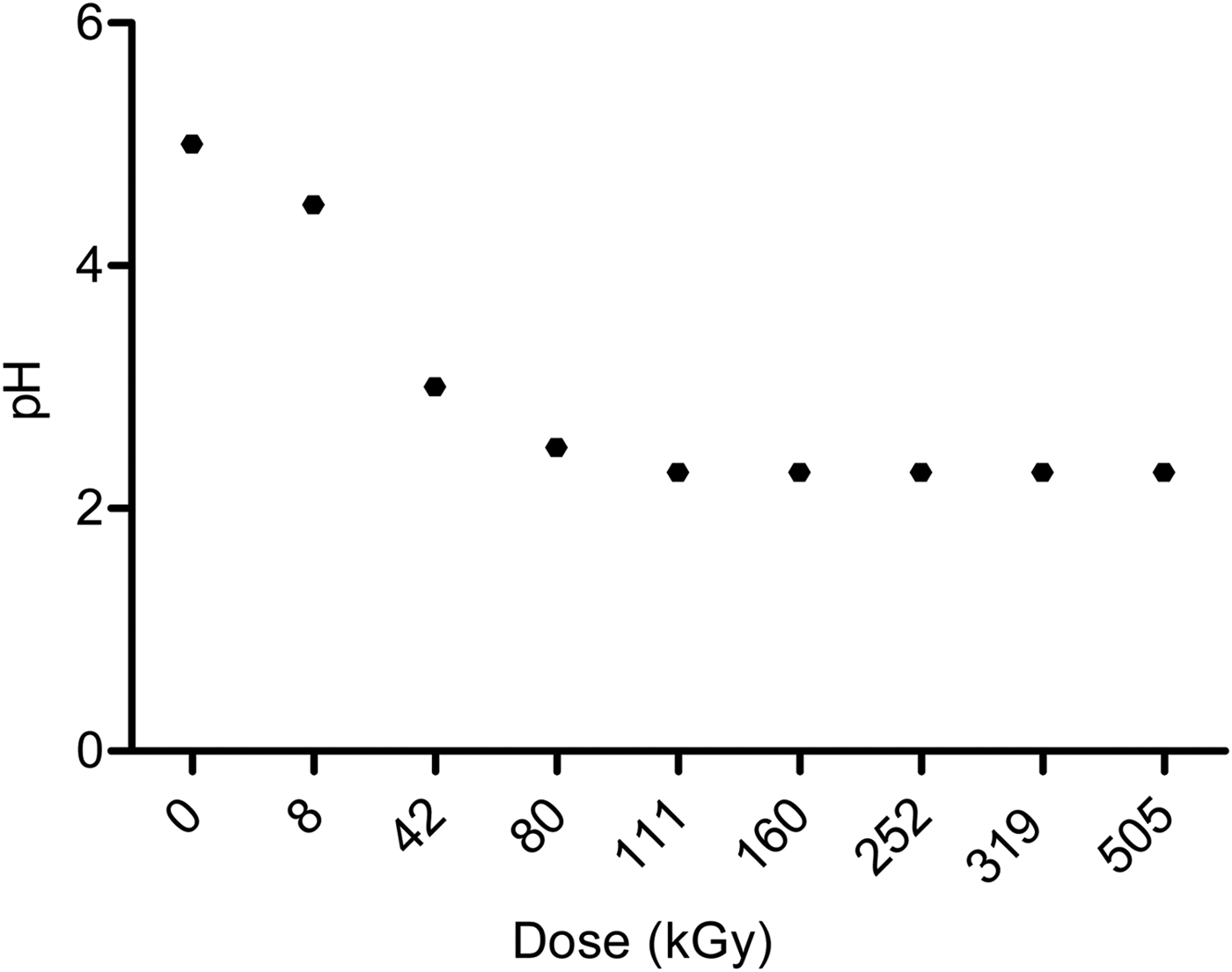
Fig. 1. pH variation of aqueous formaldehyde solutions 1.1% after γ irradiation.
Decomposition of formaldehyde
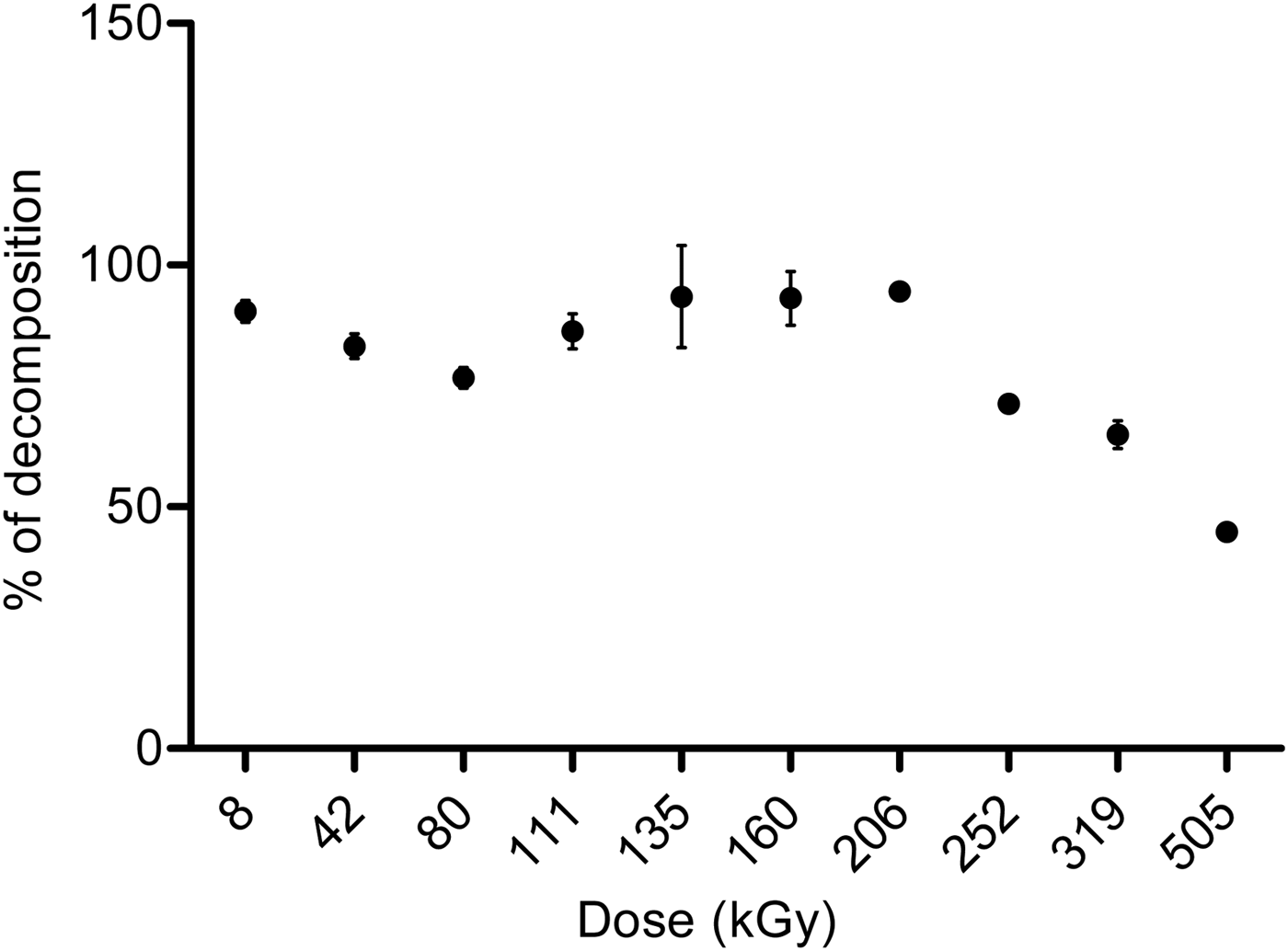
Analysis by gas chromatography showed that the percentage of decomposition increased with the irradiation dose (Fig. 2). Nonetheless, in the interval from 111 to 206 kGy, there was a significant increase in the concentration of formaldehyde, probably due to the regeneration of this molecule by its radiolytic products (formic acid). These results will be discussed later. Glycolaldehyde and formic acid were identified by CG-MS.
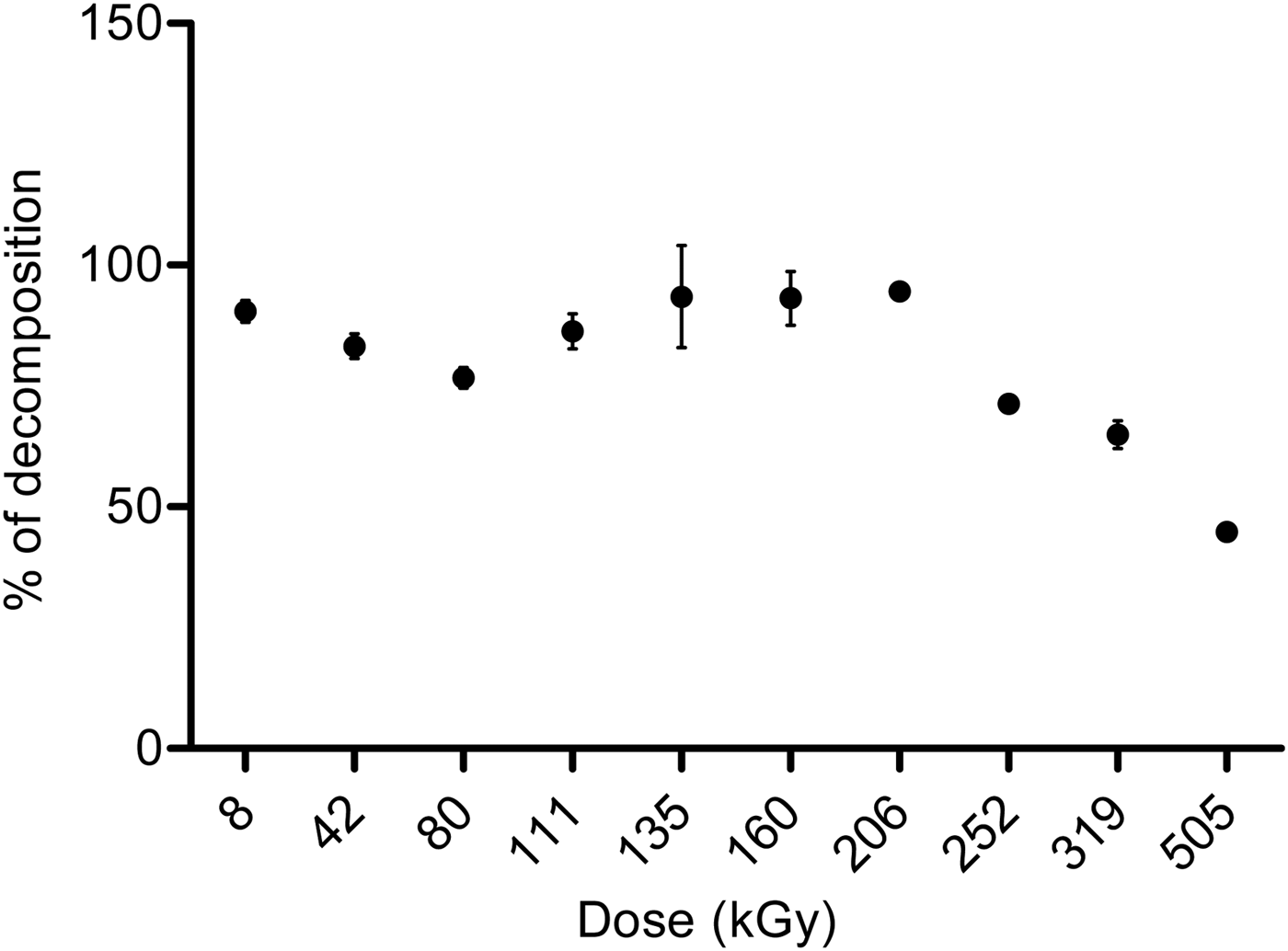
Fig. 2. Effect of the dose in the radiolysis of formaldehyde solutions, measured by gas solid chromatography.
Formation of formic acid
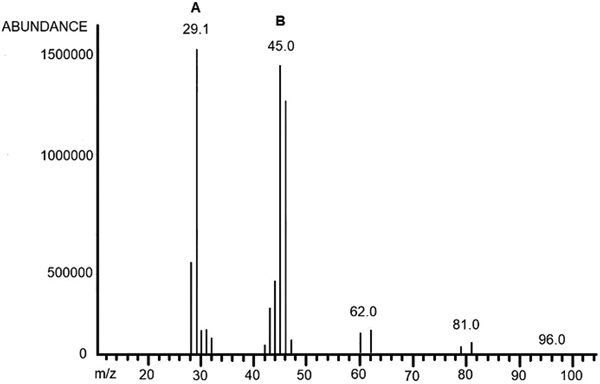
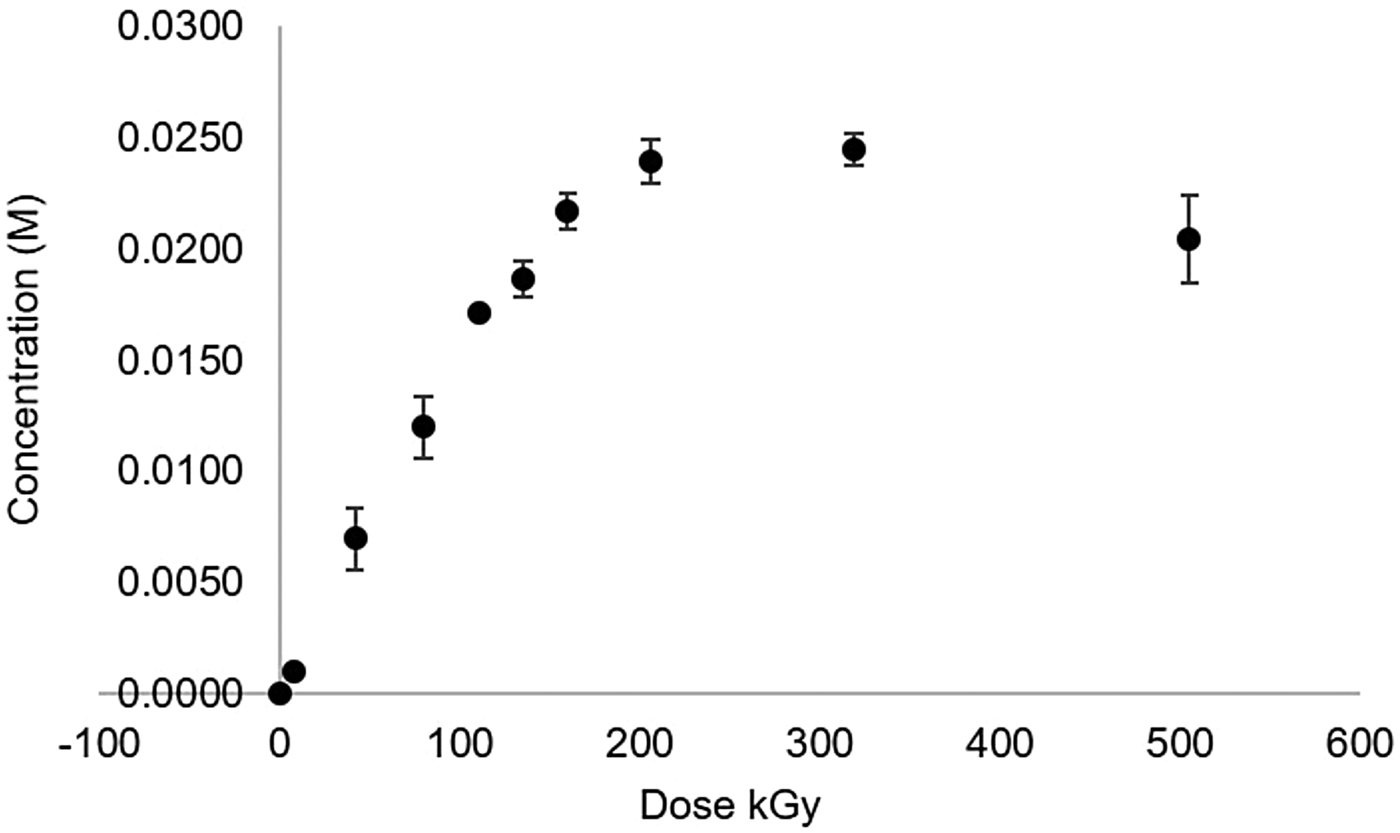
Irradiated formaldehyde was analysed by GC-MS to detect molecules with a molecular mass in the range from 30 to 90 g mol−1. A mass of 46 g mol−1 was detected and corresponded to formic acid molecule (Fig. 3). By titration, we observed a dose-dependent increase in the concentration of formic acid (Fig. 4).

Fig. 3. Mass spectrum of formic acid formed by irradiation of formaldehyde at 160 kGy. (a) Formaldehyde m/z 30, (b) formic acid m/z 46.

Fig. 4. Formation of formic acid from irradiated formaldehyde solutions as a function of the irradiated dose.
Search for aldehydes and ketones
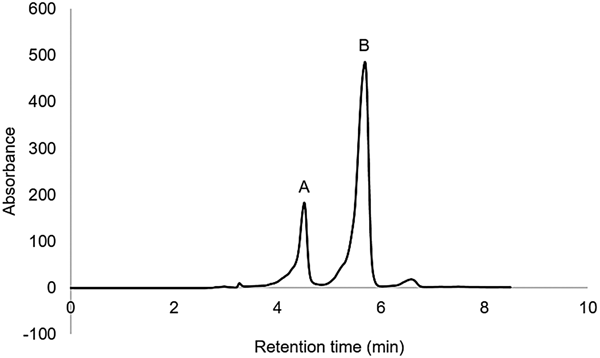
The analysis of DNPH derivatives by HPLC showed that DNPH-glycolaldehyde was formed under these conditions (Fig. 5). Irradiated samples were compared with DNPH-glycolaldehyde standard (Fig. 5(a)). At 5.5 min, another compound was detected (Fig. 5(b)), corresponding to the retention time of DNPH-formaldehyde. As mentioned above, with this technique, an increase of formaldehyde in the interval of 111–252 kGy was observed.
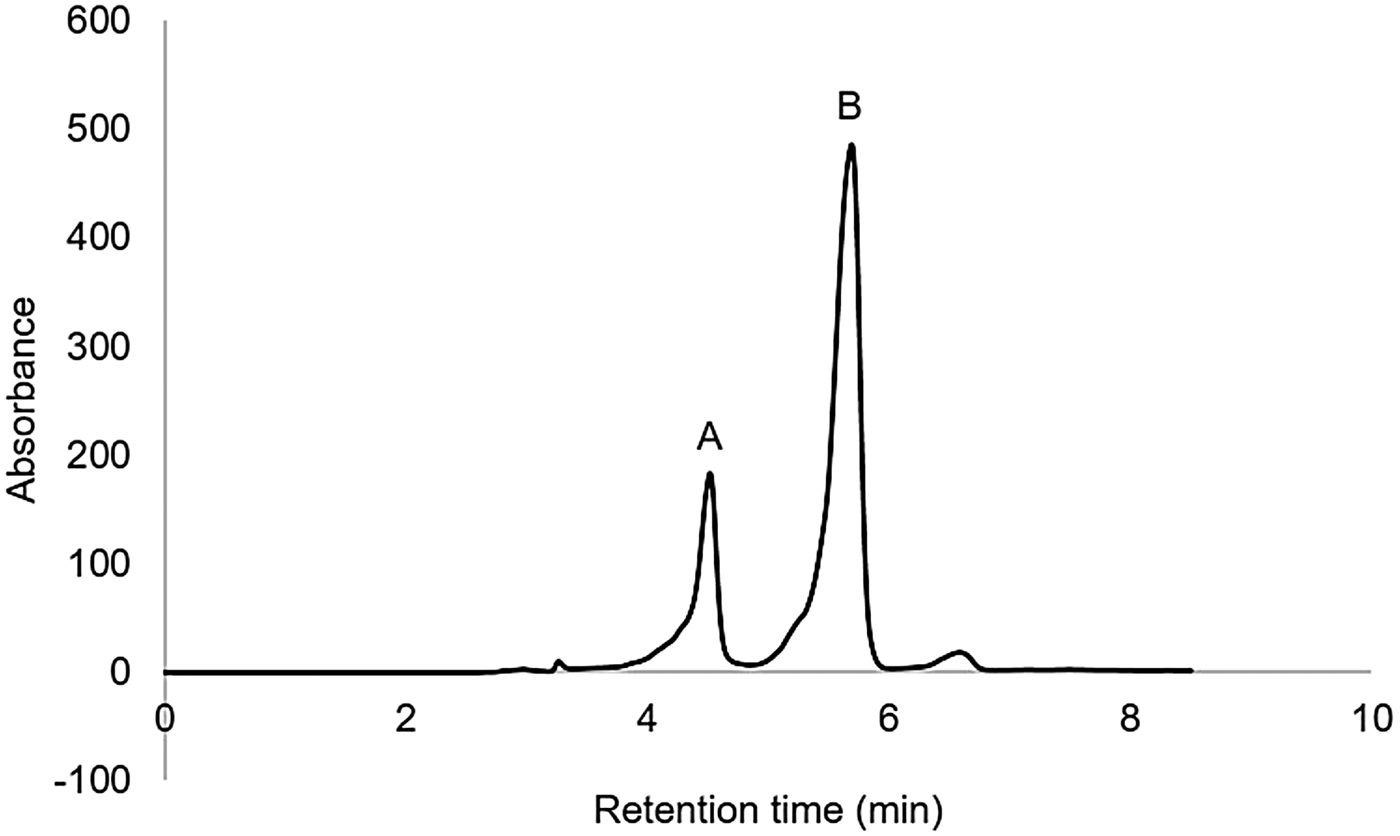
Fig. 5. HPLC chromatogram of DNPH derivatized aqueous solution of formaldehyde irradiated at 160 kGy. (a) DNPH-glycolaldehyde, (b) DNPH-formaldehyde.
Discussion
The evaluation of the stability of organic compounds in prebiotic chemistry simulations can give us an insight into the availability of these molecules to follow for further reactions and their transformation to form more complex molecules. The accumulation of organic molecules will be limited by their rate of formation/destruction. Formaldehyde is chemically reactive, and its permanence in a prebiotic environment can be critical for the synthesis of molecules with biological relevance. Formaldehyde, along with hydrocyanic acid and ammonia, is considered a key compound for pre-biological synthesis (Chang, Reference Chang, Greenberg, Mendoza-Gomez and Pirronello1993).
Formaldehyde has been proposed as an essential precursor for biomolecules, especially sugars (Cleaves, Reference Cleaves2008). It is readily formed through several simulation experiments (Bossard et al., Reference Bossard, Raulin, Mourey and Toupance1982; Stribling and Miller, Reference Stribling and Miller1987), and is observed in different extraterrestrial bodies (Chyba et al., Reference Chyba, Thomas, Brookshaw and Sagan1990; Greenberg et al., Reference Greenberg, Mendoza-Gomez and Pirronello1993). However, its stability under the possible prebiotic environments is important, and this information is scarce.
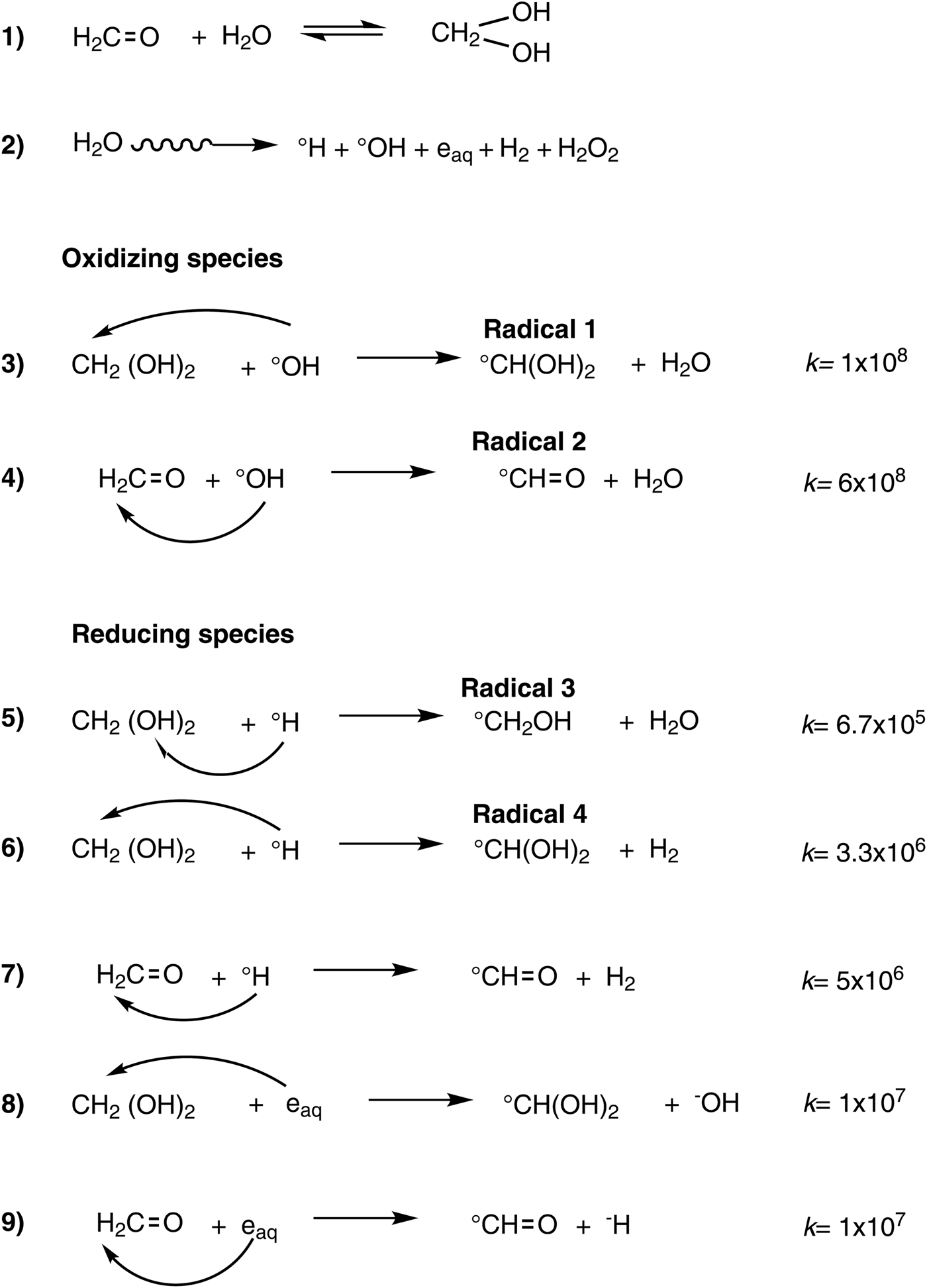
Formaldehyde in solution reacts by a nucleophilic attack of the water molecule forming the hydrated form (Scheme 1, equation 1). The position of the equilibrium, in this case K = 40, strongly favours the hydrated form due to electronic and steric effects, and only about 0.1% of the keto form is present (Baker and Engel, Reference Baker and Engel1992). The keto form adsorbs at a range of 250–350 nm, and the hydrated form does not absorb at this range. This chemical behaviour favours the permanence of formaldehyde in aqueous systems (Ferris and Chen, Reference Ferris and Chen1975). This enables protection of formaldehyde in aqueous solution from the UV light photolysis. However, with other sources of energy, this protection does not occur, and formaldehyde can be very reactive.

Scheme 1. Equation 1, hydrated form of formaldehyde. Equation 2, reactive species of water induced by γ radiation. Equations 3–9, reactions of the oxidizing and reducing species of water with formaldehyde and its hydrated form.
As mentioned previously, ionizing radiation could have been a more efficient energy source for prebiotic processes. It is ubiquitous in the Earth, abundant and efficient in inducing chemical changes via free radicals, and its way of energy deposition is very specific. Water represents the more abundant compound in the system studied. When it is exposed to an ionizing source, such as γ radiation, the energy is practically all deposited into the water molecules. The effect of the radiation is the formation of excited and ionized molecules, forming very reactive chemical species, which in a further step react with the formaldehyde molecules through a secondary process. The γ radiation induces the formation of primary products (·OH, ēaq, ·H, H2O2 and H2) in aqueous oxygen-free solutions (Draganić and Draganić, Reference Draganić and Draganić1971; Garibov et al., Reference Garibov, Melikzade, Bakirov and Ramazanova1982; Draganić, Reference Draganić2005), which in turn promote further reactions with themselves and with the solute present in the solution. A primary radiolytic product from formaldehyde is formic acid (Fig. 5), as observed previously (Navarro-González et al., Reference Navarro-González, Castillo-Rojas and Negrón-Mendoza1990; McElroy and Waygood, Reference McElroy and Waygood1991). We confirmed its formation by the changes of pH and its fragmentation pattern by GC-MS analysis of irradiated samples (Figs. 1 and 3). Formic acid concentration increased with the dose, reaching up to 0.02 M (Fig. 4). A probable pathway for its formation is the following:
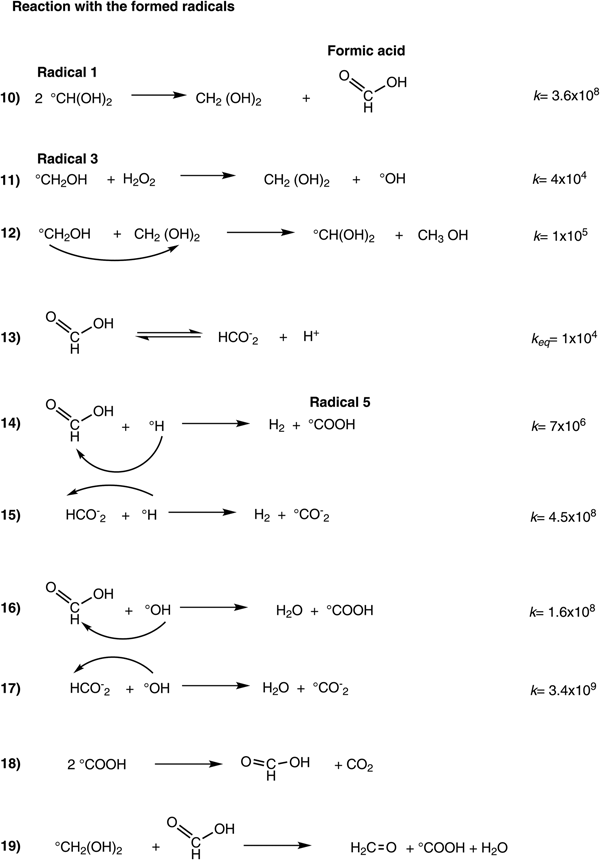
When a critical concentration of formic acid is reached in the irradiated system, in a dose interval from 111 to 206 kGy (Figs. 2 and 4), formaldehyde and formic acid compete for the free radicals of the water decomposition, resulting in the regeneration of formaldehyde. This regeneration is an important aspect that has not been observed before and ensures a longer permanence of formaldehyde in an environment exposed to a high radiation field. These data suggest two steps in the behaviour of formaldehyde under irradiation: (1) decomposition/formation and (2) regeneration. In the first step, the formaldehyde is destroyed, and formic acid is formed. In this phase, the reactive species react mainly with formaldehyde and take place in a formic acid formation, and its concentration increases gradually. The second step involves formaldehyde regeneration by the formic acid and other intermediates formed in the radiolysis. Some competitive reactions (Scheme 2, equations 12–19) take place between formaldehyde and its radiolytic product.
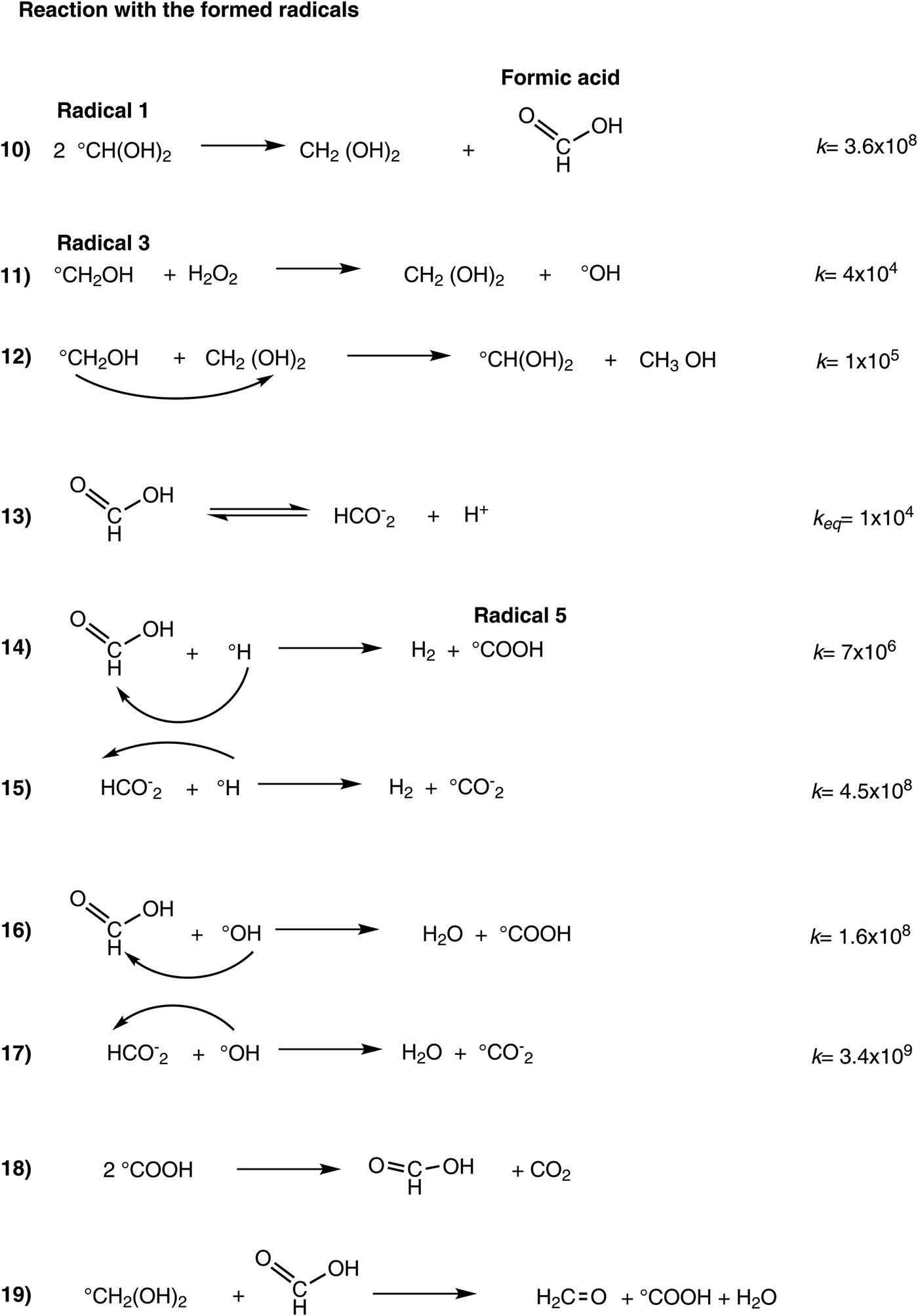
Scheme 2. Equation 10, formic acid formation. Equation 11, formaldehyde regeneration. Equations 12–19, interaction with radiolytic products.
Based on inventory kinetic data of the National Standard Reference Data Systems (NSRDS) (Anbar et al., Reference Anbar, Bambenek and Ross1975a, Reference Anbar, Ross and Farhataziz1975b; Ross and Farhataziz, Reference Ross and Farhataziz1975), the reaction mechanism involves several steps involving the radiolytic products of water radiolysis and the formed radicals (Scheme 1).
The ·OH radical is a powerful oxidant species and reacts very efficiently with the hydrated form through abstraction reactions (Scheme 1, equations 3 and 4). At the pH of the solution (2.5), the reducing species (·H and ēaq) are present, specially the .H and react with the hydrated form of the formaldehyde (Scheme 1, equations 5–9).
The disproportionate reaction of radical 1 generates the formic acid (equation 10). This reaction has a high reaction rate constant (3.6 × 108 dm3 mol−1 s−1) and it results in a very efficient method of producing formic acid. The hydrate is also regenerated by this reaction. McElroy and Waygood (Reference McElroy and Waygood1991) proposed the plausible oxidation of formaldehyde by the radical hydroxyl (·OH). Likewise, other researchers have supported this proposal (Steenken, Reference Steenken1979; von Sonntag, Reference von Sonntag1980). Because this reaction produced the radical 1, the hydrate can also be regenerated. Other reactions involved are in the Scheme 2, equations 12–19.
Once formic acid is formed and reaches a critical concentration, it starts to compete for the radiolytic species of water, with rate constants in the order of 106–108 dm3 mol−1 s−1 – much higher than the rate for formaldehyde. Formic acid is mainly in undissociated form (98%) at the pH of the irradiated solution (pKa = 3.75). After this dose interval (111–206 kGy), its concentration decreases. Garrison et al. (Reference Garrison, Bennett and Jayko1956) and Adams and Hart (Reference Adams and Hart1962) demonstrated the reduction of formic acid to formaldehyde by radiation.
Formic acid is prebiotically relevant, being a carbon source for lipid compound formation in primitive Earth (McCollom et al., Reference McCollom, Ritter and Simoneit1999). Also, formic acid has been observed in several astronomical sources such as comets, proto-stellar ices, dark molecular clouds and regions associated with the stellar formation (Andrade et al., Reference Andrade, Domaracka, da Silveira, Rothard and Boduc2011).
Glycolaldehyde detection
The formation of glycolaldehyde from formaldehyde solutions was detected starting with an irradiation dose of 160 kGy. This formation is important due that glycoladehyde is considered the simplest sugar, and it is the first intermediate in the formose reaction (Cleaves, Reference Cleaves2008; Marcellus et al., Reference Marcellus, Meinert, Myrgorodskab, Nahonc, Buhsed, d'Hendecourta and Meierhenrichb2015). It is important to point out that the formose reaction takes place under basic conditions. However, it has been reported that it can occur at neutral pH (Shwartz and De Graaf, Reference Schwartz and De Graaf1993). In our experiments, formation of glycolaldehyde occurred at 2.5 pH. This finding provides new information about the conditions for the formation of formose intermediates.
It has been proposed that glycolaldehyde synthesis from formaldehyde is initiated by condensation reactions (Scherz, Reference Scherz1970; Kochetkov et al., Reference Kochetkov, Kudrjashov and Chlenov1979; Meinert et al., Reference Meinert, Myrgorodska De Marcellus, Buhse, Nahon, Hoffmann, Le Sergeant, d'Hendecourt and Meierhenrich2016). Ricardo et al. (Reference Ricardo, Carrigan, Olcott and Benner2004) reported the ribose synthesis from formaldehyde and glycolaldehyde in the presence of borate, which reinforces our result in which both formaldehyde and glycolaldehyde coexist and could take the place in the ribose synthesis.
Glycolaldehyde has been detected in molecular clouds and protostars such as IRAS 16293-2422 (Halfen et al., Reference Halfen, Apponi, Woolf, Polt and Ziurys2006; Jørgensen et al., Reference Jørgensen, Favre, Bisschop, Bourke and van Dishoeck2012). It has also been successfully detected in ice simulations (Hudson et al., Reference Hudson, Moore and Cook2005).
Conclusion
Considering that the stability of formaldehyde is an important aspect in simulating plausible prebiotic scenarios, we studied the behaviour of formaldehyde in aqueous solution exposed to ionizing radiation and the most remarkable results are the following:
1. The yield of decomposition of formaldehyde under irradiation is high. However, it is regenerated through formic acid and other radiolytic intermediaries.
2. The radiolytic products detected from irradiated formaldehyde were formic acid and glycolaldehyde; all of them are of potential prebiotic interest because they are precursors of more complex molecules, such as lipids and sugars.
3. Maintaining a formaldehyde pool is crucial to ensure its availability for synthesis of pre-biological molecules, even with a high dose of radiation. One pathway to keep this pool is by the radiation-induce decomposition of formic acid or glycolaldehyde, which have a similar structure. Therefore, they can regenerate between them.
4. Finally, we observe the formation of glycolaldehyde at 2.5 pH. These data suggest another alternative for the synthesis of formose intermediates under acid conditions.
Acknowledgments
The authors acknowledge support from Posgrado en Ciencias Biológicas, UNAM and the CONACyT fellowship 345522. The Support from DGAPA grant IN111116 is acknowledged. The authors are grateful to Benjamin Leal, M.Sc., and Francisco Flores, Phys., for their technical assistance. Finally, they are grateful to Claudio Fuentes Carreón for the preparation of DNPH derivatives used for controls in the performed experiments, and to Claudia Camargo Raya, Chem., for support in the laboratory.