Introduction
Spatially and temporally variable methane (CH4) concentrations on the order of tens of parts per billion by volume (ppbv) were detected in the Martian atmosphere by ground-based telescopes in 2003 (Mumma et al. Reference Mumma, Villanueva, Novak, Hewagama, Bonev, DiSanti, Mandell and Smith2009) and by the Mars Express orbiter in 2004 (Formisano et al. Reference Formisano, Atreya, Encrenaz, Ignatiev and Giuranna2004). A 2010 re-analysis of earlier data from the Mars Global Surveyor orbiter also identified a seasonal CH4 signature with maxima of about 50 ppbv over the Elysium, Tharsis, and Arabia Terrae regions in the northern hemisphere (Fonti & Marzo Reference Fonti and Marzo2010). CH4 concentrations apparently peak during northern-hemisphere summer and decline thereafter, with an inferred atmospheric lifetime of less than 200 Martian days (Lefèvre & Forget Reference Lefèvre and Forget2009).
These surprising but mutually supportive results remain controversial (Zahnle et al. Reference Zahnle, Freedman and Catling2011) and await confirmation by NASA's Mars Science Laboratory and ESA's Trace Gas Orbiter (currently intended to reach Mars in 2016). If verified, the presence of CH4 indicates either or both: (1) extant biological or non-biological generation of CH4; (2) periodic release – perhaps due to seasonal pressure and temperature changes – from a vestigial reservoir of ancient biogenic or abiogenic gas.
Earth's atmospheric CH4 is predominantly biogenic, but methanogens are unlikely to be viable in the dry and oxidizing conditions at the CH4 surface. On the other hand, they could have thrived there in the geological past, and the subsurface may remain habitable today (Fisk & Giovannoni Reference Fisk and Giovannoni1999). However, whereas Earth's atmospheric CH4 is replenished by a yearly supply of 6 × 1014 g (Dentener et al. Reference Dentener and Houghton2001), the low concentrations detected in Mars' tenuous atmosphere require an influx of only 2 × 108 g per year (Geminale et al. Reference Geminale, Formisano and Giuranna2008). Hence, the production and emission mechanisms responsible for Martian atmospheric CH4 may be of such low yield that they would normally be considered insignificant on Earth.
Candidate sources for Martian CH4 include a range of geochemical processes known to reduce inorganic carbon in the terrestrial subsurface, of which serpentinization is the most significant (Oze & Sharma Reference Oze and Sharma2005). Serpentinization is the aqueous alteration of the common igneous minerals olivine and pyroxene to the hydrous mineral serpentine via several exothermic reactions favourable below 300 °C. These reactions evolve H2, which reduces ambient CO2 to CH4. The CO2 on Earth includes magmatic carbon in basalts, but also CO2-rich groundwaters which interact with basalts so readily that the reactions have potential for sequestration of greenhouse gases (Schaef et al. Reference Schaef, McGrail and Owen2009, Reference Schaef, McGrail and Owen2010). The CO2 on Mars could ultimately be derived from mantle, atmospheric or meteoritic infall sources (Grady et al. Reference Grady, Verchovsky and Wright2004). Experiments have confirmed the production of H2 and CH4 from the aqueous alteration of olivine at temperatures as low as 30–70 °C (Neubeck et al. Reference Neubeck, Duc, Bastviken, Crill and Holm2011). Olivine and pyroxene are major components of basalt, the glassy silicate rock that dominates the Martian crust. Liquid water has apparently been stable on the Martian surface for long periods of its history and may continue to circulate in the subsurface, driven by geothermal convection (Travis et al. Reference Travis, Rosenberg and Cuzzi2003). Thus, serpentinization is likely to have generated CH4 in Mars’ past and may continue to do so. Where carbon is available, e.g. from magmatic degassing, thermodynamic calculations predict that redox reactions with hydrothermal fluids could also generate CH4 from basalt or magma (Lyons et al. Reference Lyons, Manning and Nimmo2005). Other reduced organic carbon molecules have already been detected in a range of Martian meteorites (Steele et al. Reference Steele2012).
Intracrystalline ‘fluid inclusions’ and crystal boundaries in basalt and other mafic and ultramafic igneous rocks are known to incorporate and retain CH4 and other volatiles (liquids and gases) formed by serpentinization as well as other geological processes (e.g. Welhan Reference Welhan1988; Kelley Reference Kelley1996). Clay minerals in altered basalts can also adsorb CH4 (e.g. Gough et al. Reference Gough, Tolbert, McKay and Toon2010). Thus, Martian basalt could serve as both a record of ancient methanogenic processes and a reservoir from which ancient CH4 might escape. As a first step in evaluating the significance of this reservoir, we investigated the potential for sampling and detecting CH4 in basalts using an incremental-crushing mass spectrometry technique previously found to detect CH4 in serpentinites (Parnell et al. Reference Parnell, Boyce and Blamey2010), artificial impact craters (McMahon et al. Reference McMahon, Parnell, Burchell and Blamey2012a) and hydrothermal minerals (McMahon et al. Reference McMahon, Parnell and Blamey2012b). This technique has potential for application in situ on the Martian surface.
Objectives
The key questions were the following:
(i) Can CH4 always be detected by this technique in basalts from Earth?
If all or most terrestrial basalts yield CH4, we would expect the same on Mars, given evidence of comparable mineralogy (McSween et al. Reference McSween, Taylor and Wyatt2009). This expectation would be strengthened if CH4 contents were consistently high across a wide range of altered, unaltered, recently erupted and ancient basalts in different eruptive settings.
(ii) Does particle size affect the sampling or analysis of gas?
A limited range of particle sizes may be available for in situ analysis on Mars. A future rover, for example, will carry a Sample Preparation and Distribution System (SPDS) capable of crushing geological samples to a broad grain-size distribution up to 400 μm (Schulte et al. Reference Schulte, Widani, Hofmann, Bönke, Re and Baglioni2008). It is therefore of particular interest to establish how CH4 recoverability varies with particle size within this range. Most inclusion volatiles are present in relatively large (10s of μm) inclusions, which are opened and evacuated when grains are broken, whereas finer samples, having already lost these large volumes of volatiles, yield proportionally more crystal-boundary gas when crushed. CH4/CO2 ratios in hydrothermal calcite veins were previously observed to vary systematically with particle size, interpreted to suggest a difference in composition between inclusion volatiles and crystal-boundary volatiles (McMahon et al. Reference McMahon, Parnell and Blamey2012b).
(iii) Can we relate detected variations in CH4 quantity to the geological context of basalts, e.g. eruption depth and vesicularity?
An affirmative result could constrain the quantity of CH4 likely to be retained in Martian basalt and inform sampling strategies for lander missions.
(iv) Does CH4 content vary systematically with basalt age?
It is important to establish the stability and consistency of volatile retention in basalts over geological time. Any trends in CH4 recoverability might reflect: (a) secular changes in Earth's carbon cycle; (b) diffusion, weathering or diagenesis; (c) chance.
(v) Does oxidative weathering preclude or obscure measurements of ancient CH4?
Oxidative weathering pervades the Martian near-surface and may affect the composition of interstitial volatiles. Crystal-boundary CH4 in basalt may be gradually oxidized or analytically masked by CO2. Oxidized basalts can be easily identified from their penetrative red discoloration.
(vi) Do mineral veins cross-cutting basalts share the same gas composition?
An affirmative result might suggest that hydrothermal systems can remobilize CH4 from basalt, a possible venting mechanism. Hydrothermal systems are likely to occur on Mars as a result of geothermal heat (Travis et al. Reference Travis, Rosenberg and Cuzzi2003) and may be locally increased by impact cratering and magmatic activity. Previous results demonstrate that terrestrial hydrothermal calcite veins entrain CH4 in quantities varying according to the thermal maturity of organic-rich sediments through which they were injected (McMahon et al. Reference McMahon, Parnell and Blamey2012b).
Method
Sampling programme
An incremental-crushing mass-spectrometry technique was applied to a wide range of basalt samples (Table 1). To answer questions (i) and (iii) above, ancient oceanic vesicular, amygdaloidal and non-vesicular basalts were sampled from ten localities in the British Isles, one in Michigan, USA, one in Gävle, Sweden and one in Ontario, Canada; and recent subaerial basalts were collected from near Lake Myvatn in Iceland and Kilauea in Hawaii. An olivine bomb (xenolith) derived from the upper mantle was also collected from recent basalt in Tenerife. To address question (iv), Archean, Mesoproterozoic, Neoproterozoic, Carboniferous, Ordovician, Palaeocene and Recent basalts were sampled. At five localities basalts experienced contemporaneous oxidation (permeated by red discoloration due to weathering of lava flows) and were sampled to investigate question (iv). Ordovician basalt from Helen's Bay in County Down, Northern Ireland was sampled along with two generations of cross-cutting mineral veins, addressing question (v). The older vein, calcite, was sampled directly. The younger, haematite, was formed during Permo-Triassic uplift and weathering. Samples were removed from its (visibly oxidized) alteration halo.
Table 1. Details of the basalt samples analysed for CH4

Basalt samples from Helen's Bay were crushed in a press and sieved through four mesh sizes – 63, 125, 212 and 425 μm – in order to investigate question (ii). All other samples were crushed in a press to obtain grains about 3 mm in diameter. Care was taken to avoid unintentionally sampling veins, amygdales, or visible alteration halos. All samples were then washed for about 5 minutes in H2O2 to remove surface organics, rinsed and dried at room temperature.
Table 2. Relative abundance of species recovered from a basalt sample (Penryn Nefyn, Gwynedd, Wales; Ordovician) using the CFS technique. Water is set to 0; percentages represent the remainder. SO2 and organics other than CH4 are usually excluded from analysis. This sample would be reported to have a CH4/CO2 ratio of 2.67/1.24 = 2.15

CFS mass spectrometry
Extraction and analysis of volatiles were performed by the crush-fast scan (CFS) method (Norman & Moore Reference Norman and Moore1997; Moore et al. Reference Moore, Norman and Kennedy2001; Parry & Blamey Reference Parry and Blamey2010; Blamey Reference Blamey2012), which offers very low detection limits (Blamey et al. Reference Blamey, Parnell and Longerich2012). Samples were loaded into a crushing chamber under an ultra-high vacuum (approximately 10−8 Torr) and crushed in swift increments, with each crush producing a burst of mixed volatiles. A typical sample size of about 250 mg (one or two 3 mm grains) released four to ten bursts of volatiles (up to ∼2 × 10−11 litres) into the vacuum chamber, which remained there for 8–10 analyser scans (∼2 seconds) before removal by the vacuum pump. This method does not require a carrier gas and volatiles are not separated from each other but released simultaneously into the chamber.
Bursts were analysed for H2, He, CH4, H2O, N2, H2S (or O2), Ar, CO2, C2H4, C2H6, SO2, C3H6, C3H8, C4H8, C4H10 and benzene using a Pfeiffer Prisma ‘residual-gas’ quadrupole mass spectrometers operating in fast-scan, peak-hopping mode at room temperature. In multiple ion-detection modes, both isotopic peaks and fragmentation peaks are observed; the major peaks for individual gases are generally selected but some exceptions occur. O2 and H2S cannot be analysed together because O2 isotopologues at mass 34 obscure the typically much weaker H2S signal at the same mass. In this study, most samples were analysed for O2 (at mass 32) rather than H2S. The samples generally produced much lower yields than routine aqueous fluid inclusions from geothermal systems or hydrothermal ore deposits for which the system was built. In order to enhance the detection of low-yield samples, a limited gas suite was selected for most samples, comprising H2, He, CH4, H2O, N2, O2, Ar and CO2, thus removing higher organics and SO2. In preliminary work, samples were analysed for SO2, C2H4, C2H6, C3H6, C3H8, C4H8, C4H10 and benzene, which were (each) found to constitute less than 1% of non-water volatiles on average; Ar typically constituted 0.1–1% and He typically constituted less than 0.1%.
CH4 is detected at mass 15 (i.e. the CH3+ fragment; Fig. 1) to avoid interference by O− fragments from water (the CH4 peak at mass 15 is about 90% as intense as the peak at mass 16). CH3+ fragments generated by other organic compounds are typically much less abundant and can be corrected for.
Calibration was checked against commercial standard gas mixtures, atmospheric capillary tubes and three fluid-inclusion standards as described by Norman & Blamey (Reference Norman and Blamey2001). Instrumental blanks are also analysed routinely.
The amount of each species was calculated by proprietary software to provide a quantitative analysis, which is corrected for the instrumental background. Crushing does not liberate all the entrapped gas from samples, so data are generated as molar percentages rather than moles. To circumvent the dependence of individual percentages on irrelevant species, we report CH4 abundances as the ratio (mol% CH4)/(mol% CO2), hydrogen abundances as (mol% H2)/(mol% N2) and oxygen abundances as (mol% O2)/(mol% N2) (hereafter CH4/CO2, H2/N2 and O2/N2, respectively). As predicted by thermodynamic calculations of redox equilibria, CO2 is usually a dominant volatile in magmatic sources, second only to H2O (Lowenstern Reference Lowenstern2001). High values of CH4/CO2 are therefore a good indicator of elevated CH4 abundance in basalt.

Fig. 1. Output from CFS-MS technique during CH4 release. Output from CFS-MS over 80 cycles (approx. 27 seconds) at m/z = 15, representing ionized CH4 released during sample-crushing. The ionized volatiles produce a current in the detector proportional to their amount. The signal from a basalt sample (Helen's Bay, Co. Down, Northern Ireland; CH4/CO2 ≈ 4) is shown alongside a quartz blank with maxima aligned. The basalt's peak signal is about three orders of magnitude higher than the highest signal from the quartz blank.
Results
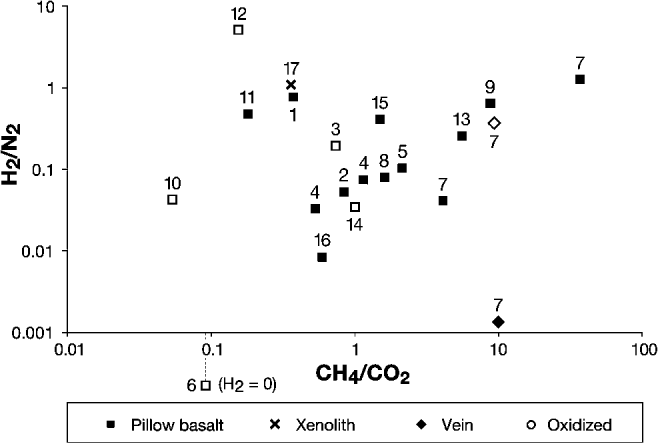
A typical set of results for a basalt sample is displayed in Table 2. H2O typically exceeded 90% of all volatiles released. Of the remaining <10%, CH4, CO2, N2 and H2 were the most abundant constituents, each commonly accounting for between 0.1% and several% of total volatiles. Typical results for a basalt sample are shown in Table 2. CH4 and CO2 were detected in all samples (Fig. 2), with CH4/CO2 ratios (means of several bursts, weighted by volatile burst size) ranging from 0.05 in oxidized Palaeocene basalt from Giant's Causeway (Co. Antrim, Northern Ireland) to 37.41 in non-oxidized Ordovician basalt from Helen's Bay (Co. Down, Northern Ireland). The mean mol% (of the remainder after subtracting H2O) CH4 was about 20. H2/N2 was weakly correlated with CH4/CO2. Both ratios increase with H2 fugacity, hence their apparent association.
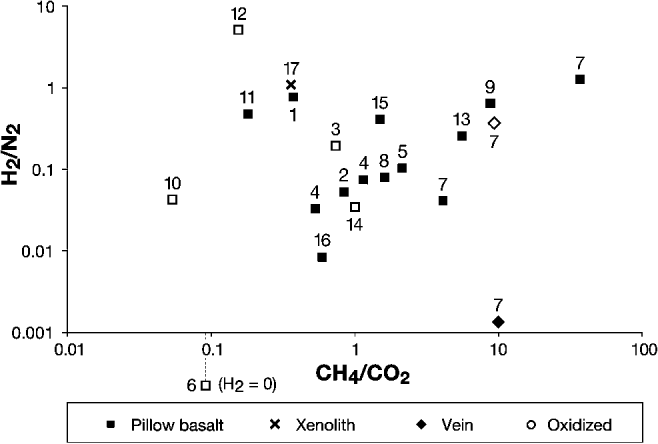
Fig. 2. Composition of volatile gases (CH4/CO2 against H2/N2 molar ratios) in basalt and related samples. Each point represents the mean molar ratios obtained from several volatiles bursts and weighted by burst size. Both ratios increase with H2 fugacity, hence their apparent association. Archean: (1) Schreiber, Ontario, Canada. Palaeoproterozoic: (2) Hamrånge, Gävle, Sweden. Mesoproterozoic: (3) Keweenaw Peninsula, Michigan, USA. Neoproterozoic: (4) Kiell's Jetty, Argyll, Scotland. (5) Penryn Nefyn, Gwynedd, Wales. Ordovician: (6) Ballantrae, South Ayrshire, Scotland. (7) Helen's Bay, Co. Down, Northern Ireland. (8) Stonehaven, Aberdeenshire, Scotland. Carboniferous: (9) Kinghorn, Fife, Scotland. Palaeocene: (10) Giant's Causeway, Co. Antrim, Northern Ireland. (11) Glen Drynoch, Skye, Scotland. (12) Newtown Crommelin, Co. Antrim, Northern Ireland. (13) Portballintrae, Co. Antrim, Northern Ireland. (14) Portree, Skye, Scotland. Recent: (15) Kilauea, Hawaii, USA. (16) Lake Myvatn, Iceland. (17) Tenerife, Spain. See Table 1 for more details.
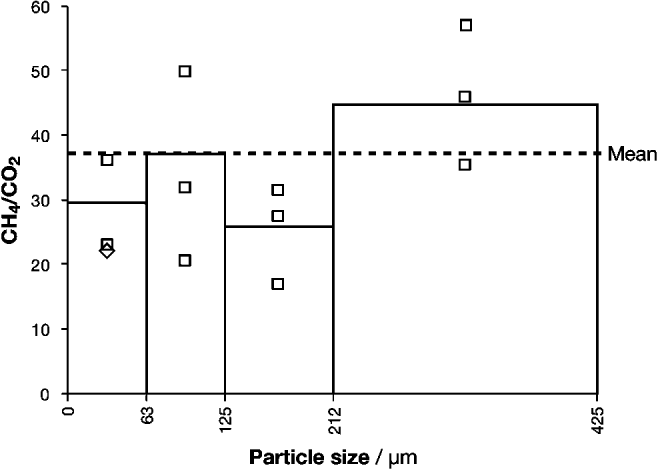
Four grain-size fractions of basalt from Helen's Bay were analysed: <63, 63–125, 125–212 and 212–425 μm (Fig. 3). The 212–425 μm grains yielded the highest weighted mean CH4/CO2 ratio (44.7) followed by the 63–125 μm (37.1), the <63 μm (29.6) and the 125–212 (26.1); hence, no clear trend was observed.
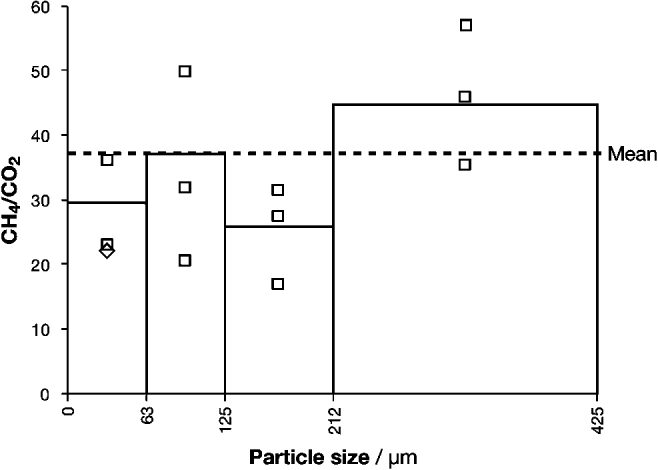
Fig. 3. Molar CH4/CO2 ratios in four particle-size fractions. The weighted mean ratio for each size fraction is presented as a bar; the individual bursts are presented as squares (or diamonds where points overlap). The dashed line represents the mean of all CH4/CO2 measurements of particles between 0 and 425 μm, weighted by burst size.
Of the ten basalt samples in which O2/N2 was measured, the three samples with the highest O2/N2 ratios were all oxidized, red basalt with low CH4/CO2 ratios. However, one other red basalt (Portree) yielded a very low O2/N2 ratio (Fig. 4). In general, pervasive reddening of basalt samples is broadly associated with lower CH4/CO2 ratios (Fig. 2). However, the reddened halo of a haematite vein in Helen's Bay basalt, in which vein volatiles had evidently infiltrated and oxidized the basalt, was found to have a similar volatile composition to the rest of the basalt. A calcite vein cross-cutting the Helen's Bay basalt also released gas of a similar composition to the rest of the basalt.

Fig. 4. O2/N2 plotted against CH4/CO2 molar ratios in oxidized and non-oxidized basalt. Archean: (1) Schreiber, Ontario, Canada. Palaeoproterozoic: (2) Hamrånge, Gävle, Sweden. Mesoproterozoic: (3) Keweenaw Peninsula, Michigan, USA. Ordovician: (6) Ballantrae, South Ayrshire, Scotland. (7) Helen's Bay, Co. Down, Northern Ireland. Carboniferous: (9) Kinghorn, Fife, Scotland. Palaeocene: (12) Newtown Crommelin, Co. Antrim, Northern Ireland. (14) Portree, Skye, Scotland. Recent: (15) Kilauea, Hawaii, USA. (16) Lake Myvatn, Iceland. Sample numbers are the same as in Figure 2. See Table 1 for more details.
Discussion
The results allow assessment against the original objectives of the study.
(i) Can CH4 always be detected by this technique in basalts from Earth?
CH4 and CO2 were detected in all samples (Fig. 2). Most CH4/CO2 ratios were >0.5. The release of the gases during crushing implies that they were sealed within the sample before crushing, e.g. in fluid inclusions and on the tight crystal boundaries. The identification of CH4-bearing fluid inclusions in olivine crystals in other studies (Sachan et al. Reference Sachan, Mukherjee and Bodnar2007; Arai et al. Reference Arai, Ishimaru and Mizukami2012) shows that significant quantities of CH4 can become trapped in olivine. CH4 can also be adsorbed on clay minerals produced by the alteration of basalt (e.g. Gough et al. Reference Gough, Tolbert, McKay and Toon2010), which could also have contributed to the CH4 released during crushing.
(ii) Does particle size affect the sampling or analysis of gas?
Variation in gas composition with grain size was small (CH4/CO2 fell within one order of magnitude) and no clear trend was observed in the basalt from Helen's Bay, Northern Ireland (Fig. 3). Given the reasonable assumption that finer particles yield proportionally more crystal-boundary (as opposed to fluid inclusion) gas when crushed, this result could suggest either that grain-boundary and inclusion volatiles shared a similar gas composition in this basalt or that one reservoir dominated the gas composition.
In previous work on hydrothermal calcite veins, grains below <63 μm yielded insufficient gas for analysis, having already liberated their volatiles during crushing and milling. It was suggested that this result provided an approximate lower limit to the particle size required for CFS. The new results are not consistent with that tentative conclusion, at least in the case of the basalt analysed. In any case, about 75–85% of the powder generated from basalt by the jaw crusher intended for the ExoMars rover (for example) falls within the range 63–400 μm (Schulte et al. Reference Schulte, Widani, Hofmann, Bönke, Re and Baglioni2008), so success in analysing the <63 μm fraction is not critical.
(iii) Can we relate detected variations in CH4 quantity to the geological context of basalts?
No consistent differences were found between vesicular and non-vesicular or subaqueous and subaerial basalts, or between the olivine bomb and the rest of the basalts. This suggests that CH4 is incorporated into basalt in a wide range of geological contexts, perhaps reflecting basalt's inherent mineralogical susceptibility to serpentinization or hydrothermal alteration, and does not depend strongly on local microbial methanogenesis; however, we did not assess the biogenicity of the gas directly. The alteration process probably involves connate water, so the distinction of subaqueous and subaerial basalts may be of negligible importance.
(iv) Does CH4 content vary systematically with basalt age?
Some samples close in age appear to share similar CH4/CO2 ratios (e.g. Recent), but the data do not demonstrate long-term changes in the quantity of CH4 included or retained by basalt. It is unknown, however, whether CH4 recovered from ancient basalt is itself ancient. Overall, similar volatile compositions were observed for basalts of all ages.
(v) Does oxidative weathering preclude or obscure measurements of ancient CH4?
A previous CFS mass spectrometry study found no evidence that oxidative weathering affected gas compositions in hydrothermal calcite veins (McMahon et al. Reference McMahon, Parnell and Blamey2012b). In this study, however, pervasive reddening of basalt samples is broadly associated with lower CH4/CO2 ratios (Fig. 2). This suggests that oxidative weathering may change the volatile composition of basalts, for instance by oxidizing adsorbed and interstitial CH4 to CO2. Three out of four reddened basalts analysed for O2/N2 yielded high O2/N2 ratios and low CH4/CO2 ratios (Fig. 4). However, one reddened basalt yielded a very low O2/N2 ratio and a CH4/CO2 close to 1. Moreover, all oxidized samples released some CH4 and the oxidation halo of the haematite vein in basalt from Helen's Bay showed no significant difference in CH4/CO2 ratio from non-oxidized basalt groundmass. Thus, the strength of the effect of oxidative weathering on gas composition is variable.
(vi) Do mineral veins cross-cutting basalts share the same gas composition?
Figure 5 shows the volatiles detected in two basalt samples and two veins, all from the Helen's Bay locality. Differences between the basalts may reflect differences in hydrothermal alteration, while the calcite vein shows a distinct volatile composition, most clearly by its lack of H2. The veins yielded similar CH4, CO2 and CH4/CO2 ratios to the host rock (Fig. 2 and Fig. 5), which is consistent with the possibility that they may have remobilized basaltic CH4, one of several plausible mechanisms for CH4 release to the surface. This is a small-scale equivalent of the release of CH4 through hydrothermal fracture systems on the sea floor (Charlou et al. Reference Charlou, Donval, Fouquet, Jean-Baptiste and Holm2002). Conversely, volatiles in hydrothermal fracture systems may also have influenced the gas composition of the basalt. This influence is less likely to have been significant at the other, less heavily veined localities.

Fig. 5. Comparison of gas compositions in two basalt samples, a calcite vein, and the oxidation halo of a magnetite vein, all from Helen's Bay, Co. Down, Northern Ireland. Percentage abundances refer only to the seven volatiles presented; water is excluded and no other volatiles were analysed. The hatched bars represent two basalt samples, the black bars represent the alteration halo of a haematite vein through basalt, and the white bars represent a calcite vein in basalt. Differences between the basalts may reflect differences in hydrothermal alteration, while the calcite vein shows a distinct volatile composition, most clearly by its lack of H2. However, CH4 and CO2 are similar for all four samples, which is consistent with gas exchange between the basalt and the veins.
Comparison with other datasets
Comparison of the basalt dataset with measurements from modern environments (sea floor hydrothermal fluids, Icelandic groundwaters and a hydrocarbon-bearing system; Charlou et al. Reference Charlou, Donval, Fouquet, Jean-Baptiste and Holm2002; Stefánsson & Arnórsson Reference Stefánsson and Arnórsson2002; Parry & Blamey Reference Parry and Blamey2010) shows that higher CH4/CO2 ratios are recorded from the basalts (Fig. 6a). This is consistent with a progressive build-up of CH4 in the basalts over time. Basalt data can also be compared with other geological materials previously analysed by the CFS technique (Fig. 6b; Parnell et al. Reference Parnell, Boyce and Blamey2010; McMahon et al. Reference McMahon, Parnell and Blamey2012b). This comparison reveals that most basalts in this study yielded lower CH4/CO2 ratios than previously studied serpentinites, which might be expected if the CH4-enrichment of the basalts is mostly due to serpentinization. Nevertheless, some basalt yielded CH4/CO2 ratios as high as some serpentinites, despite the high concentration of CO2 in magmatic systems.

Fig. 6. Comparison of gas compositions in basalts with other CH4-bearing fluids and geological materials.(a) CH4/CO2 against H2/N2 molar ratios in evacuated volatiles from unweathered basalts in the present study (filled triangles = ancient basalts; unfilled triangles = Recent basalts) compared with three modern fluids (Parry & Blamey Reference Parry and Blamey2010; Charlou et al. Reference Charlou, Donval, Fouquet, Jean-Baptiste and Holm2002; Stefánsson & Arnórsson Reference Stefánsson and Arnórsson2002). (b) CH4/CO2 against H2/N2 molar ratios in evacuated volatiles from unweathered basalts in the present study (filled triangles = ancient basalts; unfilled triangles = Recent basalts) compared with volatiles evacuated from serpentinites and hydrothermal calcite veins (Parnell et al. 2010; McMahon et al. Reference McMahon, Parnell and Blamey2012b).
Sources of CH4 in terrestrial and Martian basalt
Although the present study did not seek to determine the ultimate sources of the CH4 detected in terrestrial basalt, it would be an interesting question to explore further. Methanogens are known to thrive in hydrothermal systems associated with mid-ocean ridges, but are unlikely to have contributed significantly to the basalts formed in other settings. It is also possible that some CH4 arrived in the basalt via the thermal breakdown of biogenic organic matter (kerogen) in sediments, sedimentary rock, and circulating fluid. Such ‘thermogenic’ gas commonly includes higher alkanes; we did not test for these in all samples, but found only trace amounts during preliminary work on samples from five localities. Ultimately, the biosphere is inextricable from the Earth's carbon cycle and subducted biological material may re-emerge in magmatic gases; Sleep et al. (Reference Sleep, Bird and Pope2012) have recently shown that N2 in mid-ocean ridge basalt derives from this source.
A magmatic CH4 source is thermodynamically unlikely. Redox equilibration between basaltic magma and hydrothermal C–O–H fluids is constrained by the QFM (quartz–fayalite–magnetite) mineral redox buffer, which predicts very low CH4/CO2 ratios at typically basalt quenching temperatures: less than 10−6 at 1000 °C (Giggenbach Reference Giggenbach and Barnes1997). Where more reducing conditions occur, equilibrium is controlled by graphite and can generate CH4/CO2 ratios within the range we observed; however, it would be unusual for mid-ocean ridge basalts on Earth to form under these conditions.
In our view, the high CH4/CO2 ratios in these basalt samples are best explained by hydrothermal fluid- and/or water-rock reactions. Hydrothermal C–O–H fluids in contact with basalt are in redox equilibrium at high CH4/CO2 well below 500 °C, whether buffered by QFM or the FeO–FeO1.5 (quartz–fayalite–hematite) ‘rock buffer’ of Giggenbach (Reference Giggenbach1987). Thus, Giggenbach (Reference Giggenbach and Barnes1997) describes CH4 production in a ‘hydrothermal envelope surrounding the [volcanic] vent system.’ Additional H2 and CH4 are likely to have been supplied by the low-temperature aqueous alteration of olivine and pyroxene (serpentinization) in local cumulates, in the underlying ultramafic layers of oceanic crust, and within the basalt itself, even the young Icelandic and Hawaiian basalt. These gases have been generated in the laboratory by the serpentinization of olivine at 30–70 °C on an experimental timescale (Neubeck et al. Reference Neubeck, Duc, Bastviken, Crill and Holm2011).
Both of these mechanisms are likely to have operated on Mars. The surface of Mars is dominated by basalt (McSween et al. Reference McSween, Taylor and Wyatt2009), and evidence from orbit (Ehlmann et al. Reference Ehlmann, Mustard and Murchie2010) and meteorites (Changela & Bridges Reference Changela and Bridges2011) shows that the basalt is at least partially altered to serpentinite and other silicates. Olivine and pyroxene cumulates, as preserved in the Chassigny and Nakhla Martian meteorites, respectively, are particularly vulnerable to serpentinization. The Martian mantle is also believed to be more iron-rich than the Earth's, such that the Fe–Mg (solid-solution) minerals involved in serpentinization reactions are likely to contain more FeO and may thus release more H2 and CH4 (Sleep et al. Reference Sleep, Meibom, Fridriksson, Coleman and Bird2004; Bridges & Warren Reference Bridges and Warren2006). Moreover, the Martian mantle is expected to be saturated with graphite and more reduced than QFM (and the Earth), such that hydrothermal fluids at equilibrium will contain a higher proportion of CH4 (Lyons et al. Reference Lyons, Manning and Nimmo2005; Hirschmann & Withers Reference Hirschmann and Withers2008). Steele et al. (Reference Steele2012) have identified reduced organic macromolecular carbon in diverse Martian meteorites, which they interpret as a precipitate from C–O–H fluids.
There are several plausible mechanisms by which volatile-escape from altered ancient basalt could contribute to Martian atmospheric CH4: in the surface, wind abrasion, impact erosion due to micrometeorites and meteorites, freeze-thaw weathering, hot-cold weathering, or salt weathering; and in the subsurface, geochemical activity and hydrothermal fracturing enhanced by geothermal convection (Travis et al. Reference Travis, Rosenberg and Cuzzi2003).
The gas data emphasize that basalts are sinks for carbon. This is why they are attracting strong attention for their potential in CO2 sequestration (McGrail et al. Reference McGrail, Schaef, Ho, Chien, Dooley and Davidson2006; Oelkers et al. Reference Oelkers, Gislason and Matter2008; Schaef et al. Reference Schaef, McGrail and Owen2009, Reference Schaef, McGrail and Owen2010). On Mars, there is growing evidence that carbonate formation is widespread (Michalski & Niles Reference Michalski and Niles2010; Niles et al. Reference Niles, Boynton, Hoffman, Ming and Hamara2010; Chassefière & Leblanc Reference Chassefière and Leblanc2011) and must be contributing to CO2 drawdown. The occurrence of pre-terrestrial low-temperature carbonates in Martian meteorites (Carr et al. Reference Carr, Grady, Wright and Pillinger1985; Changela & Bridges Reference Changela and Bridges2011) shows that basalts contribute to drawdown on Mars, as on Earth. At least some of this carbonate precipitation may have been surficial (Knauth et al. Reference Knauth, Brilli and Klonowski2003; Halevy et al. Reference Halevy, Fischer and Eiler2011), as with carbonates in terrestrial basalts (Fig. 7). CO2 extracted from the atmosphere in this way is available for conversion to CH4 when coupled with serpentinization in basalts. Thus, carbon from both atmosphere and lithosphere could contribute to fuelling a putative methanotrophic biosphere on Mars.

Fig. 7. Photograph of contemporaneously weathered basalt. Weathered basalt (red coloured by the oxidation of iron) in which vesicles have been filled with calcite (white) before erosion of basalt and incorporation into conglomerate, in which fine matrix intrudes into vesicles where calcite has been dissolved. This demonstrates rapid uptake of CO2 (sequestered as calcite) into basalt, where interaction with hydrogen would yield CH4. The scale bar is 5 cm long.
Conclusions
These results show that CH4 is retained within a range of basalts, including cross-cutting hydrothermal mineral veins, which may be capable of remobilizing ancient CH4 and venting it to the atmosphere. Several other mechanisms may also plausibly release CH4 from Martian basalt. More work is needed to establish whether Martian basalts or meteorites contain quantities of ancient CH4 comparable with their terrestrial counterparts and whether they are a likely source for the spatially and temporally variable CH4 in the atmosphere. Indeed, the CFS technique demonstrated here could be adapted for use on Mars, perhaps by the modification of existing mass spectrometry techniques for Mars (e.g. the Sample Analysis at Mars package flying on NASA's Mars Science Laboratory).
Evidence of serpentinization or hydrothermal alteration on Mars would imply that liquid water, water-rock redox gradients, and gases including CO2, H2 and CH4 – all potential energy sources for metabolism – have all been present in the subsurface. These conditions are suggestive of a habitable environment. The products of serpentinization are known to support complex ecosystems in the Earth's oceanic crust and have also been implicated in the origin of life (Sleep et al. Reference Sleep, Meibom, Fridriksson, Coleman and Bird2004, Reference Sleep, Bird and Pope2011; Kelley et al. Reference Kelley2005; Hellevang Reference Hellevang2008). Biogenic CH4 on Earth is classically distinguished from abiogenic CH4 by its light carbon isotopic composition (i.e. very negative δ13C), although this test for biogenicity can yield false positives where isotopic fractionation is induced by hydrothermal processes (Horita & Berndt Reference Horita and Berndt1999), as well as false negatives in circumstances where biogenic CH4 is isotopically heavy (Takai et al. Reference Takai, Nakamura, Toki, Tsunogai, Miyazaki, Miyazaki, Hirayama, Nakagawa, Nunoura and Horikoshi2008). Moreover, interpretation of δ13C in Martian CH4 will be challenging without a deeper understanding of the Martian carbon cycle than is presently available. Thus, other criteria including geological context, hydrogen isotope composition and degree of co-occurrence with higher hydrocarbons should also be considered in the determination of biogenicity. For this purpose, CFS analysis of CH4 in basalts on Earth and Mars could be coupled with measurements of higher hydrocarbons and carbon- and hydrogen-isotopic analysis.
Acknowledgements
Dr Paula Lindgren (University of Glasgow), Mr Sam Spinks (University of Aberdeen) and Professor Martin Brasier (University of Oxford) each contributed a basalt sample for analysis. Colin Taylor assisted in the preparation of samples. Sean McMahon's PhD studentship is funded by the STFC. The manuscript was greatly improved by the comments of Norm Sleep and two anonymous reviewers.