Introduction
Titan is Saturn's largest satellite with a diameter of 5150 km, and is known to have a dense atmosphere in contact with liquids on its surface (Lunine & Atreya Reference Lunine and Atreya2008). The atmospheric pressure at the surface is about 1500 hPa at a temperature of 94 K (Samuelson et al. Reference Samuelson, Hanel, Kunde and Maguire1981; Lindal et al. Reference Lindal, Wood, Hotz, Sweetnam, Eshelman and Tyler1983). The main component of the atmosphere is nitrogen, with smaller amounts of methane and ethane. Voyager's infrared interferometer spectrometer also detected trace amounts of ethane (C2H4), acetylene (C2H2), propane (C3H8), hydrogen cyanide (HCN), acetonitrile (CH3CN), cyanoacetylene (CHCCN) and other diverse organic compounds in Titan's atmosphere (Hanel et al. Reference Hanel1981; Kunde et al. Reference Kunde, Aikin, Hanel, Jennings, Maguire and Samuelson1981; Maguire et al. Reference Maguire, Hanel, Jennings, Kunde and Samuelson1981). In 1995, the Cassini spacecraft was launched by NASA and ESA to explore the Saturn system, and continues to return remarkably detailed images of Saturn and its satellites. In 2005, the Huygens probe separated from Cassini and descended through the atmosphere to Titan's surface. During descent, a gas chromatograph-mass spectrometer (GC/MS) analysed atmospheric gases and detected HCN and NH3 after pyrolysis of captured mist particles (Israel et al. Reference Israel2005).
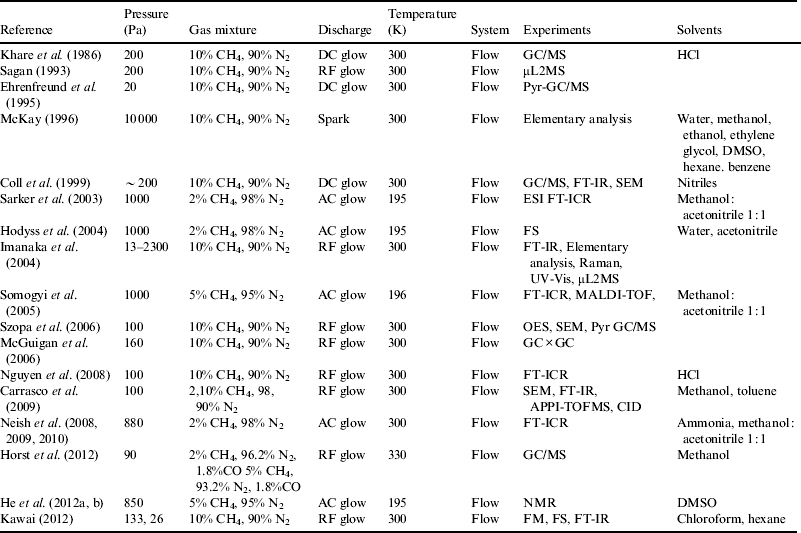
Syntheses of organic material in simulated Titan atmosphere were first performed by Sagan & Khare (Reference Sagan and Khare1979) who irradiated mixtures of nitrogen and methane with ultraviolet light and electrical discharge. A viscous brown organic polymer formed, which they named tholin after the Greek word for ‘muddy’. The tholin was pyrolysed and the products were analysed with a Py-GC/MS. A variety of aliphatic and aromatic hydrocarbons, nitriles and nitrogen-containing compounds such as pyrrole and pyridine were detected after pyrolysis at 900 °C. It was suggested that tholins were kerogen-like substances having complex structures. The initial observations were followed by later studies in which a variety of gas mixtures were irradiated with different energy sources and the products analysed (Khare et al. Reference Khare, Sagan, Ogino, Nagy, Er, Schram and Arakawa1986; Sagan et al. Reference Sagan, Khare, Thompson, McDonald, Wing, Bada, Vo-Dihn and Rakawa1993; Ehrenfreund et al. Reference Ehrenfreund, Boon, Commandeur, Sagan, Thompson and Khare1995; Coll et al. Reference Coll, Coscia, Smith, Gazeau, Ramõrez, Cernogor, Israel and Raulin1999). (See Table 1 for a list of relevant publications.)
Table 1. Parameters of tholin synthesized by an electrical discharge

Hydrocarbon lakes on the surface on Titan were first predicted by a photochemical model (Lorenz Reference Lorenz1994). The Huygens probe revealed that Titan's surface temperature was 93.7 K (Fulchignoni et al. Reference Fulchignoni2005), at which ethane and methane can remain liquid, and the Cassini radar observations showed that there are lakes composed of hydrocarbons (mainly liquid ethane and methane) at both high and low latitudes (Stofan et al. Reference Stofan2007; Brown et al. Reference Brown, Soderblom, Soderblom, Clark, Jaumann, Barnes, Sotin, Buratti, Baines and Nicholson2008; Griffith et al. Reference Griffith, Lora, Turner, Penteado, Brown, Tomasko, Doose and See2012). Furthermore, if water ice on the surface of Titan is melted by a meteorite strike, as suggested by computer simulations, a liquid water lake would remain for 102–104 years (O'Brien, et al. Reference O'Brien, Lorenz and Lunine2005). Over the 108 years history of Titan, it is estimated that 1022 kg of carbonaceous chondrites have delivered organic compounds to its surface, including possible lakes (Jones & Lewis Reference Jones and Lewis1987). Biologically important elements such as P, S, K and Na are contained in C1 chondrites (Anders & Grevesse Reference Anders and Grevesse1989), and would also be supplied to Titan's liquidospheres.
Ammonia frost has been observed on the surface of Titan (Nelson et al. Reference Nelson2009), and the existence of sub-surface ammonia-water solutions was suggested by simulation models (Grasset et al. Reference Grasset, Sotin and Deschamps2000; Tobie et al. Reference Tobie, Grasset, Lunine, Mocquet and Sotin2005).The pH of an ammonia–water solution with 15 wt% ammonia is ∼11.4 (Engel et al. Reference Engel, Lunine and Norton1994; Fortes, Reference Fortes2000) but the pH is expected to decrease at high pressure to a value closer to ∼10.5 (Krauskopf & Bird Reference Krauskopf and Bird1995). Given that both tholins and a liquidosphere are present on Titan, what happens when tholins come into contact with liquids like ammonia-water? There have been several reports on the solubility of tholins. McKay (Reference McKay1996) synthesized tholins by spark discharges in a mixture of 10% CH4 and the balance N2, and tested their solubility in several solvents. Tholins could be dissolved in water, methanol and ethanol. Coll et al. (Reference Coll, Coscia, Smith, Gazeau, Ramõrez, Cernogor, Israel and Raulin1999) examined the solubility of tholins that were formed by direct current (DC) glow discharges in a gas mixture of N2/CH4 (98 : 2), and found that they were soluble in nitriles such as acetonitrile, isobutyronitrile, propionitrile and benzonitrile. When a mixture of CH4 and N2 was exposed to an alternating current (AC) electrical discharge at 195 K, the products were soluble in a methanol/acetonitrile mixture (1 : 1) for ESI-MS and FT-ICR-MS measurements in order to measure their molecular weights and elemental compositions (Sarker et al. Reference Sarker, Somogy, Lunine and Smith2003; Somogyi et al. Reference Somogyi, Oh, Smith and Lunine2005). He et al. (Reference He, Lin, Upton, Imanaka, Mark and Smith2012a,Reference He, Lin, Upton, Imanaka, Mark and Smithb) synthesized tholins in a similar way but with 13C and 15N isotopic labelling, and dissolved them in DMSO to investigate structures of tholins by nuclear magnetic resonance (NMR) spectroscopy.
The presence of a liquidosphere on Titan containing an admixture of tholins suggests that it would be worth investigating how tholins behave in aqueous dispersons. Trainer et al. (Reference Trainer, Pavlov, DeWitt, Jimenez, McKay, Toon and Tolbert2006) irradiated a gas mixture composed of nitrogen and methane, and observed spherical particles several nm in diameter by transmission electron microscopy. However, there have been no studies of tholins exposed to conditions simulating an ammonia-water ocean. The purpose of the present research is to examine self-assembly properties of tholins under these conditions. Since both hydrophilic and hydrophobic liquids are presumed to be present in Titan, we studied solutions of tholins in non-polar solvents and possible self-assembly of tholins in a polar ammonia-water solvent. Hexane and chloroform were used in place of methane and ethane because they can exist as liquids at room temperature. Solutions of tholins in these solvents were analysed by FT-IR, and fluorescence spectrometry. Samples in ammonia-water were observed by microscopy to undergo self-assembly into coacervates.
Materials and methods
Chemicals
Deionized water was further purified with Milli-Q (Easy pure UF compact bioresearch water system) to remove both inorganic ions and organic contaminants. A gas mixture of CH4/N2 (10/90) was purchased from Matheson Tri-Gas/Aeris (USA). All other chemicals used were of analytical grade. Glassware and a metal rod were heated in high-temperature oven at 500 °C in order to eliminate possible organic contaminants.
Synthesis of Tholins
Tholins were synthesized by plasma discharge into the gas mixture at 1 Torr (133 Pa) for 72 hours. The discharge device at NASA Ames was RFX-600 with a glass slide attached to the lower electrode. The chamber was flushed with oxygen to clean it before the gas mixture was injected. After plasma discharge for 72 hours, the products on the plate were scraped off with a metallic rod. The collected tholins were dissolved in either non-polar solvents such as hexane and chloroform, or dispersed in an ammonia-water solution, followed by analysis.
FT-IR
FT-IR spectra of tholin samples in chloroform (1 mg ml−1) were obtained with a Nicolet Nexus 670 spectrometer over the range of 4000–500 cm−1 with a resolution of 1 cm−1. After filtration through a PTFE membrane filter (pore size; 2 μm, Varian Inc.), the filtrate was completely dried, then 50 μl of chloroform was added. Twenty μl of the solution was dried on a KBr window (PIKE Technologies). The FT-IR sample chamber was constantly purged with dry air to remove moisture.
Fluorescence spectrometry
Tholin samples were dispersed in 10 mM NH4OH at a concentration of 0.67 mg ml−1. The dispersion was filtered through cellulose acetate filter (pore size; 0.2 μm, ADVANTEC), and the filtrate was put in a quartz cell (Scientific Ltd.). Three-dimensional fluorescence spectra were measured with a JASCO FP-6300 fluorescence spectrometer. Excitation and emission were scanned over the range of 220–600 nm at 1 nm intervals with the excitation slit set at 20 nm.
Fluorescence microscopy
A Zeiss Axiovert 135 M fluorescence microscope was used to monitor possible self-assembly properties. Tholin samples dissolved either in chloroform or hexane were dried on a clean glass slide, followed by addition of 10 μl NH4OH and a cover slip. The initial drying process was designed to allow self-assembly of tholins into aggregates which could then be visualized. The same field was observed by differential interference contrast (DIC) mode and in fluorescence mode with a filter (excitation: 365BP25) and a dichroic mirror (370). Images were taken with a digital camera (Canon EOS Kiss X3).
Results
Solubility of tholins
The samples described here were synthesized in gas mixtures either at high pressure (1 Torr, 133 Pa) or low pressure (0.2 Torr, 26 Pa). Hereafter, the former product is referred to as HP tholins and the latter as LP tholins. Tholins could be partially dissolved in chloroform to produce a pale yellow-orange solution, but solubility of tholins in hexane was quite low.
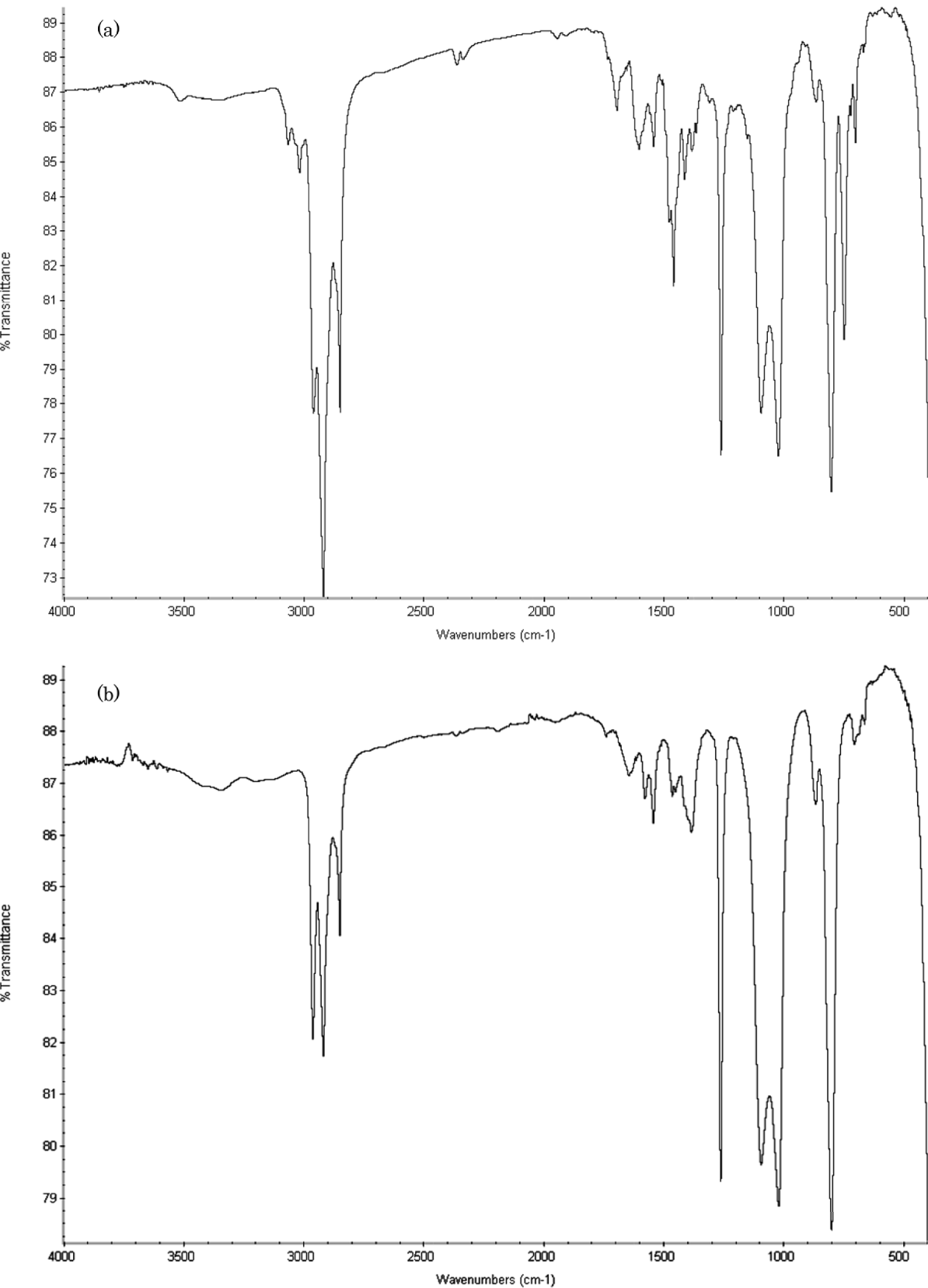
FT-IR
Infrared spectra of chloroform soluble fraction of HP and LP tholins are shown in Fig. 1(a) and (b). The bands at 3324–3366 cm−1 (Fig. 1(a)) and at 3355 cm−1 (Fig. 1(b)) were assigned to stretching of N-H groups. Other bands at 3019, 2959, 2918 and 2849 cm−1 (Fig. 1(a)) and at 3019, 2962, 2918 and 2849 cm−1 (Fig. 1(b)) were assigned to stretching of C-H groups. In addition, tholins gave peaks of unsaturated bonds such as C=C and C=N bonds (1600–1670 cm−1) and C≡N bond (2200–2260 cm−1). LP tholins gave stronger peaks of –NH and –CN groups compared with the chloroform-soluble fraction of HP tholins.

Fig. 1. FT-IR Spectra of HP (a) and LP (b) Tholin (Chloroform fraction) on KBr.
Fluorescence spectrometry
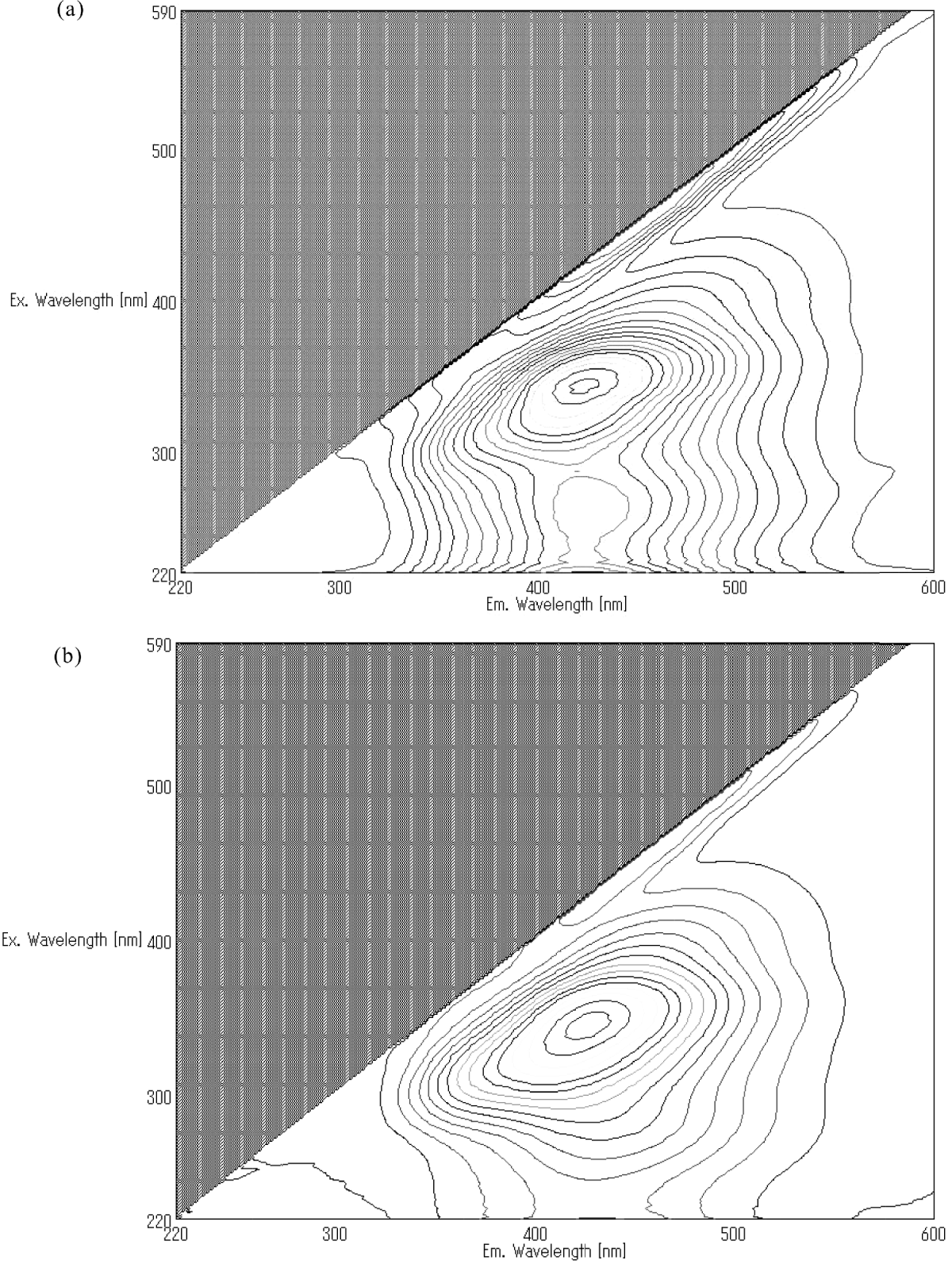
Three-dimensional fluorescence spectra of ammonia-water extracted of HP tholins and LP tholins are similar (Fig. 2(a) and (b)). Maximum excitation and emission wavelengths of the HP tholins were 346 and 426 nm, respectively, and those of LP tholins were 345 and 428 nm.

Fig. 2. Two-dimensional fluorescence spectra of HP (a) and LP (b) tholins (ammonia-water fraction).
Microscopy
We used DIC microscopy and fluorescence microscopy to investigate the behaviour of tholins exposed to aqueous ammonia solutions. After tholin samples were dried from chloroform or hexane solutions onto glass slides, the HP tholins adhered to the slide as brown particles, whereas LP tholins were black. Following addition of 10 mM NH4OH, the tholins dried from chloroform solutions assembled into spherical structures in the range of 1–15 μm diameters. Tholins dried from hexane solutions produced somewhat larger and more complex globular structures (Fig. 3). Since chloroform is more polar than hexane, the chloroform extract of tholins contained higher concentrations of polar molecules, while hexane extracts contained more non-polar molecules. The difference in polarity probably accounts for the observed variations in the self-assembled structures.

Fig. 3. (a) The DIC image when 10 mM ammonia solution was added to tholins dried from a chloroform solution onto slide glass (×400). (b) Fluorescence image of the same field. (c) The DIC image when 10 mM ammonia solution was added to the tholins dried from a hexane solution onto slide glass (×400). (d) Fluorescence image of the same field.
Discussion
In order to understand the self-assembly properties of tholins, it is essential to have general knowledge of their synthesis and composition. Possible energy sources available on Titan include ultraviolet light, electrical discharge and cosmic rays. Ultraviolet light is not absorbed by molecular nitrogen gas and therefore does not photochemically activate a nitrogen-methane mixture (Yung et al. Reference Yung, Allen and Pinto1984), hence synthesis of organic compounds containing nitrogen requires higher energy sources such as electrical discharges.
A variety of two to four ring polycyclic aromatic hydrocarbons (PAHs) were identified by two step laser mass spectrometry (L2MS) in tholins that were formed from a 90% N2/10% CH4 gas mixture by DC discharges (Sagan et al. Reference Sagan, Khare, Thompson, McDonald, Wing, Bada, Vo-Dihn and Rakawa1993). Ehrenfreund et al. (Reference Ehrenfreund, Boon, Commandeur, Sagan, Thompson and Khare1995) synthesized tholins by plasma discharge of a mixture of 90% nitrogen and 10% methane at 0.2 hPa, and characterized the products by Py-GC/MS. They reported that tholins were composed of hydrogen cyanide, acetonitrile, long-chained unsaturated nitriles, aromatic compounds such as benzene and toluene and nitrogen-containing heteroaromatics such as pyridine and pyrrole. The tholins had peaks at 14 m/z intervals, suggesting that a major component of tholins were methylene groups (-CH2-). Since Py-GC/MS analysis provides such rich information about chemical composition, the Huygens probe loaded an Aerosol Collector and Pyrolyser (ACP) for in situ characterization of aerosols in Titan's atmosphere.
Imanaka et al. (Reference Imanaka, Khare, Elsila, Bakes, McKay, Cruikshank, Sugita, Matsui and Zare2004) analysed the tholins synthesized by plasma discharge in a mixture of methane (10%) and nitrogen (90%) at varying pressures (13–2300 Pa), and reported that nitrogen-containing polycyclic aromatic compounds more easily formed at lower pressure. Somogyi et al. (Reference Somogyi, Oh, Smith and Lunine2005) analysed tholins synthesized by an AC electrical discharge in a gas mixture of 95% nitrogen and 5% methane, and the resulting tholins were analysed by high resolution Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS). These authors reported that tholins were complex molecules composed of carbon, hydrogen and nitrogen, with molecular weights in the range of several hundred Daltons. Szopa et al. (Reference Szopa, Cernogora, Boufendi, Correia and Coll2006) analysed atoms and radicals in the discharged plasma with optical emission spectroscopy (OES). The emission spectra obtained showed that atoms and radicals produced by discharges were growing into particles in the plasma. McGuigan et al. (Reference McGuigan, Waite, Imanaka and Sacks2006) analysed the tholins by pyrolysis-two-dimensional gas chromatography/time of flight mass spectrometry (GC×GC-TOF-MS). They identified several organic compounds of nitriles, pyrroles, hydrocarbons, benzenes and PAH compounds. PAHs may also play a significant role in synthesis of tholins (Wilson & Atreya Reference Wilson and Atreya2003).
Tholins have also been characterized with several techniques including estimation of their molecular weights. Gas mixtures of CH4 and N2 (1 mbar, 2 : 98 or 10 : 90, v/v) were irradiated with a radio frequency (RF) plasma discharge. The products were dissolved in methanol or toluene, and identified by atmospheric pressure photoionization (APPI) – hybrid quadrupole time-of-flight mass spectrometry (QTOFMS) with a tandem collision induced dissociation (CID) method. The spectra showed peak clusters with 13–14 m/z intervals in 50–800 m/z area (Carrasco et al. Reference Carrasco2009). Elementary analysis of tholins produced by RF discharge from 13–2300 Pa gas mixtures of CH4 and N2 (10 : 90, v/v) showed that the C/N ratio was 1.45–2.76 (Imanaka et al. Reference Imanaka, Khare, Elsila, Bakes, McKay, Cruikshank, Sugita, Matsui and Zare2004). These previous analytical results suggested that the Titan tholins we synthesized would have hydrophobic groups that were large enough to undergo self-assembly.
Finally, it is significant that biomolecules used in terrestrial life have also been detected in tholins synthesized when a gas mixture of 10% methane and 90% nitrogen at 0.2 mbar was exposed to DC discharge. The products were hydrolysed with 6 M HC1, then derivatized with N-trifluroacetyl isopropyl esters, and 16 amino acids were identified (Khare et al. 1986). In more recent studies, tholins were synthesized by RF discharge in a gas mixture (N2:CH4=98 : 2), hydrolysed in 2 M HCl, and derivatized with N-tert-butyldimethyl-silyl-N-methyltrifluoroacetamide (MTBSTFA) for GC/MS. A variety of organic compounds including amino acids and carboxylic acids were identified (Nguyen et al. Reference Nguyen, Raulin, Coll, Derenne, Szopa, Cernogora, Israe and Bernard2008). Neish et al. (Reference Neish, Somogyi, Imanaka, Lunine and Smith2008, Reference Neish, Somogyi, Lunine and Smith2009, Reference Neish, Somogyi, Lunine and Smith2010) also synthesized tholins in a similar way as reported by Sarker et al. (Reference Sarker, Somogy, Lunine and Smith2003) and Somogyi et al. (Reference Somogyi, Oh, Smith and Lunine2005), and dissolved the tholins in ammonia solution at 253 K or 293 K: They identified almost all amino acids except sulfur-containing species such as cysteine and methionine and nucleobases such as adenine, guanine, thymine, cytosine and uracil in the solution without acid-hydrolysis. Horst et al. (Reference Horst2012) also identified various organic compounds including amino acids and nucleotide bases without hydrolysis for tholins synthesis in a similar way with Szopa et al. (Reference Szopa, Cernogora, Boufendi, Correia and Coll2006), Nguyen et al. (Reference Nguyen, Raulin, Coll, Derenne, Szopa, Cernogora, Israe and Bernard2008) and Carrasco et al. (Reference Carrasco2009)
Fluorescence properties of tholins
Hodyss et al. (Reference Hodyss, McDonald, Sarker, Smith, Beauchamp and Beauchamp2004) synthesized tholins by AC electrical discharge in gas mixtures of CH4 and N2 (1 mbar, 2 : 98 or 10 : 90, v/v) at 195 K. When the synthesized tholins were dissolved in acetonitrile, the fluorescence excitation spectra showed a peak at 410 nm, with an emission peak at 471 nm. When water was used, tholins showed complex fluorescence spectra with multiple peaks. The effects of solvents, temperature and synthesis conditions had only minor effects on the excitation and emission wavelengths. From the Py-GC/MS results, it was suggested that tholins by both DC and RF discharge contain aromatic compounds such as benzene, toluene and heterocyclic compounds like pyrrole and pyridine (Ehrenfreund et al. Reference Ehrenfreund, Boon, Commandeur, Sagan, Thompson and Khare1995; McGuigan et al. Reference McGuigan, Waite, Imanaka and Sacks2006). It seems likely that the fluorescence characteristics of our tholins were mainly due to the presence of aromatic hydrocarbon groups and heterocyclic groups. Since the tholins had fluorescence, it was possible to image self-assembled tholin structures by fluorescence microscopy.
Mechanism of self-assembly of amphiphilic molecules
Tholins are composed of C (carbon), N (nitrogen) and H (hydrogen) present in the original gas mixture, but lack O (oxygen). Most hydrophilic organic compounds have O-containing groups such as hydroxyl group (–OH) and carboxyl group (–COOH), but they cannot be expected to be present in our tholins. For this reason amino or imino groups are likely to be hydrophilic groups in Titan tholins. In fact, FT-IR spectra of chloroform solutions of HP and LP tholins revealed the presence of both hydrophilic amine groups and hydrophobic alkyl groups. In preliminary experiments, we confirmed amphiphilic properties of tholins by spreading a few microlitres of a chloroform solution of HP tholin on the air-water interface of a Langmuir trough. The tholin readily spread to form a film at the interface which produced a measurable surface pressure. When the film was compressed it went through a transition to a solid phase. This result clearly indicates that tholins have surface active properties expected of an amphiphile and are likely to undergo self-assembly into more complex structures.
Self-assembly of ordinary amphiphilic compounds such as fatty acids and phospholipids is related to the length of carbon chains in the molecule. Amphiphiles with hydrophobic groups having more than six carbons in length chains form micelles, and those with eight carbon chains can assemble into vesicles bounded by bilayer membranes (Apel et al. Reference Apel, Deamer and Mautner2002). Long alkyl chains with 13–23 carbons were detected in CM2 chondrites by gas chromatography (Studier et al. Reference Studier, Hayatsu and Anders1968; Smith & Kaplan Reference Smith and Kaplan1970; Anders et al. Reference Anders, Hayatsu and Studier1973). FT-IR spectra showed that the extracted molecules had both hydrophilic groups such as carboxyl and hydroxyl groups and hydrophobic groups such as alkyl groups. If sufficiently long amphiphilic molecules are present, it is likely that they will undergo self-assembly into more complex structures. For instance, Deamer & Pashley (Reference Deamer and Pashley1989) demonstrated that amphiphilic components in chloroform–methanol extracts from the Murchison meteorite produced membranous vesicles and coacervates. From such observations, it is likely that similar self-assembly processes could occur in Titan's liquidosphere.
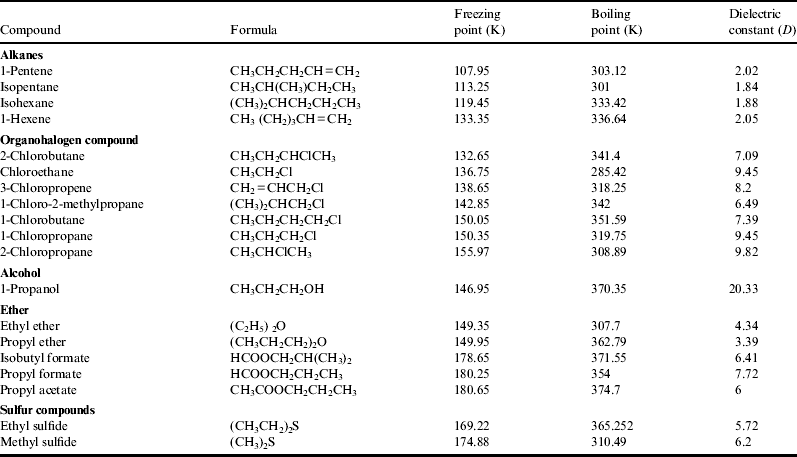
When ammonia solution was added to tholins dried from chloroform solutions, self-assembled microspheres were observed. If hexane was used instead of chloroform, clear spheres were not observed, but some aggregates of tholins were observed. Small fluorescent particles were observed in the surface part of the aggregates in the hexane case, while whole spheres showed fluorescence in the chloroform case. The aggregates obviously looked different from the self-assembly of chloroform-dissolved tholins (Fig. 3). It seems that it is due to the polarity of solvents. It has been shown that the solubility of tholins is higher in polar solvents such as ethanol (ε=24) than in non-polar solvents such as hexane (ε=1.9), toluene (ε=2.4) and benzene (ε=2.3). Among so-called non-polar solvents, chloroform has a larger dielectric constant (ε=4.8) than hexane, and tholins are more soluble in chloroform than in non-polar solvents such as hexane (McKay Reference McKay1996; Carrasco et al. Reference Carrasco2009). Table 1 summarizes the characteristics of organic solvents, and Table 2 shows characteristics of molecules found in Titan environments. If Titan lakes are composed of liquid methane and ethane, our results suggest that only small amounts of tholins could dissolve in them. However, if Titan lakes have more polar molecules as minor constituents, solubility of tholins would be markedly increased. Some of the compounds shown in Table 3 do exist in Titan's atmosphere, and those with oxygen atoms would increase dielectric constant of Titan's lakes if they are components. It would be interesting to examine the solubility and behaviour of Titan tholins in actual low temperature environments like liquid methane (boiling point −161.6 °C, Melting Point −182.5 °C) and ethane (boiling point −89 °C, melting point −172 °C) lakes.
Table 2. Boling point, melting point and dielectric constant of organic solvents Riddick, J. A. & Bunger, W. B. (Reference Riddick and Bunger1970)

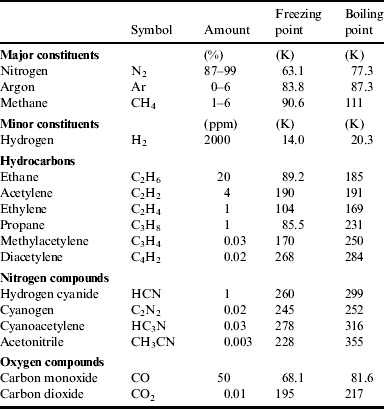
Table 3. Composition of Titan Atmosphere and their Boiling point and Freezing point (http://sci.esa.int/science-e/www/object/index.cfm?fobjectid=31187)

Coacervates
Formation of self-assembled structures is an important first step of chemical evolution which separates an internal molecular system from the external environment. Oparin (Reference Oparin1957), Oparin et al. (Reference Oparin, Orlovskii, Bukhlaeva and Gladilin1976) discussed the relevance of coacervates to the origin of life in the context of colloid chemistry. Coacervates are aggregates formed by colloidal macromolecules in aqueous solvent systems. In his pioneering studies, Oparin had little knowledge of the actual macromolecular composition of cytoplasm, hence he used polymers such as gum arabic and gelatin to produce coacervates. More recent studies employed polymers of amino acids, such as the proteinoid microspheres described by Fox et al. (1963). Egami (Reference Egami1974) proposed that transition metal ions in seawater would have played significant roles, such as catalysis in the process of chemical evolution in primitive seawater. Marigranules were produced with a mixture of amino acid in modified seawater (the concentrations of transition metal ions were drastically increased from the present seawater level) at 105 °C for 4 weeks (Yanagawa & Egami Reference Yanagawa and Egami1980).
The self-assembled structures formed from the Titan tholins described here are best defined as coacervates. The fact that they are fluorescent when activated by near-UV wavelengths is significant, because it is possible that the energy absorbed from UV photons could drive chemical evolution further by photochemical processes. Future studies directed towards this possibility are likely to be fruitful.
The significance of the results for astrobiology
The results reported here show that tholins synthesized in simulated upper atmosphere of Titan can undergo self-assembly in ammonia-water solutions to form spherical coacervates. A possible scenario of self-assembled coacervates in Titan is that complex organic compounds are formed in Titan's atmosphere by energy sources such as high-energy electrons impinging on Saturn's magnetosphere, the equivalent of the plasma discharge used in simulations. The tholins are carried by precipitation into surface lakes composed of liquid methane and ethane. The dissolved tholins then come into contact with ammonia–water jets associated with cryovolcanic activities and assemble into the coacervates described here.
Assuming that the coacervates represent the first steps in evolution towards living systems, is it possible that microbial organisms could emerge and thrive in Titan's environment? For instance, Fortes (Reference Fortes2000) estimated that Titan's underground sea is composed of 15% ammonia aqueous solution, with a pH of 11.5. Some terrestrial microbes can in fact survive in such highly alkaline solutions. Alkaliphiles isolated from water samples in a gold mine near Carletonville, South Africa can grow at pH∼12.5 (Takai et al. Reference Takai, Moser, Onstott, Spoelstra, Pfiffner, Dohnalkova and Fredrickson2001), hence the pH of subsurface water in Titan does not prohibit the emergence of life. Another interesting question is whether life might be able to adapt to non-polar hydrocarbon solvents. In this regard, it is interesting that microbial populations have been found in the Pitch Lake of Trinidad and Tobago, where the liquid mostly consists of asphaltenes (Schulze-Makuch et al. Reference Schulze-Makuch, Haque, de Sousa Anto, Hosein, Song, Yang, Zaikova, Guinan, Lehto and Hallam2011). It would be of great interest to study possible life forms (including membranes and their systems) in alkaline environments and in non-polar solvent environments present in Titan.
Acknowledgements
We would like to thank the scientists at NASA Ames Research Center for their cooperation in sample collection. Dr Hiroshi Imanaka is acknowledged for useful comments of plasma discharge. Drs Delphine Nna Mvondo and Patrick Wilhite are appreciated for helpful discussions of infrared spectra.