Introduction
Sulphur-bearing organic compounds such as the amino acids cysteine and methionine are basic constituents of biochemistry. From which classes of compounds and how might they form in astronomical environments? Model theories usually consider reactions between neutral species, ion–atom interactions or ion–molecule reactions (see, for example, Smith et al. (Reference Smith, Adams, Giles and Herbst1988)). Photolytic processes are considered less frequently, even though the thresholds of CS2→CS+S and of OCS→CO+S are in the near-ultraviolet at 277.8 and 397.3 nm (Lee Reference Lee1984) and the photolytic dissociation of H2S yields S2 (Grim & Greenberg Reference Grim and Greenberg1987). The present study addresses this issue by investigating a hypothesis of photochemical synthesis involving carbon molecules with triple C≡C bonds and sulphur atoms or molecules. Hydrogen end-capped polyyne C10H2 was chosen to represent reactive carbon compounds in part because it can be easily synthesized in organic solvents (Cataldo Reference Cataldo2003), in part because C10H2 has a strong absorption band at 252 nm near the 253.6 nm emission line of the ultraviolet lamp and, lastly, because acetylene and some polyacetylenes and/or sulphur are known to occur in interstellar and circumstellar media, in cometary comas, in the atmosphere of the planet Jupiter, on Saturn's largest satellite Titan and on Jupiter's satellite Io (A'Hearn et al. Reference A'Hearn, Feldman and Schleicher1983; Cernicharo et al. Reference Cernicharo, Heras, Tielens, Pardo, Herpin, Guélin and Waters2001; Coustenis et al. Reference Coustenis, Bézard and Gauthier1989, Reference Coustenis, Schmitt, Khanna and Trotta1999; Moses et al. Reference Moses, Bézard, Lellouch, Gladstone, Feuchtgruber and Allen2000; Shindo et al. Reference Shindo, Benilan, Guillemin, Chaquin, Jolly and Raulin2003; Thaddeus et al. Reference Thaddeus, McCarthy, Travers, Gottlieb and Chen1998; Vuitton et al. Reference Vuitton, Gée, Raulin, Bénilan, Crépin and Gazeau2003). Octacyclosulphur, c-S8, was chosen as the source for reactive sulphur species. The formulae c-Sn (cyclic) and o-Sn (open) are used to ensure textural distinction between cyclic and open molecular sulphur structures. Octacyclosulphur when exposed in CS2 to ultraviolet radiation from a high-pressure Hg lamp yields substantial amounts of c-S6 and c-S7 plus lesser amounts of c-S5, c-S9, c-S10 and c-S12, presumably via the unstable open o-S8 diradical (Strauss & Steudel Reference Strauss and Steudel1987). The photolysis of c-S6 in CS2 yields c-S8 and c-S7, and the photolysis of c-S7 in CS2 yields c-S8 and c-S6 as major products (Strauss & Steudel Reference Strauss and Steudel1987). The results strongly suggested that significant amounts S and S2 were also formed. As Strauss and Steudel (Reference Strauss and Steudel1987) did not calibrate their high-pressure Hg lamp, they could not determine photolytic yields of c-S8 and c-S6 ‘destruction’ or yields of the respective photolytic products. The photolyses of c-S8 and c-S6 in n-hexane are therefore quantitatively studied here with a calibrated low-pressure Hg source. S and S2 formed by photolysis were used for reaction with C10H2 in n-hexane.
Experiment
C10H2 was obtained by the immersed carbon-arc method (Cataldo Reference Cataldo2003) and was purified by high-pressure liquid chromatography (HPLC) on a MetaChem Technologies Inertsil octadecylsylil (ODS; 5 μm; 4.6×250 mm) column with n-hexane (95% n-hexane, 5% methylcyclohexane) as mobile phase. Photolyses of C10H2 with and without sulphur were done in n-hexane. Octacyclosulphur was sublimated sulphur that contained a trace of c-S6 but no detectable c-S7. Its photolysis was studied in n-hexane and methanol. Weighable amounts of hexacyclosulphur were made by reacting sodiumthiosulfate with hydrochloric acid. The solid product, recrystallized from n-heptane, was c-S6 with traces of c-S7 and about 1 mol% c-S8. Its photolysis was studied in n-hexane.
The solutions for photolysis were placed in a quartz cuvette with a 10 mm light path. The radiation source was an 11SC-1 low-pressure Hg pen-ray lamp, whose strongest ultraviolet lines are at 184.9 and 253.6 nm. As the photolysis rate of C10H2 was relatively slow, the lamp was placed centrally against a 3 mm quartz cuvette that contained an aqueous KBr absorber, which, in turn, was placed against the sample cuvette. The absorber was used to prevent any 184.9 nm radiation from entering the sample. The same geometry was used for calibrations of the 253.6-nm radiation with the potassium ferrioxalate actinometer (Hatchard & Parker Reference Hatchard and Parker1956). The 253.6-nm photon flux through the cuvette's entry face was 8.8±0.9×1015 photons cm−2 s−1. Owing to the dimensions of the cuvette, this was also the number of photons entering 1 cm3 of solution every second. For the much faster c-S8 and c-S6 photolyses, the distance between the sample cuvette and lamp was increased to 38 mm and the KBr absorber was not used. The photon flux was adjusted for the change in distance. Exposure times were controlled with a manual shutter. Aliquots of 0.05 ml were taken for HPLC analysis from each irradiated sample after various exposure times. A series of ‘blank’ irradiations of neat n-hexane and methanol were also done to check whether detectable products might interfere with analyses of the sulphur species or C10H2. No interference was found.
C10H2 concentrations were monitored with the HPLC system mentioned above. The C10H2 absorptivity from the literature was used (Eastmond et al. Reference Eastmond, Johnson and Walton1972). Concentrations of c-S8, c-S7 and c-S6 were monitored by HPLC with either a Nacalai Tesque buckyprep (bp) column (4×250 mm) with hexanes (86.1% n-hexane, 9.7% methylcyclopentane and 4.2% methylpentanes) as mobile phase or the ODS column with methanol as mobile phase. Owing to peak-overlap of c-S7 and c-S8 on the bp-column, the concentrations of c-S7 and c-S8 were obtained with a peak-resolving program using absorptions at two different wavelengths. However, when the c-S8 peak area overwhelmed that of c-S7, the c-S7 concentration could not be reliably obtained. The absorptivities of c-S6 (ε6) and c-S8 (ε8) in n-hexane were determined for this study by HPLC at 234 nm (5642 dm3 mol−1 cm−1) and 263 nm (6445 dm3 mol−1 cm−1), respectively. The ε7 value in methylcyclopentane of 5550 dm3 mol−1 cm−1 at 255 nm from the literature was used (Steudel et al. Reference Steudel, Jensen, Goebel and Hugo1988).
Results and discussion
Solutions of C10H2 with c-S8 and c-S6 were stored in the dark and at room temperature for 24 hours to check whether C10H2 reacted measurably with the sulphur-bearing molecules in the absence of radiation. No changes in polyyne or sulphur concentrations were found.
Figure 1 shows the photolysis of c-S8 in methanol. From 0 to 15 s, the concentration of c-S8 decreases while those of c-S6 and c-S7 increase. From 15 to 30 s, the concentrations remain roughly unchanged, but there is a slight and real concentration maximum of c-S8 near 25 s. Apparently, a quasi-stationary parti-closed photolytic system develops from which sulphur atoms are only lost by the formation of other Sn species such as S, S2, c-S5, c-S9, c-S10, c-S12 and the photosulphur polymer: 

Fig. 1. Photolysis of c-S8 in methanol: concentration versus exposure time.
Owing to this ‘cycle’, quantitative analyses of the functions of Figure 1 and analogous functions from the additional experiments in n-hexane are extremely complex. However, since only one sulphur species is present in solution at the start of the photolysis, its rate of ‘destruction’ and the rates of formation of its major photolytic products at this time were obtained by differentiation of the respective functions at t 0. Although the total irradiated volumes of solutions were 3 ml, the actual calculations were done for the processes occurring in a 1 cm3 volume of the irradiated solutions. For every photolysis, normalized yields Φ (normalized to a standard concentration of 1 μmol cm−3) of the ‘destruction’ of c-S8 or c-S6 to assumed open intermediate diradicals or the formation of detectable reaction products were computed with the equation:
R 0 is either the initial destruction rate of c-S8 or c-S6, or the initial formation rate of products c-S6, c-S7 or c-S8 in μmol cm−3 s−1. C 0 is the initial concentration of c-S8 or c-S6 in μmol cm−3. F is the number of photons entering the 1 cm3 reaction volume each second. A 0 is fraction of 253.6-nm photons absorbed by the reactant c-S8 or c-S6 molecules at the start of the photolysis. At that time the reaction products do not yet absorb. n-Hexane was essentially 100% transparent at 253.6 nm. Table 1 summarizes the results. Major contributions to uncertainties of Φ-values arise from the calibration of the photon flux (±10%), the manual shuttering (±10%), HPLC peak area determinations (±3%) and of the ε6 and ε8 determinations (±3%). The compounded uncertainty of the data in Table 1 could well be in the range of 20–40%.
Table 1. Summary of normalized photolytic yields
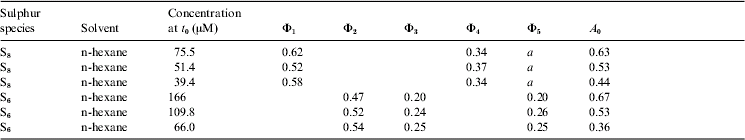
Notes: Φ1=S8 ring-opening to intermediate; Φ2=S6 ring-opening to intermediate; Φ3=S8 formation; Φ4=S6 formation; Φ5=S7 formation; a=dc 7/dt resulting from small differences of two large numbers, due to peak-overlap, hence not reported.
Exploratory photolysis of C10H2 without and with c-S8 present was performed with the ultraviolet lamp actually immersed in the solutions contained in an air-cooled Pyrex tube. Short exposures times were measured from immersion to removal of the lamp. The results are shown in Figure 2. More than 90% of the c-S8 initially present was converted mostly to c-S6 and c-S7 after only 30 s of exposure. The exploratory runs were kept short for that reason. The following three observations can be made.

Fig. 2. Photolysis of C10H2 in n-hexane with the lamp immersed in the solutions. The c-S8 concentrations shown are those at the start of the irradiation.
1. The rate of C10H2 photolysis without c-S8 present was real but slow, which is a confirmation of earlier work by Cataldo (Reference Cataldo2004). To test whether the photolysis of C10H2 with 253.6-nm radiation might have been impeded by O2 quenching, an identical experiment was performed after thorough purging of the solution with dry N2. No detectable difference was observed.
2. When c-S8 was present, C10H2 was transformed into one or several compounds whose composition could not be ascertained (see below).
3. The initial rate of transformation of C10H2 was proportional to the initial c-S8 concentration.
For subsequent experiments the lamp was placed outside the cuvette that contained the solutions. A solution of 1.36 μM of C10H2 without sulphur was exposed for a total of 14 min. Samples were taken and analysed after 2, 6 and 14 min. The initial conversion rate of C10H2 in 1 cm3 volume was −2.7×10−13 μM s−1. Equation (1) was used with R 0, the initial destruction rate of C10H2, and A 0, the fraction of 253.6-nm photons absorbed by C10H2. Φ was 3×10−5. An analogous experiment with a solution initially containing 0.885 μM C10H2 and 31.25 μM c-S8 yielded an initial C10H2 conversion rate of −3×10−11 μM s−1, two orders of magnitude faster than the transformation rate without sulphur present. The absorption of photons by c-S8 did not require corrections for A 0. Φ was 7×10−3.
As C10H2 absorbed at least 50% of all 253.6-nm photons that entered the solutions in all experiments with sulphur present, the question arose of whether the observed faster transformation in the presence of sulphur implied that the polyyne molecules themselves had to be in an electronically excited state. To examine this, analogous experiments were carried out with C8H2, also obtained by the immersed carbon-arc method, whose solutions in n-hexane were essentially 100% transparent at 253.6 nm (the strongest C8H2 absorption band in n-hexane above 200 nm is at 227 nm). In the presence of sulphur, C8H2 was transformed to unidentified product(s) with a rate similar to that of C10H2. Obviously, the reaction of C8H2 occurred with that molecule in the ground state. By inference it is suggested that the reaction of C10H2 with sulphur also occurred with that reactant in the ground state.
A search was made in all HPLC chromatograms for peaks due to products of putative reactions of C10H2 with sulphur. None were detected, perhaps because their molar extinction coefficients were too small, because their retention times were too long or because many different products were formed each with an undetectably small concentration. Nothing significant could be gained by increasing the C10H2 concentration because of its large molar extinction coefficient. Successful searches for products will require much more powerful ultraviolet sources.
Strauss and Steudel (Reference Strauss and Steudel1987) argued that ultraviolet radiation ‘opens’ the c-S8, c-S7 and c-S6 rings to produce ‘linear’ diradical intermediaries, which then form Sn polymers as well as several other Sn molecules. The hypothesis of ‘ring opening’ is accepted here.
The most salient observations of the sulphur photolyses are as follows.
1. The Φ values are in the range 0.2–0.7 (Table 1), which implies that the 253.6-nm photons are very efficient in opening c-S8 and c-S6 rings.
2. The Φ values are independent of concentration, hence the ring openings are first-order processes.
3. The sum of the Φ values of the primary products from c-S8 and c-S6 appear to be roughly equal to the Φ value of the intermediary, which implies that little, if any, photopolymer is formed.
4. The number of atoms of c-S6 and c-S7 formed is roughly equal.
When only traces of photopolymer are formed, the most likely initial reactions of the photolysis of c-S8 are
However, one may expect the rate of polymer formation, which is at least a bimolecular process, to increase for higher initial concentrations of c-S8 than those used in this study.
Theoretical calculations (Millefiori & Alparone Reference Millefiori and Alparone2001) show that reactions (2) and (3) each consume more than 100 kJ mol−1 of energy. To obtain a theoretical energy of the c-S8 ‘ring opening’, the total energies of the c-S8 ring in the singlet ground state and of the o-S8 biradical in the triplet ground state were calculated here with the B3LYP method and the 6-311G* basis set (Spartan ‘04 program; Wavefunction Inc., Irving, California, USA). The equilibrium configuration of the o-S8 biradical turns out not to be linear but ‘kinked’ and the molecule has an electric dipole moment of 0.78 Debye. The ring opening requires 143.7 kJ mol−1. The energy of a 253.6-nm photon is 417.7 kJ mol−1. The biradical obviously has enough internal energy for additional S–S bond ruptures to eventually yield c-S6+S2 and c-S7+S through channels of essentially equal and surprisingly large transmission factors.
Strauss and Steudel (Reference Strauss and Steudel1987) did not attempt to prove that S and S2 were actually formed in solution by photolysis of c-S8. These sulphur species can be detected by chemoluminescence (Richter et al. Reference Richter, Rosendahl, Hynes and Lee1998) and fluorescence (Grim & Greenberg Reference Grim and Greenberg1987), but only at very low temperatures. In the course of the present study the smell of H2S was detected above freshly photolysed solutions of c-S8 in n-hexane and methanol. The formation of that compound was confirmed by the precipitation of PbS in a solution of lead nitrate in water into which the evolved H2S gas was absorbed. Although the formation of H2S is not absolute proof that the photolysis of c-S8 produced S and/or S2, the abstraction by these species of hydrogen from n-hexane, methanol or dissolved water is the most compelling explanation for the H2S formation. S, S2, o-S8 and other biradicals are arguably the most reactive sulphur species in solution of c-S8 photolysis.
The energy of a 253.6-nm photon is also ample to open the c-S6 ring whose bond energy per sulphur atom is actually 0.06 eV less than that of c-S8 (Millefiori & Alparone Reference Millefiori and Alparone2001). However, c-S6 has fewer sulphur atoms than both of its major products c-S7 and c-S8, hence its photolysis cannot be accounted for by a set of three dissociations analogous to those for c-S8. Perhaps this photolytic process produces copious numbers of S, S2, S3, S4 and c-S5 species, which, in turn, react with mainly c-S6 or o-S6 to form the products. Once again it is remarkable that these processes are so efficient in the dilute solutions of this study. The photolysis of c-S7 produces c-S6 and c-S8 (Strauss & Steudel Reference Strauss and Steudel1987). c-S6 might simply form by c-S7+hν→o-S7*→c-S6+S, but the formation of c-S8 requires a formal scheme of c-S7+S→c-S8. It is revealing that the major products of the photolysis of c-S8 are not c-S7 and c-S9 but c-S6 and c-S7. Apparently, the dissociations of the excited o-S8* to c-S6 and c-S7 overwhelm processing through all other possible channels.
The data obtained strongly suggest that C10H2 reacts with some sulphur species when exposed to 253.6-nm radiation. A firm assignment of such species is still somewhat speculative because the sulphur-bearing products could not be identified. Nevertheless, it seems reasonable to assume that C10H2 reacted with the most chemically active photolytic sulphur species S and S2, which suggests that facile photolytic reaction may occur with the astronomically abundant molecules H2S, CS2 or COS, which are known to produce S by ultraviolet photolysis. Some astrochemical consequences have been discussed elsewhere (Cataldo Reference Cataldo2000; Cataldo & Heymann Reference Cataldo and Heymann2001; Heymann et al. Reference Heymann, Cataldo, Thiemens, Fokkens, Nibbering and Vis2000).
The simplest imaginable scheme is the reaction of S with C10H2 forming an HCSC ring on the polyyne molecule as shown in Figure 3. Dr. N.M.M. Nibbering (Private communication, 2006) suggested a scheme beginning with the attachment of an S atom at C-atoms 2, 3 and an S atom at C-atoms 8, 9 of C10H2 that following C—C cleavage of the three-member S-containing rings would give HCCSCC-CCCCSCCH. The latter molecule might then undergo four ring closures and one ring cleavage to eventually form a central benzene ring with two dehydrothiophene rings attached. C10S2H2, C4H2 and C6H2, the polyynes that have actually been detected in astronomical environments, are more likely to yield dehydrothiophene-type molecules.

Fig. 3. A hypothetical product of the reaction of S with C10H2.
Cysteine and methionine were mentioned in the introduction as examples of basic constituents of biochemistry. Both have odd numbers of carbon atoms whereas C10H2 obviously has an even number. The salient point, however, is that possibly many carbon molecules with triple or even double C—C bonds, including molecules with odd numbers of carbon atoms such as the carbenes C3H2, C5H2, C7H2 and C9H2, all four surprisingly abundant interstellar molecules (Thaddeus et al. Reference Thaddeus, McCarthy, Travers, Gottlieb and Chen1998), may react with photolytically produced S and/or S2 to produce precursors of organic sulphur-bearing molecules.
COS, CS, H2S and C3S are identified interstellar molecules (Irvine et al. Reference Irvine, Ohishi and Kaifu1991). Diacetylene and triacetylene were discovered in the proto-planetary nebula CRL 618 (Cernicharo et al. Reference Cernicharo, Heras, Tielens, Pardo, Herpin, Guélin and Waters2001). Hence, circumstellar and interstellar media with ample ultraviolet radiation might well be favourable ‘breeding grounds’ for biochemically interesting S-bearing organic molecules. The Solar-System satellites Titan and Io are intriguing in this respect. The atmosphere of the former clearly contains C2H2, C4H2 and C6H2, but the molecular forms in which the element sulphur occurs are less clear. The atmosphere of the latter is periodically rich in S and S2 due to volcanic outbursts, but polyynes are not known to occur. Polyynes and sulphur are known to occur in the atmosphere of Jupiter, but ultraviolet photolysis can only happen in the very outer reaches owing to the high density of the Jovian atmosphere.
Note added in proof
Continuing study of the polyyne suggests that its photolysis is strongly quenched by dissolved oxygen gas.
Acknowledgments
The author thanks readers Eduard Adema, Franco Cataldo, Bob Curl, Julianne Moses, Nico Nibbering and Bruce Weisman for their critical reviews of two manuscripts, one on sulphur photolysis and one on C10H2 photolysis, which were eventually combined into this one paper.






