Introduction
There is arguably no more intriguing scientific question than whether we are the only life forms that have ever evolved. We know that some of the building blocks for life that led to the origin of life on Earth are still elsewhere, even in our own Solar System. What about more complex molecules, such as polypeptides? And, if there are polymers present, are they homochiral as they are in terrestrial life? To answer these critical questions missions are required that deploy non-destructive analytical instrumentation beyond the current state-of-the-art for flight. We know that the Earth has been a home to chemical evolution and still is. These precursor organic materials were synthesized endogenously on the Earth, as well as likely delivered by extraterrestrial objects including carbonaceous meteorites, comets and interplanetary dust particles (Sephton and Botta, Reference Sephton and Botta2008). Diverse organic molecules such as amino acids, alcohols, sugar, ketones, lipids, various hydrocarbons have been discovered from the direct analysis of carbonaceous meteorites (Pizzarello, Reference Pizzarello2006; Yabuta et al., Reference Yabuta, Williams, Cody, Alexander and Pizzarello2007; Schmitt-Kopplin et al., Reference Schmitt-Kopplin, Gabelica, Gougeon, Fekete, Kanawati, Harir, Gebefuegi, Eckel and Hertkorn2010; Cooper et al., Reference Cooper, Reed, Nguyen, Carter and Wang2011; Cooper and Rios, Reference Cooper and Rios2016), and through the observation of interstellar clouds using near infrared spectroscopy (Belloche et al., Reference Belloche, Muller, Menten, Schilke and Comito2013). Other weakly polar organic compounds such as aliphatic hydrocarbons and monocarboxylic acids also have been identified from carbonaceous meteorites. These findings show that extraterrestrial environments can produce complex molecules. Among these extraterrestrial organics, a family of hydrocarbon molecules known as polycyclic aromatic hydrocarbons (PAHs) were found to be dominant and ubiquitous in the interstellar medium (ISM), contributing to the formation of small hydrocarbon radicals (Tielens, Reference Tielens2008). Thus, PAHs are considered the major organic carbon supplier for Earth and other planetary bodies, although the direct contribution of aromatic structures to the origin of life is still under debate (Ehrenfreund et al., Reference Ehrenfreund, Rasmussen, Cleaves and Chen2006).
What molecules would astrobiologists ideally like to detect? Purines and pyrimidine bases are the essential units of nucleotides, molecules that form base pairs to store and copy genetic information to pass on to the next generation. Although a complete nucleotide molecule has not yet been found in any prebiotic context, several possible chemical pathways that lead to nucleotide formation are known (Powner et al., Reference Powner, Gerland and Sutherland2009; Patel et al., Reference Patel, Percivalle, Ritson, Duffy and Sutherland2015). Biologically-common purines such as adenine, guanine, hypoxanthine and xanthine have been reported from various carbonaceous meteorites along with nucleobase analogues that are rare on the Earth (Callahan et al., Reference Callahan, Smith, Cleaves, Ruzicka, Stern, Glavin, House and Dworkin2011). The mechanism of purine formation has been predicted as the oligomerization of HCN or from other observed interstellar precursor molecules such as C3NH and HNCNH (Jung and Choe, Reference Jung and Choe2013).
A major class of organic compounds fundamental to terrestrial life is the amino acids and their precursors. It has been proposed that the Strecker reaction can occur in the ISM forming amino acids during the aqueous alteration of the parent body (Lerner et al., Reference Lerner, Peterson and Chang1993), suggesting that amino acids are found in space. Well over 80 different amino acids have been detected from various carbonaceous meteorites (Glavin et al., Reference Glavin, Callahan, Dworkin and Elsila2010). Further, outer planet icy moons such as Europa and Enceladus are made of icy planetesimals initially produced in the solar nebula, and likely harbour amino acids and their precursors in their subsurface oceans (Mousis et al., Reference Mousis, Lunine, Waite, Magee, Lewis, Mandt, Marquer and Cordier2009). Amino acids have been long considered as one of the plausible molecular biosignatures for extraterrestrial life detection on Mars and other planetary bodies (Summons et al., Reference Summons, Albrecht, McDonald and Moldowan2007), however chemical studies of extraterrestrial matter have often been challenged due to the risk of terrestrial contamination. For example, several criteria including amino acid distribution, enantiomeric ratio and stable isotopic analysis have been suggested to assess the true origins of amino acids from a Murchison meteorite (Engel and Macko, Reference Engel and Macko1997). The attempt to capture and return extraterrestrial organic materials, as a part of cometary dust grains to the earth was first achieved by the NASA Stardust spacecraft targeting the comet 81P/Wild 2 (Brownlee et al., Reference Brownlee, Tsou, Aléon, Alexander, Araki, Bajt, Baratta, Bastien, Bland, Bleuet, Borg, Bradley, Brearley, Brenker, Brennan, Bridges, Browning, Brucato, Bullock, Burchell, Busemann, Butterworth, Chaussidon, Cheuvront, Chi, Cintala, Clark, Clemett, Cody, Colangeli, Cooper, Cordier, Daghlian, Dai, D'Hendecourt, Djouadi, Dominguez, Duxbury, Dworkin, Ebel, Economou, Fakra, Fairey, Fallon, Ferrini, Ferroir, Fleckenstein, Floss, Flynn, Franchi, Fries, Gainsforth, Gallien, Genge, Gilles, Gillet, Gilmour, Glavin, Gounelle, Grady, Graham, Grant, Green, Grossemy, Grossman, Grossman, Guan, Hagiya, Harvey, Heck, Herzog, Hoppe, Hörz, Huth, Hutcheon, Ignatyev, Ishii, Ito, Jacob, Jacobsen, Jacobsen, Jones, Joswiak, Jurewicz, Kearsley, Keller, Khodja, Kilcoyne, Kissel, Krot, Langenhorst, Lanzirotti, Le, Leshin, Leitner, Lemelle, Leroux, Liu, Luening, Lyon, Macpherson, Marcus, Marhas, Marty, Matrajt, McKeegan, Meibom, Mennella, Messenger, Messenger, Mikouchi, Mostefaoui, Nakamura, Nakano, Newville, Nittler, Ohnishi, Ohsumi, Okudaira, Papanastassiou, Palma, Palumbo, Pepin, Perkins, Perronnet, Pianetta, Rao, Rietmeijer, Robert, Rost, Rotundi, Ryan, Sandford, Schwandt, See, Schlutter, Sheffield-Parker, Simionovici, Simon, Sitnitsky, Snead, Spencer, Stadermann, Steele, Stephan, Stroud, Susini, Sutton, Suzuki, Taheri, Taylor, Teslich, Tomeoka, Tomioka, Toppani, Trigo-Rodríguez, Troadec, Tsuchiyama, Tuzzolino, Tyliszczak, Uesugi, Velbel, Vellenga, Vicenzi, Vincze, Warren, Weber, Weisberg, Westphal, Wirick, Wooden, Wopenka, Wozniakiewicz, Wright, Yabuta, Yano, Young, Zare, Zega, Ziegler, Zimmerman, Zinner and Zolensky2006). The Stardust spacecraft carried an aerogel known as a low-density amorphous SiO2 to decelerate and capture particles intact. The idea of using aerogel for hypervelocity capture and recovery of particles such as interplanetary dust has been previously investigated from the 90s with early studies and development of techniques conducted in a laboratory set up as well as in space (Barrett et al., Reference Barrett, Zolensky, Horz, Lindstrom and Gibson1992; Brownlee et al., Reference Brownlee, Hörz, Hrubsh, McDonnell, Tsou and Williams1994; Burchell et al., Reference Burchell, Thomson and Yano1998; Hörz et al., Reference Hörz, Cintala, Zolensky, Bernhard, Davidson, Haynes, See, Tsou and Brownlee1998; Kitazawa et al., Reference Kitazawa, Fujiwara, Kadono, Imagawa, Okada and Uematsu1999; Hörz and Zolensky, Reference Hörz and Zolensky2000; Westphal et al., Reference Westphal, Snead, Borg, Quirico, Raynal, Zolensky, Ferrini, Colangeli and Palumbo2002, Reference Westphal, Snead, Butterworth, Graham, Bradley, Bajt, Grant, Bench, Brennan and Pianetta2004; Tsou et al., Reference Tsou, Brownlee, Sandford, Hörz and Zolensky2003). The analysis of the small cometary dust particle by Stardust revealed signatures of diverse suites of cometary organic compounds including simplest form of amino acid glycine (Sandford et al., Reference Sandford, Aléon, Alexander, Araki, Bajt, Baratta, Borg, Bradley, Brownlee, Brucato, Burchell, Busemann, Butterworth, Clemett, Cody, Colangeli, Cooper, D'Hendecourt, Djouadi, Dworkin, Ferrini, Fleckenstein, Flynn, Franchi, Fries, Gilles, Glavin, Gounelle, Grossemy, Jacobsen, Keller, Kilcoyne, Leitner, Matrajt, Meibom, Mennella, Mostefaoui, Nittler, Palumbo, Papanastassiou, Robert, Rotundi, Snead, Spencer, Stadermann, Steele, Stephan, Tsou, Tyliszczak, Westphal, Wirick, Wopenka, Yabuta, Zare and Zolensky2006; Elsila et al., Reference Elsila, Glavin and Dworkin2009). Hence a series of hypervelocity impact experiments (HIE) have been conducted after Stardust mission focusing on the fate of organic molecules during and after the impact using aerogel as a capture medium (Burchell et al., Reference Burchell, Foster, Ormond Prout, Dupin and Armes2009, Reference Burchell, Cole, Price, Kearsley and Nixon2010, Reference Burchell, Bowden, Cole, Price and Parnell2014; Spencer et al., Reference Spencer, Clemett, Sandford, Mckay and Zare2009; Nixon et al., Reference Nixon, Burchell, Price, Kearsley and Jones2012). However, aerogel capture is a complicated process and thus impact-induced alteration and isolation of carbon-bearing molecules needs to be treated cautiously to access the original organic material.
One of the potential biosignature is peptides, which are the chains of amino acids linked by a peptide bond. Terrestrial life produces polypeptides that are homochiral, thus indicative of a biological synthesis on Earth. The exogenous abiotic synthesis of peptides is known by the detection of diglycine in Murchison meteorite, and irradiation of interstellar model ices has demonstrated free radical formation of several dipeptide and amino acid species upon irradiation (Kaiser et al., Reference Kaiser, Stockton, Kim, Jensen and Mathies2013). Impact synthesis of alanine tripeptides has been shown with no enantiomeric selection (Sugahara and Mimura, Reference Sugahara and Mimura2015). Peptide serves as an attractive candidate to evaluate the ongoing state of abiotic chemical evolution or possible biological reaction in extraterrestrial context. However, unlike terrestrial polypeptides consist of homochiral L-amino acids, separation and characterization of numerous peptide stereoisomers remains technically challenging. For space mission instruments, gas chromatography combined with mass spectrometry (GC-MS) has a long and distinguished heritage as a flight instrument, and is still one the most sensitive bioanalytical instruments. GC-MS has played a major analytical role in space missions such as NASA's Viking, Phoenix, Curiosity missions to the Martian surface and ESA's Rosetta/Philae mission to a comet nucleus. However, the pyrolytic procedure required in gas chromatography destroys thermally labile molecules including biologically significant polymers such as polypeptides, oligonucleotides and polysaccharides. Recently this became the major issue for detecting organics from the Martian soil, since chlorinated organics were detected through Curiosity's SAM instrument which are likely to be a derivative of organic molecules reacting with perchlorate inside the oven. Thus, the very polymers that are indicative of life are rendered undetectable with GC-MS (Poinot and Geffroy-Rodier, Reference Poinot and Geffroy-Rodier2015). Other analytical strategies proposed for space missions include biosensors (Coussot et al., Reference Coussot, Moreau, Faye, Vigier, Baqué, Le Postollec, Incerti, Dobrijevic and Vandenabeele-Trambouze2017) and antibody-based systems such as the Life Marker Chip (Rix et al., Reference Rix, Sims and Cullen2011). However, these approaches lack flexibility as the instruments must be built with specific molecular targets. Thus far, liquid-based separation techniques are the only flexible alternative to GC.
Electro-migration techniques are especially advantageous due to their unquestionable separation power, low consumption of solvents and ease of miniaturization. The Urey instrument, known as Mars Organic Analyzer, was the first fabricated microchip electrophoresis (μCE) system proposed for the first liquid-based organic detection on Mars (Aubrey et al., Reference Aubrey, Chalmers, Bada, Grunthaner, Amashukeli, Willis, Skelley, Mathies, Quinn, Zent, Ehrenfreund, Amundson, Glavin, Botta, Barron, Blaney, Clark, Coleman, Hofmann, Josset, Rettberg, Ride, Robert, Sephton and Yen2008). μCE using a combination of laser-induced fluorescence with a fluorescent amine reactive probe can detect amino acids with minute concentration of sub-parts-per-trillion as well as aldehydes, ketones, organic acids and thiols (Mora et al., Reference Mora, Stockton and Willis2012). This system has now been adopted for exploring potential life in the Planetary In Situ Capillary Electrophoresis System (Willis et al., Reference Willis, Creamer and Mora2015; Creamer et al., Reference Creamer, Mora and Willis2017), and on icy moons (Mathies et al., Reference Mathies, Razu, Kim, Stockton, Turin and Butterworth2017; Mora et al., Reference Mora, Jones, Creamer and Willis2018). The drawback is it lacks the capability to prove the structure and mass of detected peaks. The downstream addition of MS detection is the most convenient solution of this problem. However, the MS approach excludes the usage of surfactants like in the Urey prototype (Aubrey et al., Reference Aubrey, Chalmers, Bada, Grunthaner, Amashukeli, Willis, Skelley, Mathies, Quinn, Zent, Ehrenfreund, Amundson, Glavin, Botta, Barron, Blaney, Clark, Coleman, Hofmann, Josset, Rettberg, Ride, Robert, Sephton and Yen2008) while simple capillary zone electrophoresis (CZE) mode faces difficulty in separating neutral species and enantiomers that are potential biomarkers (Kim et al., Reference Kim, Jensen, Stockton and Mathies2013).
To overcome these difficulties, we have been developing a hybrid technique known as capillary electrochromatography (CEC), allowing a combination of electro-driven migration and chromatographic separation mechanism using newly developed monolithic stationary phases. Electrochromatography provides a chemical separation with a combination of chromatographic selectivity and electrophoretic mobility. The ability of the monolithic column to withstand radiation, and its ability to separate neutral, hydrophilic, hydrophobic and chiral molecules as well as amino acids and proteins, suggest a clear application for in situ analysis during the space flight missions (Carbonnier et al., Reference Carbonnier, Guerrouache, Denoyel and Millot2007; Guerrouache et al., Reference Guerrouache, Carbonnier, Vidal-Madjar and Millot2007, Reference Guerrouache, Millot and Carbonnier2009, Reference Guerrouache, Millot and Carbonnier2011). Here we demonstrate the utility of CEC through two case studies. First, we introduced CEC technology to separate neutral and biologically relevant organic molecules to evaluate the performance of CEC for future biosignature detection in space. Second, we performed a diastereomeric separation of various tripeptides under CE with HIEs to explore the feasibility of capture and detection of peptides using ultra-low density aerogel. This report describes combinatorial technical basis for liquid-based separation and detection of various astrobiologically relevant organic compounds as well as assessment on post impact alteration of short peptides as a potential biosignature.
Materials and methods
Synthesis of generic monolith P(EDMA-co-NAS)
A generic monolith was synthesized in a capillary tube via photo-triggered free radical polymerization of 200 mg N-acryloxysuccinimide (NAS) and 110 µl ethylene glycol dimethacrylate (EDMA) using (700 µl) dry toluene as porogen. Azobisisobutyronitrile was added to the monomer solution as an initiator with a fraction of 1% in mass with respect to the total number of monomers. The polymerization mixture was sonicated for about 5 min at room temperature to obtain a homogeneous solution and then filtered through a polytetrafluoroethylene membrane (pore size = 0.45 µm). The filtered mixture was pushed through a UV-transparent capillary under a pressure of 3 bar of nitrogen. Prior to filling, the capillary was silanized to ensure the covalent attachment of the monolithic structure onto the inner capillary wall as previously reported (Guerrouache et al., Reference Guerrouache, Carbonnier, Vidal-Madjar and Millot2007). Both ends of the filled capillary were sealed with rubber septa. Then the capillary was exposed to UV irradiation (365 nm, 6 × 15 W) for 800 s to initiate the polymerization step. The monolith-containing capillary was then flushed extensively with acetonitrile (ACN) (2 µl min−1, 1 h) to remove the porogenic solvent. A fused silica capillary with a UV-transparent external coating (75 µm id × 325 µm od) from InnovaQuartz (Phoenix, ZA, USA) was used for CEC experiments and polyimide-coated fused silica capillaries (50 µm id × 363 µm od) from Polymicro Technologies (Phoenix, AZ, USA) were used for CZE experiments. A glass microfluidic 50 µm depth device was purchased from Trianja Company (USA). The inner wall of the glass chip channel was first flushed with 1.0 M NaOH for 30 min at room temperature. The microchannel was then successively flushed with deionized water for 15 min and 0.1 M HCl for 30 min, and rinsed with deionized water for 10 min and then with acetone for 15 min. Thereafter, the glass chip channel was purged with dry nitrogen gas for 1 h at a temperature of 120°C. (Trimethoxysilyl)propyl methacrylate 50% (v/v) solution in acetone was allowed to react overnight with silanols present in the inner wall of the glass chip channel at room temperature. Finally, the chip microchannel was rinsed with acetone for 15 min and dried under a stream of nitrogen for 1 h. The pretreated microchip was then filled with a polymerization mixture under pressure (2 bar) and the same polymerization conditions were used to prepare the monolith inside the glass chip microchannel.
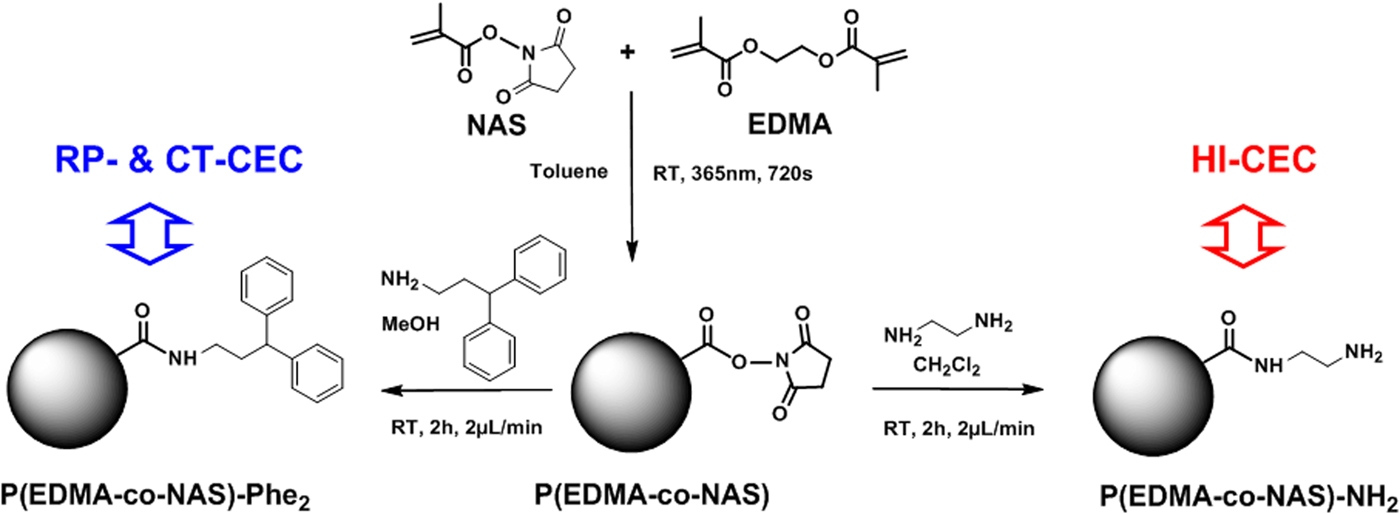
Synthesis of phenyl-like monolithic stationary phase for reversed-phase charge transfer (RP-CT) CEC P(EDMA-co-NAS)-Phe2
A solution of 3,3-diphenylpropylamine (1 M in CH2Cl2) was continuously (2 µl min−1) pumped through the generic monolith column for 2 h at room temperature to produce monolith bearing phenyl rings attached via an aliphatic arm (Fig. 1). Subsequently, the monolithic column was washed with ACN (1 h, 2 µl min−1) and kept at room temperature until use with sealed ends for CEC.
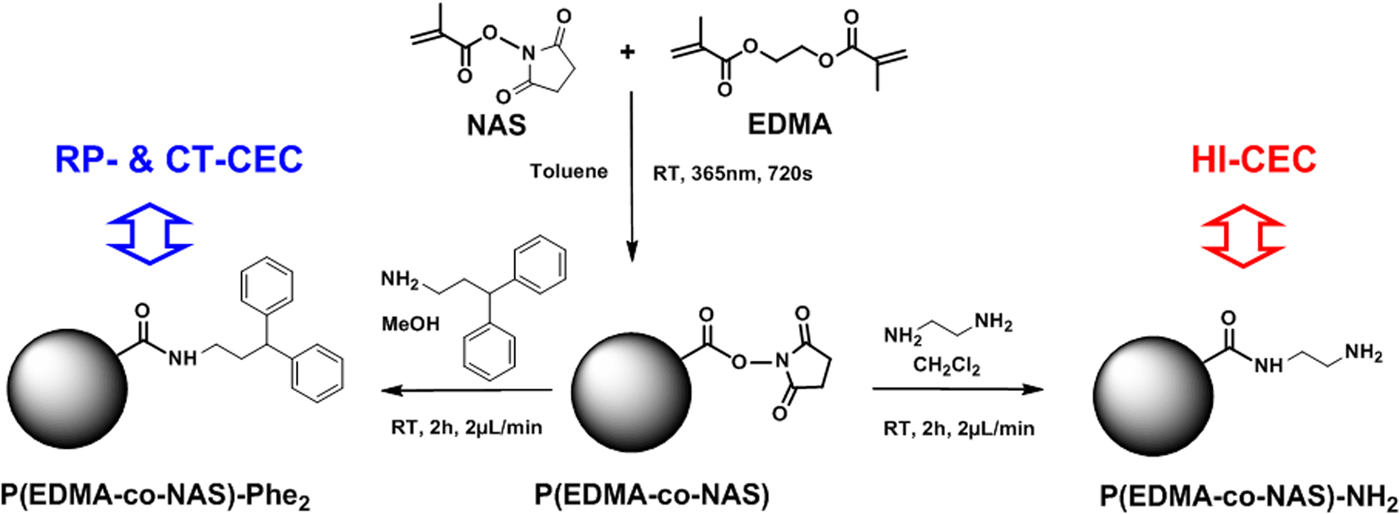
Fig. 1. Schematic illustration of the synthetic path applied to the synthesis of monolithic stationary phases for RP-CT and HI CEC. The monolithic stationary phases were obtained via the surface functionalization of a generic monolith P(EDMA-co-NAS) based on NAS and EDMA.
Synthesis of amino-like monolithic stationary phase for hydrophilic interaction (HI) CEC P(EDMA-co-NAS)-NH2
A solution of ethylenediamine (1 M in CH2Cl2) was pumped continuously (2 µl min−1) through the generic monolith column for 2 h at room temperature to functionalize the surface of the monolithic stationary phase with hydrophilic amino groups (Fig. 1). After functionalization, the monolith column was washed with ACN (1 h, 2 µl min−1) and kept at room temperature until use with sealed ends for CEC.
Separation of extraterrestrial organic compounds using CEC
All the electrochromatographic experiments were performed on a P/ACE MDQ (Beckman, Fullerton, CA, USA) equipped with 32 Karat software (version 4.0) for data acquisition. The analyte solutions (0.2 mg ml−1) were introduced in the monolith capillary column by electrokinetic injection. The separation runs were carried out in reversed mode while the detection wavelength was set at 214 nm and the temperature of the cassette compartment was kept at 25°C. A pressure of 3.5 bar was applied to both ends of the capillary to prevent bubble formation during CEC analysis. The length of the columns was 10 cm to detector, and 31 cm overall. Dimethylformamide was selected as un-retained compound for electroosmotic flow (EOF) determination.
Tripeptides and montmorillonite rock sample preparation
A total of 17 tripeptide enantiomers consisting of N- and C-termini L-tyrosine with various canonical and non-canonical amino acid in the centre (second residue) were purchased from Elim Biopharmaceuticals, Inc. (Hayward, CA, USA) with >90% purity. Types of the peptides are as follows: L-Tyr-L-Val-L-Tyr, L-Tyr-ABA-L-Tyr, L-Tyr-NorV-L-Tyr, L-Tyr-L-Ala-L-Tyr, L-Tyr-IsoV-L-Tyr, L-Tyr-GABA-L-Tyr and L-Tyr-βAla-L-Tyr, L-Tyr-L-Ser-L-Tyr, L-Tyr-AIB-L-Tyr, L-Tyr-D-Val-L-Tyr, L-Tyr-Gly-L-Tyr, L-Tyr-D-Ala-L-Tyr, L-Tyr-D-Ser-L-Tyr, L-Tyr-L-Glu-L-Tyr, L-Tyr-D-Glu-L-Tyr, L-Tyr-L-Asp-L-Tyr and L-Tyr-D-Asp-L-Tyr. Lyophilized peptide samples were dissolved in a 1 : 1 water/ACN solution at a stock concentration of 1 mg ml−1. Montmorillonite rock with a mean density of 2.45 g cm−3 (Tantora) was crushed into small particles by using a hammer. Particles between 100 and 200 µm in diameter were collected using a nylon mesh filter. Particles were rinsed with MilliQ water and dry-heat sterilized at 150°C for 2 h. The ‘all tripeptide’ solution was prepared by mixing an equal volume of each of the 17 peptide stock solutions. From this solution, 10 µl was added to the 10 mg rock projectiles in a test tube to be fully soaked resulting in 0.59 µg of each peptide per projectile. Likewise, an 11 peptide solution consisting of equal quantities of L-Tyr-(Gly, D-Ala, L-Ala, D-Asp, L-Asp, D-Glu, L-Glu, D-Ser, L-Ser, D-Val, L-Val)-L-Tyr were used to make the ‘L + D tripeptide’ solution, and 10 µl of this solution was used to soak the 10 mg rock particles resulting in 0.91 µg input of each peptides per projectile. In both cases, particles were further dried down using a centrifugal vacuum concentrator to produce a projectile. Based on the mean density, a total of 10 mg of rock projectiles with a diameter distribution from 100 to 200 µm consisting of ~2000 particles was produced for each of the two peptide solutions.
Hypervelocity impact experiment
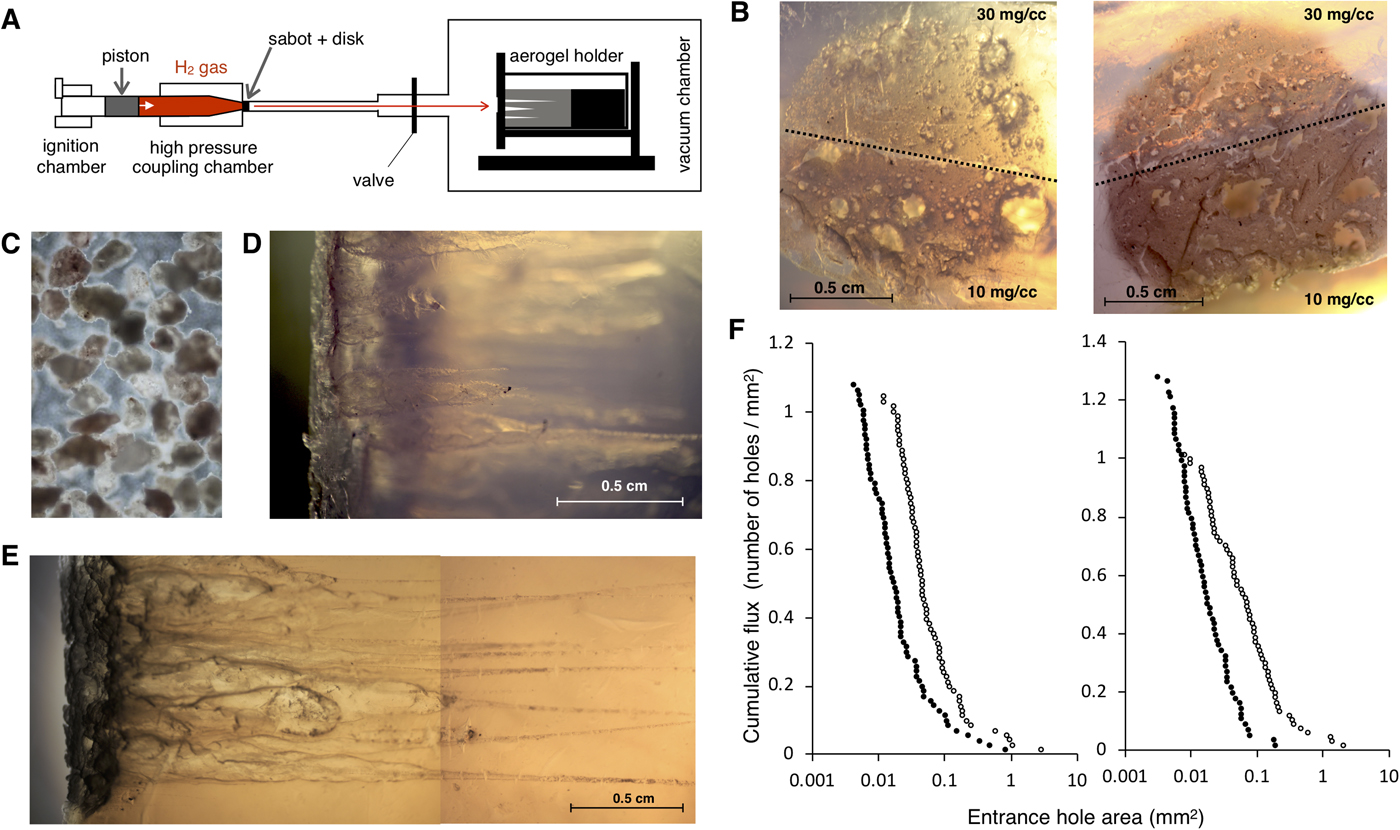
HIEs were performed using a two-stage light-gas gun (Fig. 2(a)) at the Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency (ISAS/JAXA). A powder of microparticles containing tripeptides was placed into a spherical cavity inside a double-split cylindrical ‘sabot’ projectile. Shots were set to reach hypervelocity ranging between 5.5 and 6 km s−1, a similar velocity to that experienced during collection on the NASA Stardust mission, and exceeding the estimated encounter velocity of the spacecraft if flying through the plume of Enceladus while orbiting Saturn at ~4 km s−1 (Sekine et al., Reference Sekine, Takano, Yano, Funase, Takai, Ishihara, Shibuya, Tachibana, Kuramoto, Yabuta, Kimura and Furukawa2014). Further, to minimize the thermal alteration of native organic samples and eliminate possible organic contaminants during intact capture, we utilized ultraclean, methylated, two-layered hydrophobic amorphous silica aerogel with a bulk density of 30 and 10 mg cm−3. This aerogel was originally developed for the Tanpopo mission in a class 1000 clean booth in order to capture micrometeoroids and orbital debris intact at low Earth orbit onboard the International Space Station-Japan Experiment Module (ISS-JEM) exposed facility (EF) (Mita et al., Reference Mita, Yamagishi, Yano, Okudaira, Kobayashi, Yokobori, Tabata, Kawai, Hashimoto and Wg2009; Tabata et al., Reference Tabata, Yano, Kawai, Imai, Kawaguchi, Hashimoto and Yamagishi2015; Yano et al., Reference Yano, Yamagishi, Hashimoto, Yokobori, Kebukawa, Kawaguchi, Kobayashi, Yabuta, Tabata and Higashide2015). So far, 10 mg cm−3 is the lowest density achieved among all space missions utilizing silica aerogels. For the preparation, the aerogel was sliced into a working size of 3 × 2.5 × 4 cm cuboid using a micro-feather blade, and then stabilized with a target holder designed to fit in the vacuum target chamber of the light gas gun. The aerogel holder was fixed inside the vacuum chamber with atmospheric pressure reduced to <3 Torr (Fig. 2(a)). We performed two HIEs, one with the all tripeptide-soaked (shot 1), and other with L + D tripeptide-soaked (shot 2) size-selected montmorillonite rocks as explained in the previous section. After the bulk shots of these peptide-containing microparticles hit the aerogel, the chamber was slowly pressurized to maintain the fragile structure of the aerogel. The target holder with the aerogel was then removed from the vacuum chamber. Impact craters and track images were recorded, and the aerogels were stored in safety cases for downstream analysis.
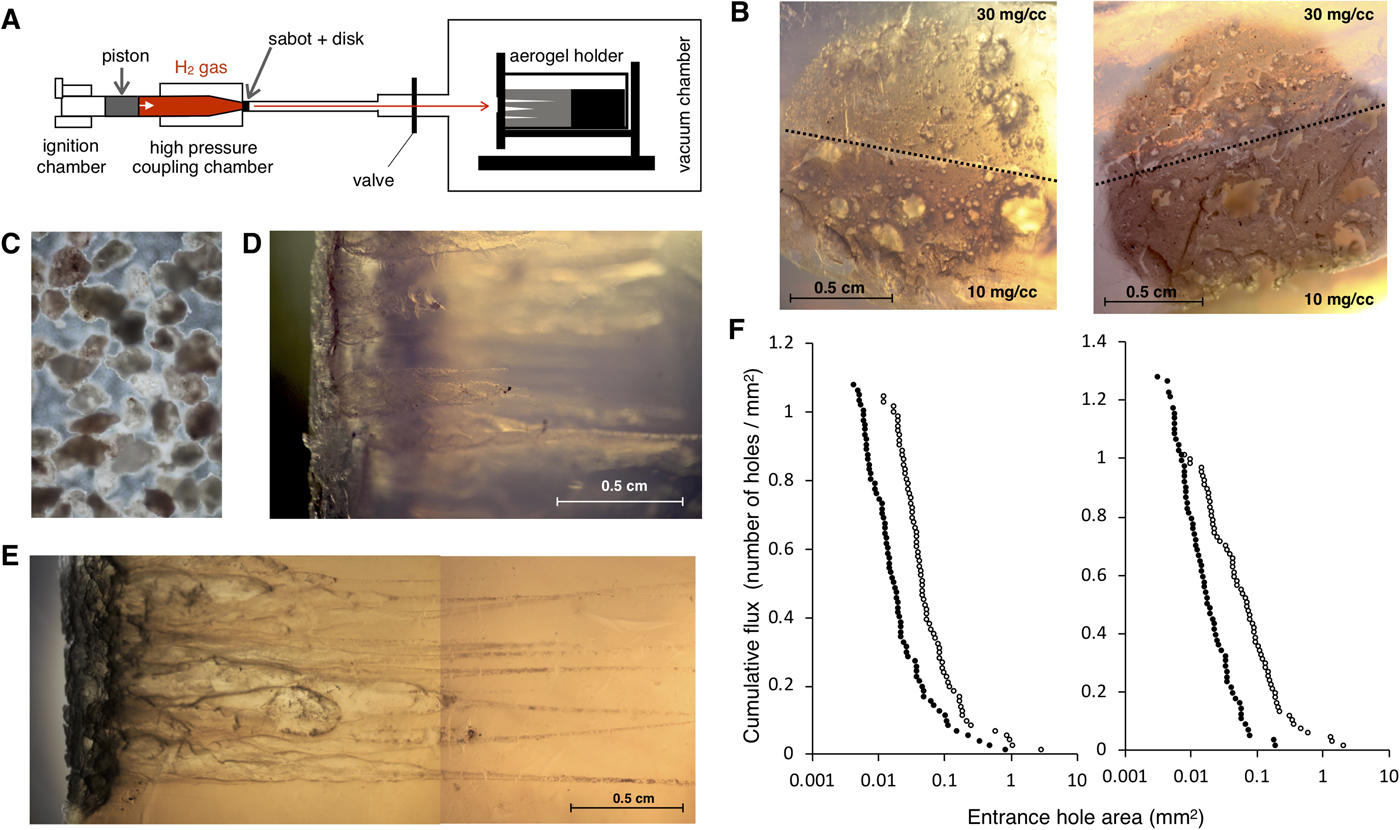
Fig. 2. HIE using hydrophobic silica aerogel as a target. (a) Schematic representation of the light gas gun setup at ISAS. Piston is powered through ignition of gunpowder which propel piston to compress the downstream hydrogen gas. Once hydrogen accumulates enough pressure, it bursts the metal disc, accelerating the sabot with projectile (montmorillonite particle with peptides) inside. Sabot rotates and releases the projectile reaching a speed of up to 6 km s−1. Projectiles are captured by the aerogel placed inside the holder within the vacuum chamber. (b) Light microscopy image of post impact aerogel surface. Aerogel targets were shot separately with projectiles containing all tripeptides at a velocity of 5.56 km s−1 (shot 1, left) and L + D tripeptides at a velocity of 5.61 km s−1 (shot 2, right). Upper half consists of 30 mg cm−3 bulk density aerogel and lower half consists of 10 mg cm−3 bulk density aerogel. (c) Microscopy image of size-selected montmorillonite particles (100–200 µm in diameter) that were used as a peptide carrier during the HIE. (d) and (e) represents aerogel side view after exposed to HIE (shot 1 and shot 2), respectively. Projectile impacts have resulted in ‘carrot-shaped’ tracks by releasing the kinetic energy towards breaking the silica matrix of aerogel. Outermost layers of the montmorillonite particles are fragmented during the impact but large particles remain intact at the end of the track. (f) Cumulative flux plot for two individual HIE. ImageJ software (Schneider et al., Reference Schneider, Rasband and Eliceiri2012) was used to calculate the area size for all detectable entrance holes for shot 1 (left) and shot 2 (right). The x-axis represents entrance hole area in the log scale and y-axis represents the cumulative flux of the projectile (cumulative number of holes per total impact area). Entrance holes made in aerogel bulk density 10 and 30 mg cm−3 are denoted by open circle and closed circle, respectively.
Extraction of peptides from rock and aerogel
As a control experiment, liquid-based extraction was conducted from the peptide adsorbed 10 mg rock particles prepared in the previous section to explore the recovery rate of each peptide from the particle. Particles were re-soaked in 50 µl of 40% ACN solution in a standard 2 ml test tube, and the tube was sonicated for 10 min using an ultrasonic bath to enhance the diffusion speed and efficiency of the extraction process. A rock sample with aerogels was prepared with the same procedure, but with an addition of 1 cm3 aerogel fragment to the test tubes. The tube then was centrifuged to remove aerogel debris. The supernatant was directly analysed using CZE.
The aerogel shot with rock particles soaked with the all tripeptide solution was carefully cut into small pieces. The pieces were squeezed into a glass 10 ml Hamilton syringe (Hamilton, Reno, NV, USA), and soaked with 2 ml of 40% ACN solution inside the syringe for 5 min at room temperature. The solution was then pushed out and subsequently back-aspirated to the syringe to perfuse the rock sample trapped within the aerogel. The repetitive action was performed manually, and after 5 min the piston was pushed fully to crush the aerogel in order to recover the majority of the liquid retained in aerogel. The extract was centrifuged to remove aerogel debris, and the supernatant was transferred to a new tube and subjected to evaporation at 50°C. The evaporation resulted in about a 10-fold volume reduction to ~200 µl and the removal of ACN. Likewise, aerogel shot with particles containing the canonical L-/D- tripeptide solution was soaked with 400 µl of 40% ACN solution in a syringe, repeatedly mixed with solution, and pushed out for evaporation at 50°C. Evaporation resulted in about a 10-fold volume reduction to ~40 µl.
Peptide separation using capillary electrophoresis
All CZE experiments were conducted using an Agilent 7100 CE System (Agilent Technologies, Santa Clara, CA, USA) equipped with a UV diode-array detector. The analysis was monitored at 214 and 280 nm corresponding to the absorbance of the tyrosine aromatic ring. The separation process was performed using bare-fused silica capillaries of 60 cm long with 50 µm id (Polymicro Technologies, Phoenix, AZ, USA). Hydrodynamic injection of an aqueous standards solution was performed for 8 s at 50 mbar with a separation buffer composed of 40 mM ammonium acetate (pH 3.65) for hydrodynamic injection or 100 mM Tris, 95 mM citric acid (pH 3.65) for the head-column injection field-amplified sample injection (FASI) technique due to the fact that the concentration of peptides in samples was expected to be at the ng ml−1 level (Zhang and Thormann, Reference Zhang and Thormann1996; Dziomba et al., Reference Dziomba, Kowalski, Slominska and Baczek2014). Tripeptide standard solutions were injected at a concentration of 5 µg ml−1 for each peptide using the FASI technique with a detection limit estimated at 40 ng ml−1. In the case of standard hydrodynamic injection, the concentrations of peptides used as standards were 50 µg ml−1 with detection limit estimated at 1 µg ml−1. The sample analytical matrix was composed of 20 µl tripeptide sample solution (rock extract, rock + aerogel extract, post HIE aerogel extract) mixed with 5 µl of 5-fold diluted separation buffer adjusted to pH 2.5 using 0.1 M HCl to provide positive ionization of the analytes, and to facilitate the stacking effect (Quirino and Terabe, Reference Quirino and Terabe2000). A short plug of sample was introduced hydrodynamically (3 s, 50 mbar) followed by electrokinetic sample injection at 10 kV. Injection for as long as 50 s can be performed without any significant band broadening effect. Further extension of injection time resulted in loss of separation efficiency and resolution. At the beginning and at the end of each day, the capillaries were sequentially flushed with 0.1 M NaOH and water (2 bar, 10 min). Additionally, before the first run each day, the capillary was conditioned with a separation buffer for 10 min at 2 bar. Between each analysis, there was a 2 min rinse with 0.1 M NaOH, water and separation buffer.
Results and discussion
Synthesis of amino-like monolithic stationary phase
CZE provides for the highly efficient separation with small volumes of samples, and is adaptable to miniaturization, however it is restricted to the separation and detection of ions and compounds that bear charges at the running pH of the analysis. Here, we demonstrate an electrochromatographic approach based on the use of monolithic stationary phases to perform separation under EOF. The generic P(EDMA-co-NAS) monolith stationary phase can be chemically grafted by different chromatographic selectors to achieve molecular-level controlled surface properties tailored to the chemical structure of target organic molecules. Figure 1 illustrates the preparation of phenyl-like P(EDMA-co-NAS)-Phe2 and amino-like P(EDMA-co-NAS)-NH2 monolith columns created via grafting of 3,3-diphenylpropylamine or ethylenediamine on the surface of P(EDMA-co-NAS), respectively.
Separation of PAHs, pyrimidines and aromatic acids via monolithic CEC
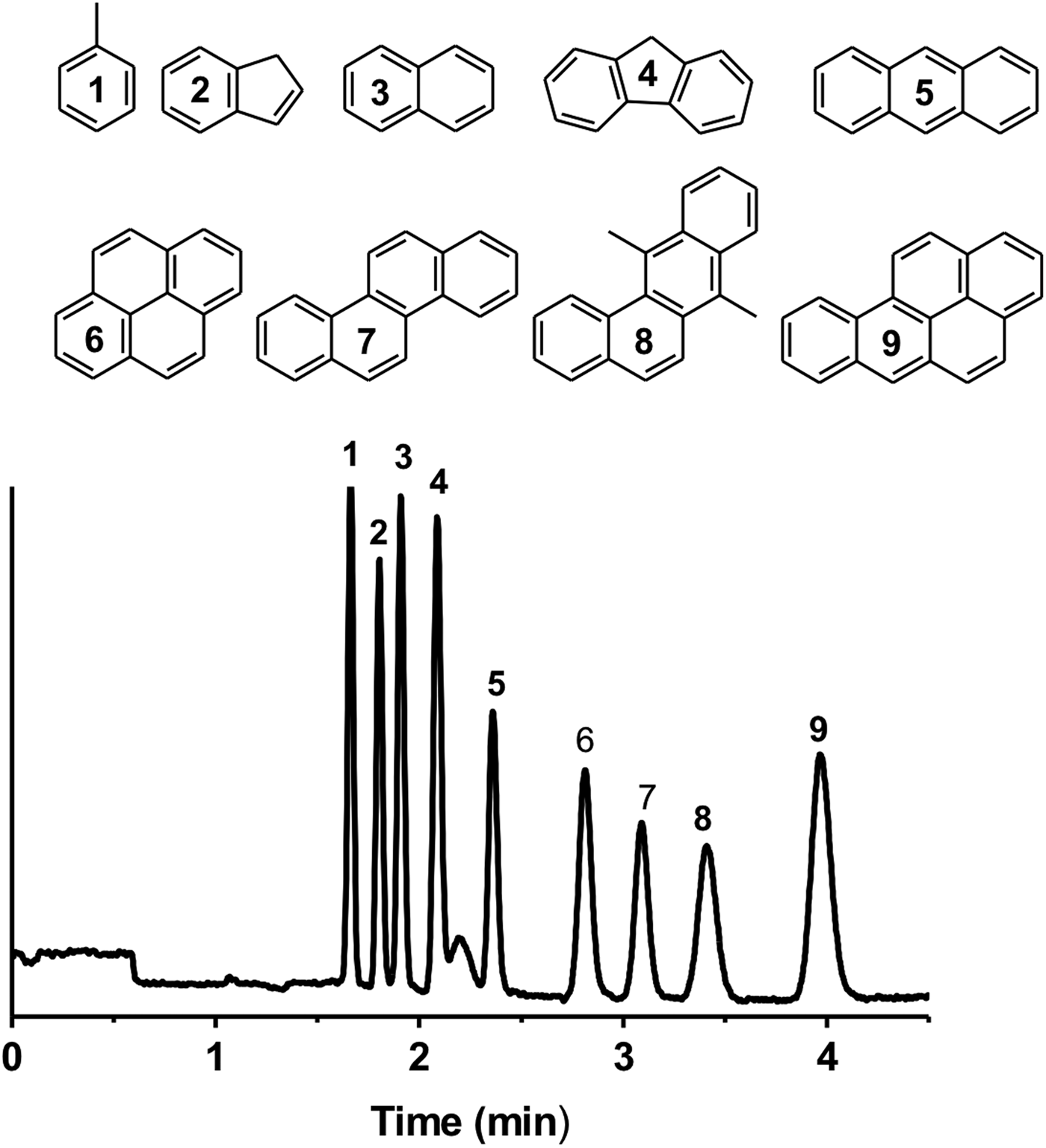
The chromatographic selector 3,3-diphenylpropylamine was chosen for the separation of PAHs because it allows combining the hydrophobic interaction because of the presence of aliphatic spacer arm, and charge transfer because of the electron-rich phenyl rings. With this setup, a mixture of nine PAHs composed of toluene, indene, naphthalene, fluorene, anthracene, pyrene, chrysene, 7,12-dimethylbenz[α]anthracene and benzo[α]pyrene, was separated in fewer than 4 min and with high efficiencies (up to 208 000 plates per m) and under a simple isocratic elution mode on the phenyl-bearing monolithic column (Fig. 3). The retention of PAHs increased with the size and the number of aromatic rings in the molecules confirming the separation mechanism. Large planar aromatic structures favour interaction with the phenyl ring grafted on the monolith surface via a flexible aliphatic spacer arm. Previously, the direct separation of neutral PAHs was achieved in a CE by implementing negatively charged cyclodextrin as a pseudostationary phase (Stockton et al., Reference Stockton, Chiesl, Scherer and Mathies2009) or through micellar electrokinetic chromatography where neutral solutes are separated based on their differential partitioning between micelles and the background electrolyte (Akbay et al., Reference Akbay, Shamsi and Warner1997). P(EDMA-co-NAS)-NH2 monoliths exhibit pH-dependent electro-osmotic generation ability depending on the charge state of the primary amine. At pH 8, cathodic EOF is obtained similarly to what was obtained in the case of the phenyl-like monolith. However, the separation mechanism of the amino-like monolith is dictated by the hydrated nature of the stationary phase and such a separation mechanism is well-suited to the separation of polar and hydrophilic solutes. This separation mode has been utilized in HI liquid-chromatography, well-known for separating biology relevant organic molecules such as nucleobases and nucleosides (Alpert, Reference Alpert1990; Marrubini et al., Reference Marrubini, Mendoza and Massolini2010; Guo et al., Reference Guo, Duan, Qian, Wang, Tang, Qian, Wu, Su and Shang2013).
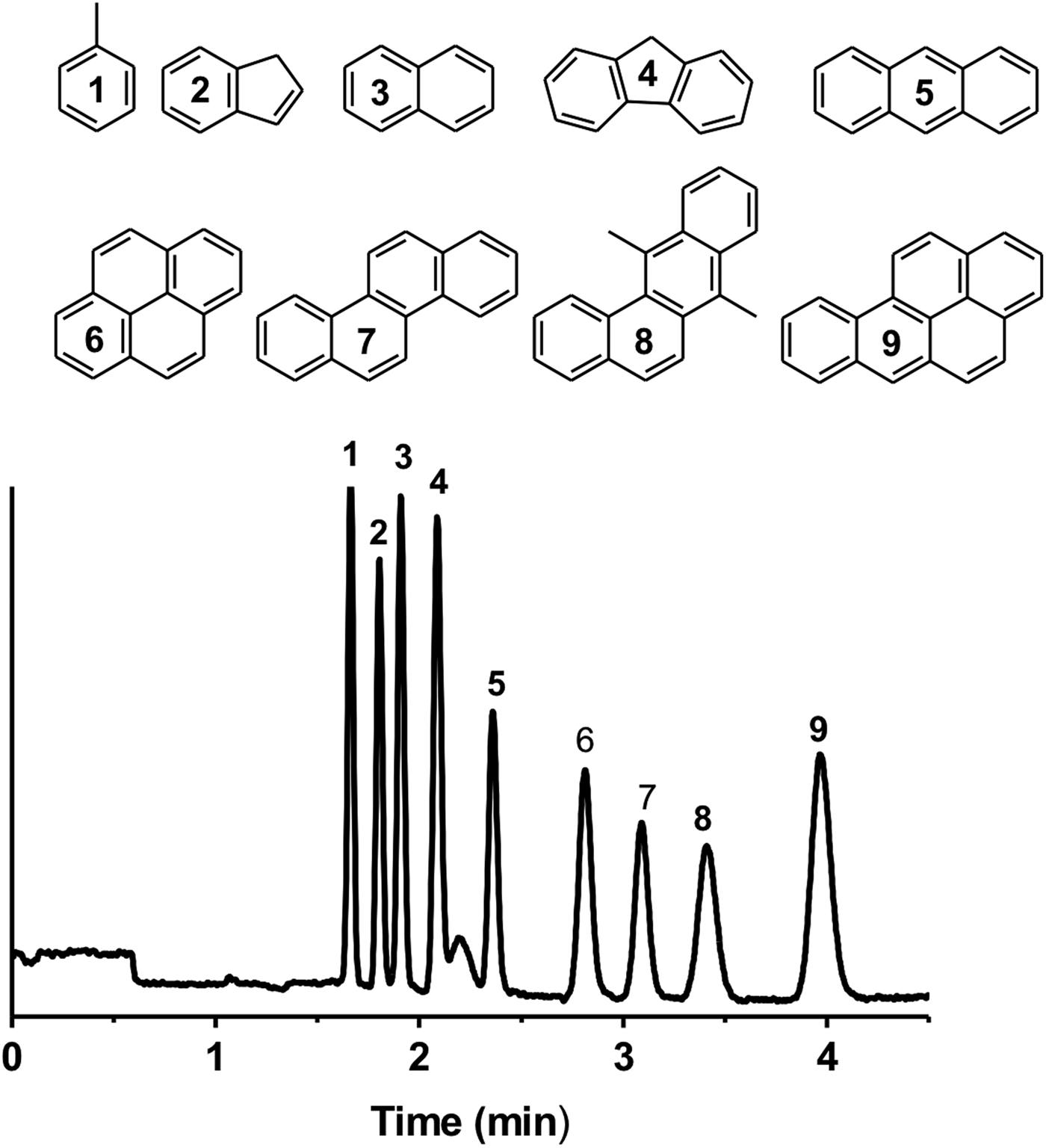
Fig. 3. Electrochromatographic separation of a mixture of nine PAHs: (1) toluene, (2) indene, (3) naphthalene, (4) fluorene, (5) anthracene, (6) pyrene, (7) chrysene, (8) 7,12-dimethylbenz[α]anthracene and (9) benzo[α]pyrene on a phenyl-like monolithic column (CEC conditions: mobile phase 60/40 ACN/PBS (2.5 mM pH 8); injection −5 kV/5 s, voltage −30 kV, detection 214 nm, total and effective lengths of the monolithic column are 31 and 10 cm, respectively).
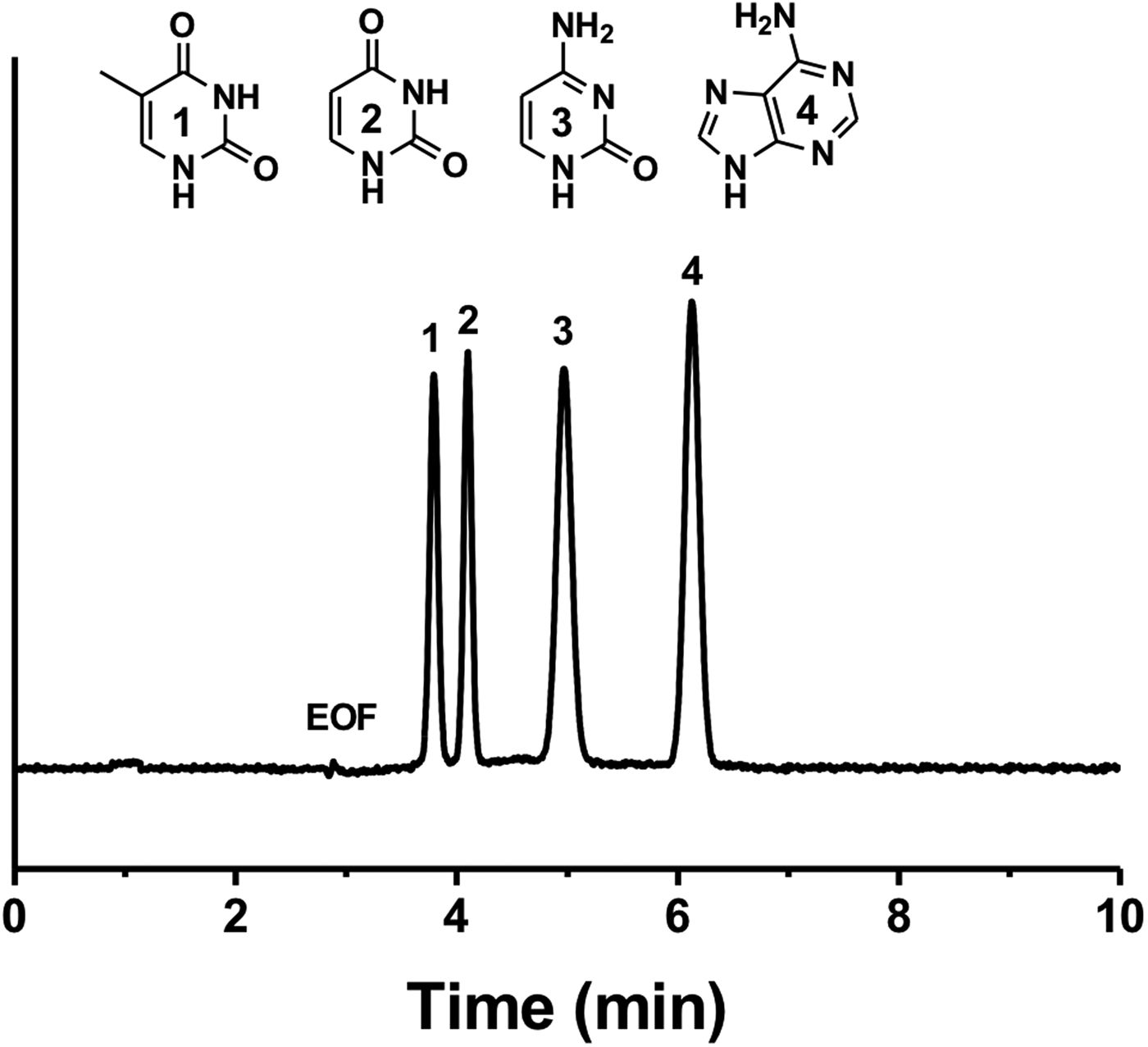
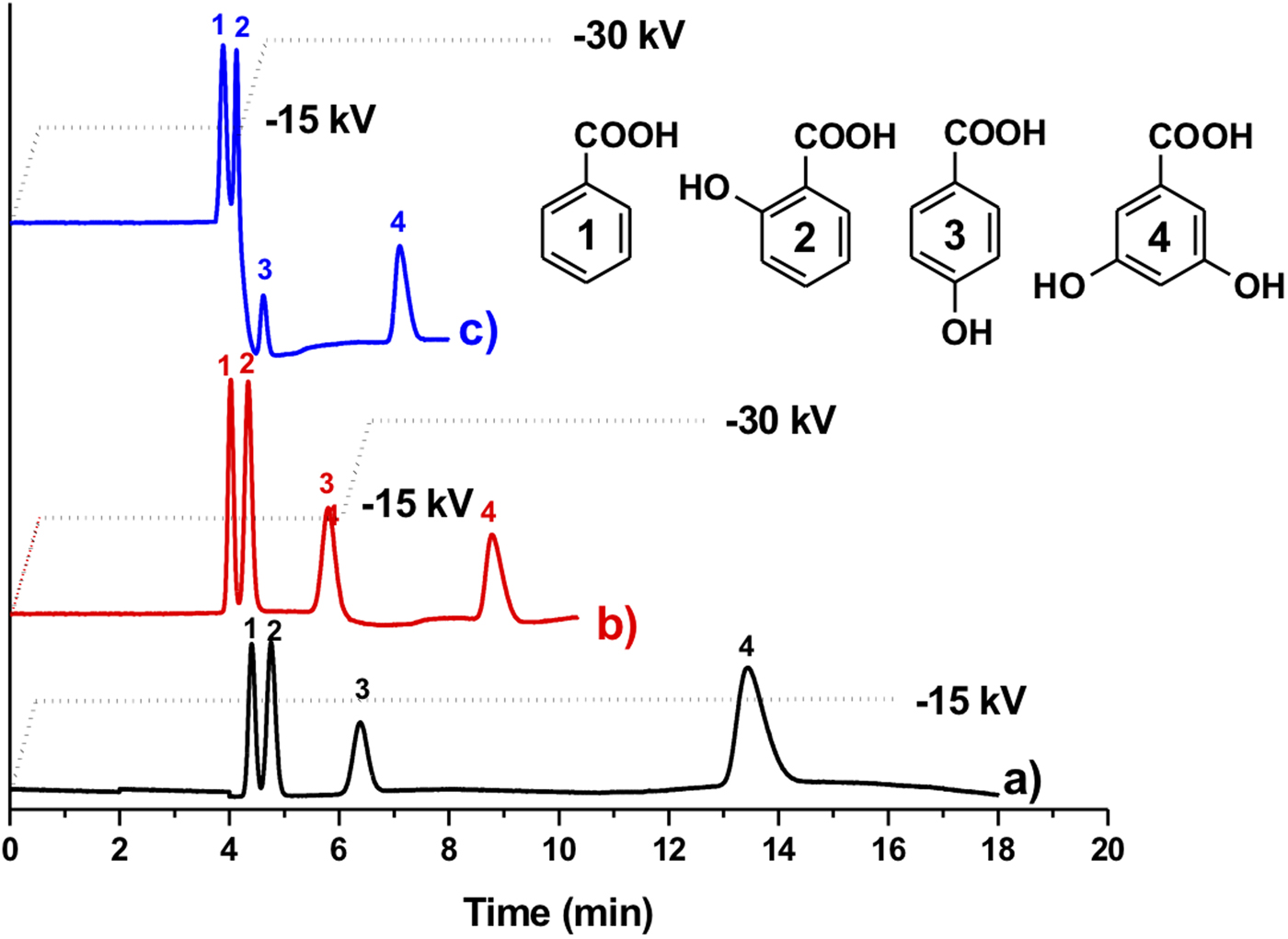
Figure 4 shows the electrochromatographic separation of four different nucleobases: thymine, uracil, adenine and cytosine. We observed that the electrochromatographic resolution of thymine and uracil increased with the ACN content in the mobile phase, confirming the proposed separation mechanism. Note that nucleobases possess different pK as and their acid-base properties depend on the pH (Hiller and Strobel, Reference Hiller and Strobel2011). Therefore, it has been shown that nucleobases can be separated by conventional CE at high pH (pH > 10; Chen et al., Reference Chen, Shi, Wang, Li and Wang2015). However, in this study nucleobases were separated in their neutral form at the same pH value (pH 8) as the one used for the PAH separation. To further demonstrate the potential of CEC, we tested the separation of a mixture of benzoic acid derivatives, namely benzoic acid, 2-hydroxybenzoic acid, 4-hydroxybenzoic acid and 3,5-dihydroxybenzoic acid (Fig. 5). In a first attempt, the four benzoic acids were separated using a constant voltage (−15 kV) leading to a rather long analysis time of about 15 min. In concordance with the hydrophilic character of the P(EDMA-co-NAS)-NH2 monolithic stationary phase, the dihydroxylated benzoic acid was eluted last, while benzoic acid was eluted first. The monohydroxylated benzoic acids exhibit intermediate retention and their separation can be rationalized by the possibility in the case of 2-hydroxybenzoic acid of the formation of intermolecular hydrogen bonding limiting the interaction with the amino group of the monolithic stationary phase. Thus 4-hydroxybenzoic acid is retained more than 2-hydroxybenzoic acid. With the aim of shortening the analysis time and to improve separation efficiency (narrowing of the peak width), further analyses were performed using a voltage gradient (electropherograms (b) and (c) in Fig. 5). Increasing the separation voltage from −15 to −30 kV in a stepwise fashion decreased the analysis time by half and improved efficiency (from 26 500 plates per m in plot (a) to 40 000 plates per m in plot (c) for 4-hydroxybenzoic acid).
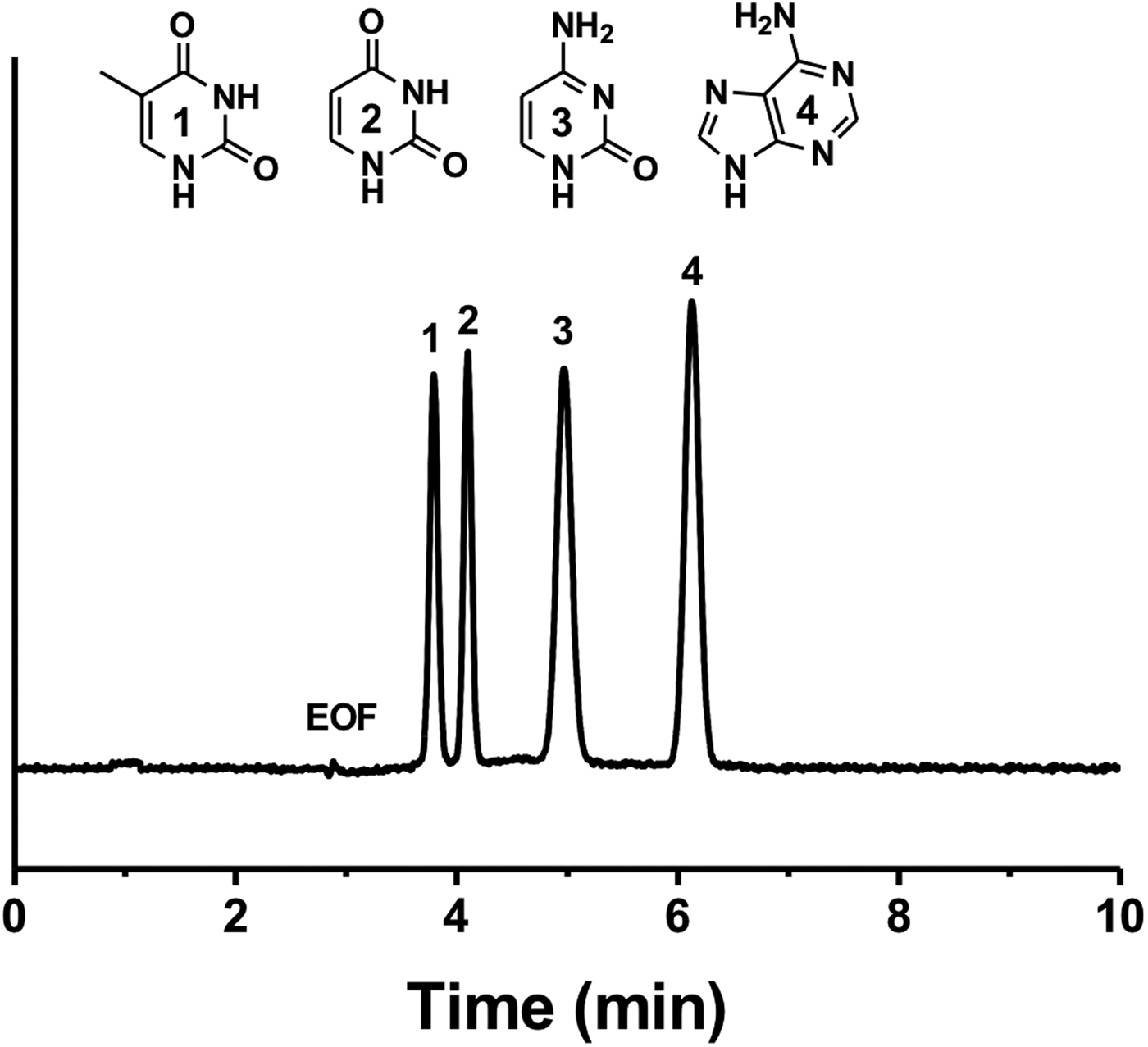
Fig. 4. Electrochromatographic separation of four pyrimidines: (1) thymine, (2) uracil, (3) cytosine and (4) adenine on the amino-like monolithic column (CEC conditions: mobile phase 80/20 ACN/PBS (5 mM pH 8), injection −5 kV/5 s).
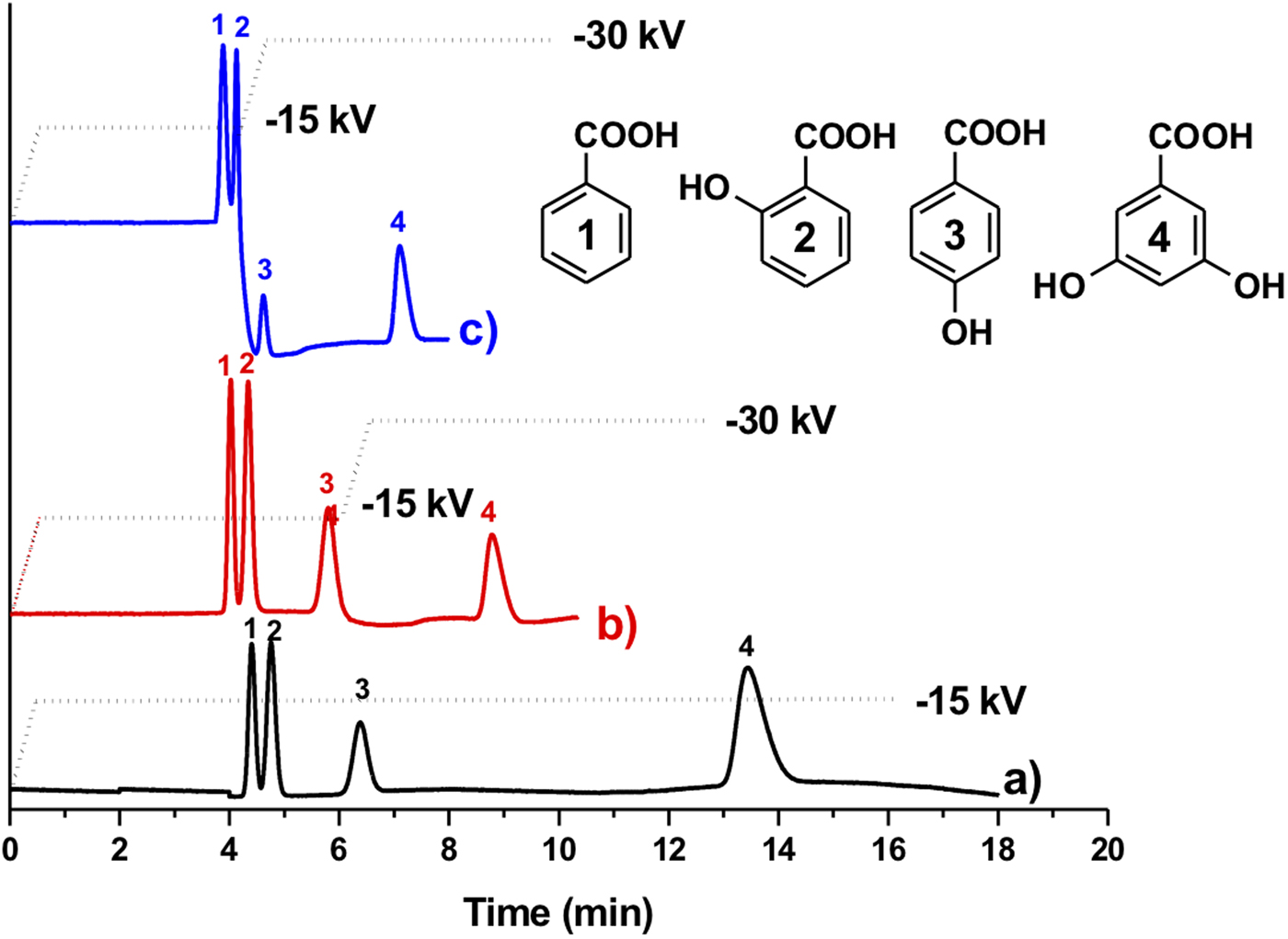
Fig. 5. Electrochromatographic separations of aromatic acids: (1) benzoic acid, (2) 2-hydroxybenzoic acid, (3) 4-hydroxybenzoic acid and (4) 3,5-dihydroxybenzoic acid on the amino-like monolithic column. The analyses were performed under three different voltage conditions as indicated. The electrochromatogram (a) was obtained using a constant voltage of −15 kV while electrochromatograms (b) and (c) were obtained using voltage gradients (stepwise increase to −15 and −30 kV at a voltage rate of 30 kV min−1) (other CEC conditions: mobile phase 80/20 ACN/PBS (5 mM pH 8), injection −5 kV/5 s, detection 214 nm, total and effective lengths of the monolithic column are 31 and 10 cm, respectively).
Separation of tripeptides including diastereomers using CZE
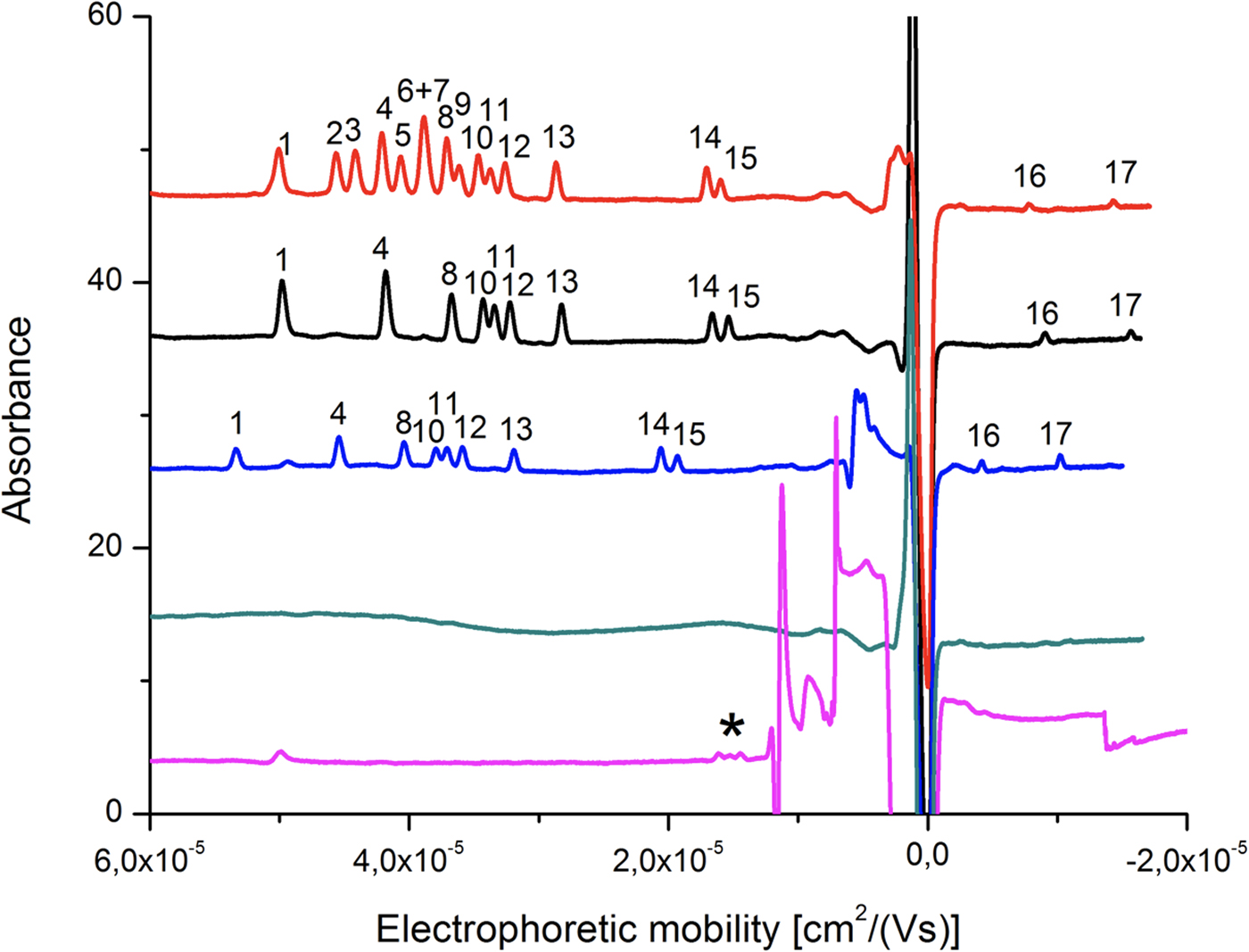
Seventeen tripeptides including five pairs of diastereomers (see ‘Materials and methods’ section for details) served as both a simulant for extraterrestrial organic compounds and for analytes. Separation and identification of such isomeric peptides is challenging, and requires highly efficient chromatographic or electrokinetic separation. Therefore, CZE was chosen as a technique that features extremely high-separation efficiency and whose usefulness in stereoisomeric peptide analysis has been proved in a number of studies (Kasicka, Reference Kasicka1999; Reference Kasicka2006; Scriba, Reference Scriba2006; Reference Scriba2009; Ali et al., Reference Ali, Al-Othman, Al-Warthan, Asnin and Chudinov2014). Separation of the compounds in CZE is possible because of the difference in the migration velocities of the ions. Only peptides containing glutamic and aspartic acid in their structure are predicted to feature lower pK a values compared with other analytes. We investigated the influence of the buffer pH in the range from 2.50 to 7.00 on separation. The baseline separation of eight out of 11 tripeptides containing biogenic amino acids was initially achieved with 50 mM ammonium acetate buffer at pH 3.65. However, due to the high-electrophoretic mobility of co-migrating ammonium ions (μammonia = 76.2 × 10−9 m2 V−1 s−1), we observed peak asymmetry and deteriorated separation efficiency and resolution. This was due to the electrophoretic dispersion phenomenon. We therefore used tris(hydroxymethyl)aminomethane (Tris) instead of ammonium, as the former ions feature lower electrophoretic mobility (Tris = 29.5 × 10−9 m2 V−1 s−1). Tris ions have been reported to coat the capillary wall dynamically, and protect peptides and proteins from adsorption to silica in CZE (Kasicka, Reference Kasicka1999). To obtain the proper buffering effect in the desired pH range, citric acid was used to replace volatile acetate. An increase of buffer concentration was found to enhance separation efficiency and detection sensitivity due to the stacking effect (Burgi and Chien, Reference Burgi and Chien1991). Modification of this parameter also had an effect on peak resolution. Dynamic coating of the capillary by Tris ions decreases EOF in the capillary simultaneously extending the separation time. Under the optimized conditions of 100 mM Tris and 95 mM citric acid (pH 3.65) with FASI, we were able to distinguish 16 signals originating from 17 tripeptide compounds including diastereomers (Fig. 6, red trace). Under these conditions most compounds migrated into the cationic form while Tyr-Asp-Tyr diastereomers were resolved as anions. While co-migration was observed in the case of the gamma amino acid γ-aminobutyric acid (GABA) and β-alanine-containing tripeptides, separation of the diastereomer was achieved and thus the same separation condition was applied for the samples obtained from the HIE.
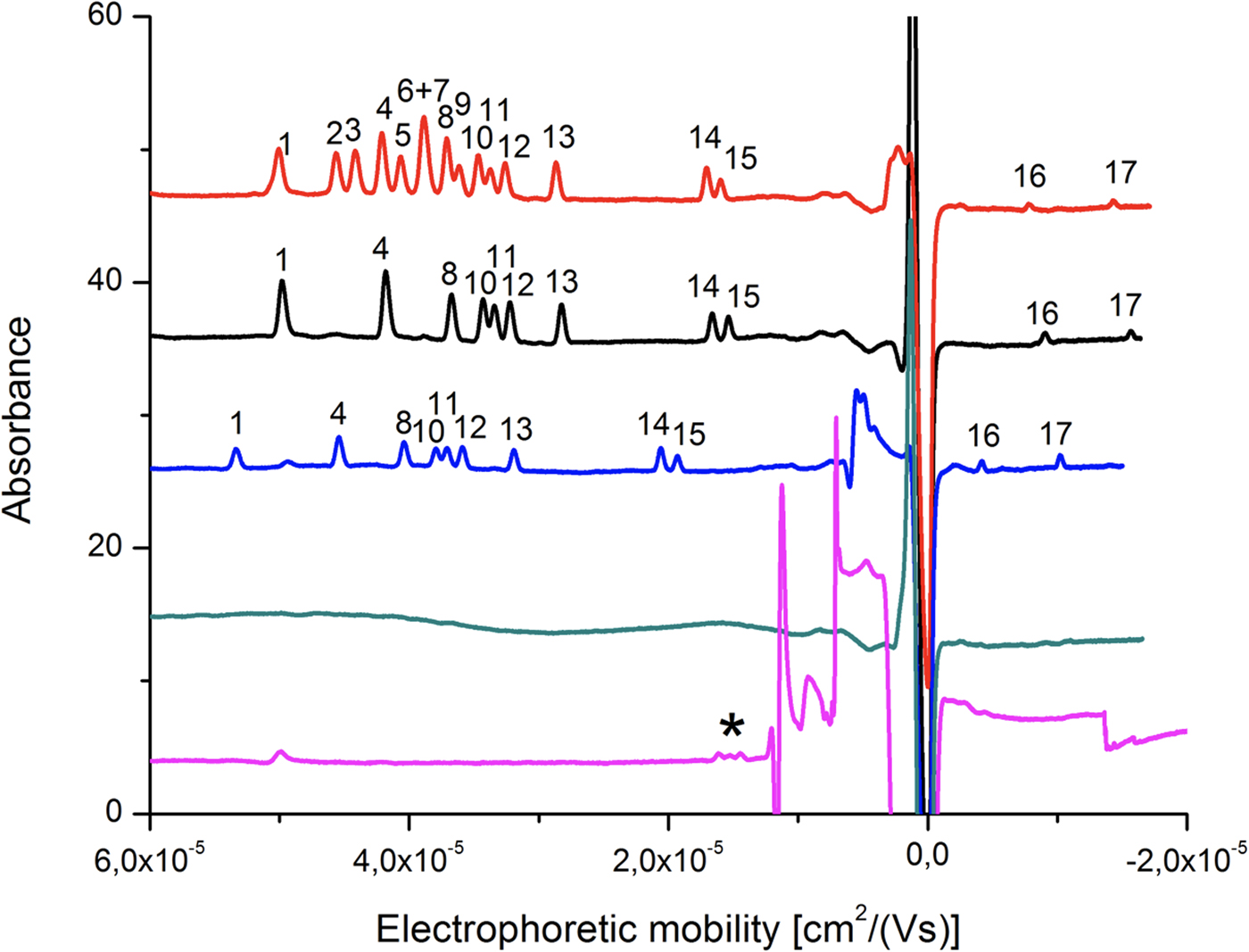
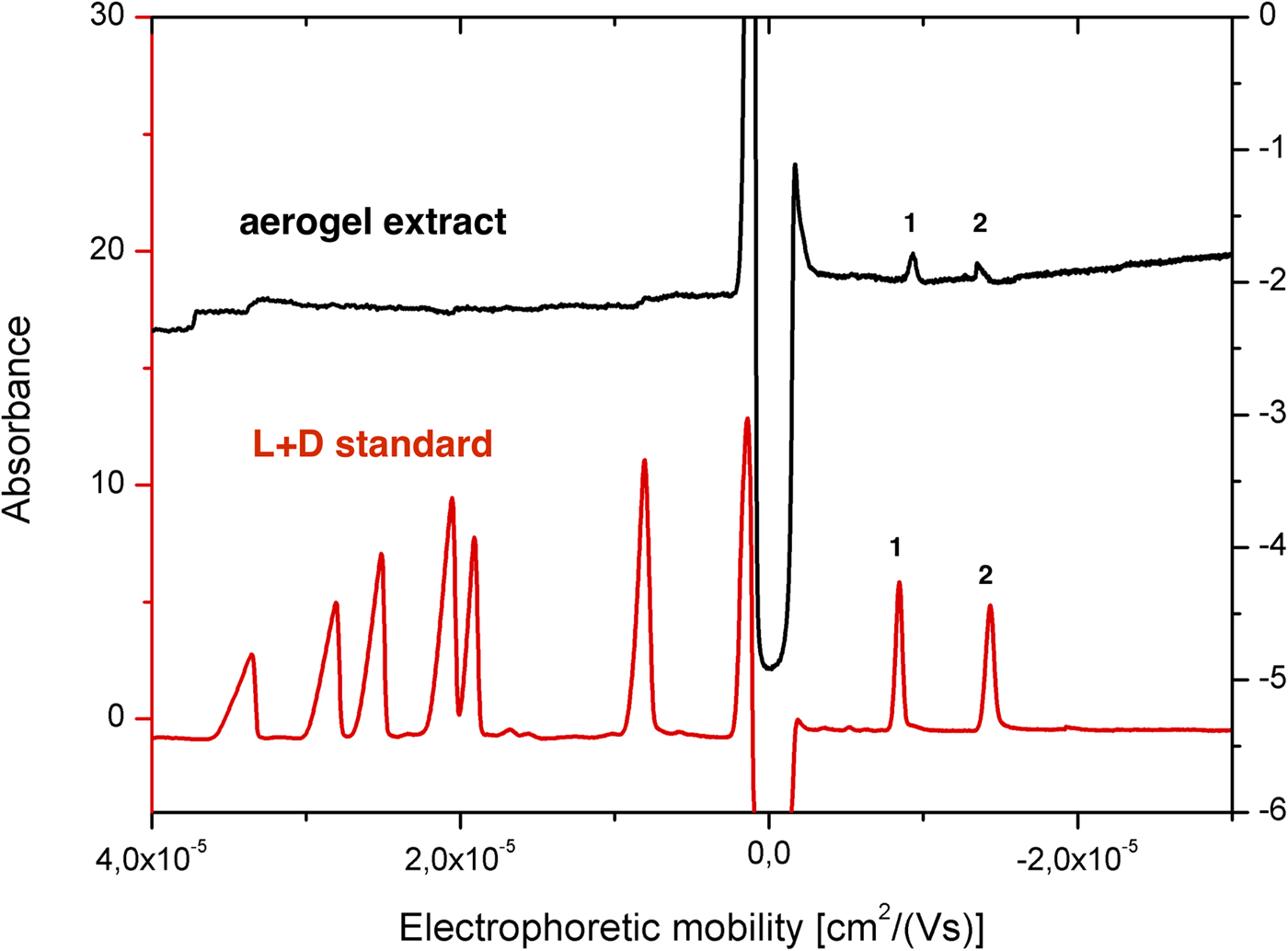
Fig. 6. CZE separation of YNY-tripeptides. Each peak corresponds to (1) L-Tyr-L-Val-L-Tyr, (2) L-Tyr-ABA-L-Tyr, (3) L-Tyr-NorV-L-Tyr, (4) L-Tyr-L-Ala-L-Tyr, (5) L-Tyr-IsoV-L-Tyr, (6) L-Tyr-GABA-L-Tyr, (7) L-Tyr-βAla-L-Tyr, (8) L-Tyr-L-Ser-L-Tyr, (9) L-Tyr-AIB-L-Tyr, (10) L-Tyr-D-Val-L-Tyr, (11) L-Tyr-Gly-L-Tyr, (12) L-Tyr-D-Ala-L-Tyr, (13) L-Tyr-D-Ser-L-Tyr, (14) L-Tyr-L-Glu-L-Tyr, (15) L-Tyr-D-Glu-L-Tyr, (16) L-Tyr-L-Asp-L-Tyr and (17) L-Tyr-D-Asp-L-Tyr. The electrochromatograms are all tripeptide standards (red), canonical L-/D-tripeptides extracted from the montmorillonite sample (black), canonical L-/D-tripeptides extracted from the montmorillonite rock sample mixed with aerogel (blue), extract of HIE blank sample (green) and extract of HIE sample (violet). Background electrolyte was composed of 100 mM Tris and 95 mM citric acid (pH 3.65). The injection was performed electrokinetically for 20 s using 10 kV except for the HIE sample extract analysis for which 10 kV were applied for 50 s. *unidentified peaks.
Extraction and detection of tripeptides resulting from the HIE
The in situ analysis of diverse extraterrestrial organic molecules is a challenging goal for astrobiology, given the limitation of size, weight, types of chemicals and energy required for the on-board instrumentation. Also, any flyby space mission would require extra level of difficulty given the expected trace amounts of organic molecules, which can further undergo thermal decomposition and impact alteration during the encounter. Therefore, assessment of HIE-exposed organic materials using different capture materials has been discussed. We used two tripeptide-soaked montmorillonite bulk particles (one with all 17 tripeptides and one with 11 L + D tripeptides) as a simulant of solid material with biology relevant molecules and performed independent impact experiments reaching a speed of 5.56 km s−1 (shot 1 with all tripeptides) and 5.61 km s−1 (shot 2 with L + D tripeptides), respectively. Microscopic observation of the aerogel impact surface revealed a total 149 (shot 1) and 141 (shot 2) detectable impact craters (Fig. 2(b)). Given the initial total particle numbers to be around 2000, we estimate that approximately 7.5% (shot 1) and 7% (shot 2) of the bulk shot rock particles (Fig. 2(c)) were captured in the aerogel. Longer tracks were obtained from the ultra-low density 10 mg cm−3 aerogel compared with 30 mg cm−3 aerogel (Fig. 2(d) and (e)), suggesting less energy and momentum loss per unit length for lower density aerogel. Indeed, clear difference in the particle entrance hole area was observed between 10 and 30 mg cm−3 aerogel (Fig. 2(f)). An unbiased logarithmic distribution with only several anomalies (clumped and fragmented particles) indicating that majority of the aerogel encountered particles fall within the diameter size range of 100–200 µm.
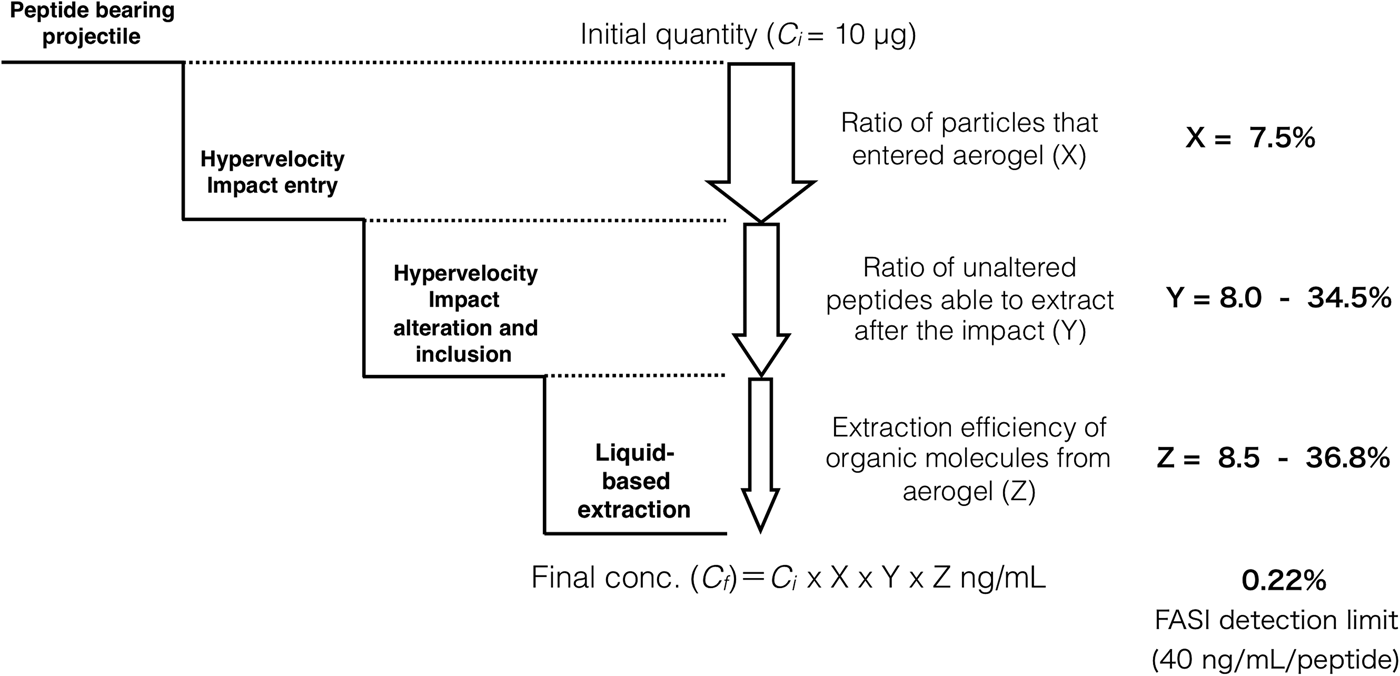
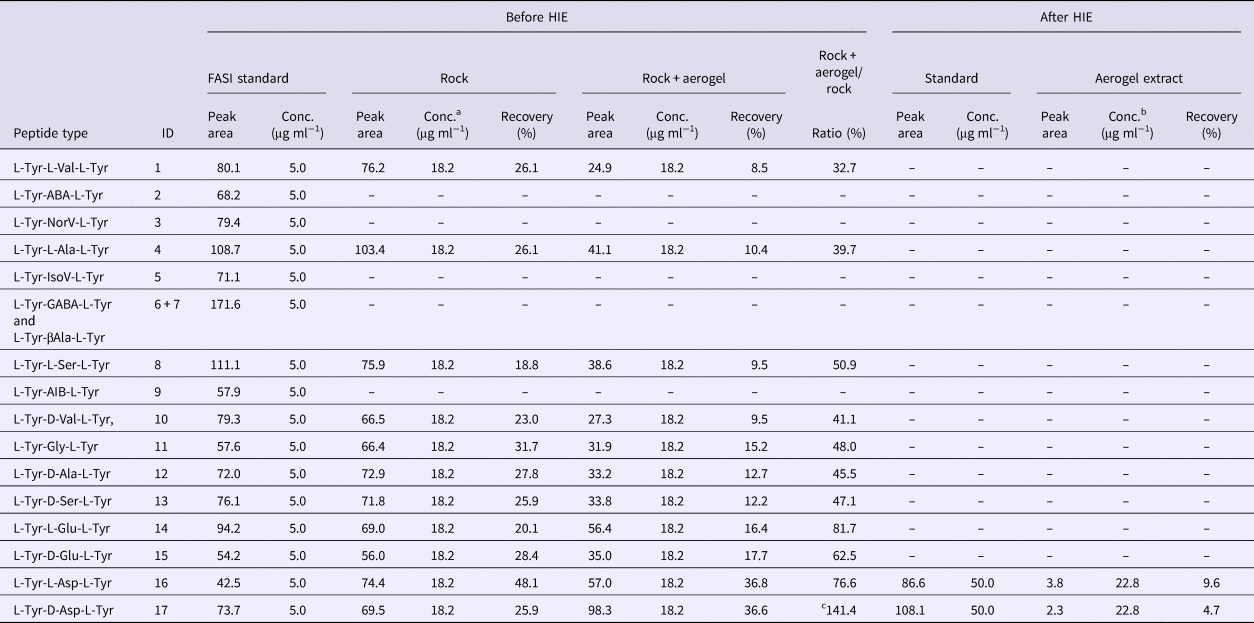
After the impact, the aerogel targets were perfused with 40% ACN to extract the peptides according to the procedure described in the ‘Materials and methods’ section. As opposed to handling and analysis of individual aerogel captured grains (Westphal et al., Reference Westphal, Snead, Butterworth, Graham, Bradley, Bajt, Grant, Bench, Brennan and Pianetta2004), we soaked the entire aerogel to see if bulk liquid extraction and concentration procedure is feasible for detection of captured organic compounds. Due to the methylated surface of silica aerogel, 40% ACN solution in water was selected to provide good solubility of the peptides and sufficient permeability to the crushed aerogel. Prior to this experiment, we had shown that the 40% ACN is capable of dissolving peptides from the montmorillonite particles with a recovery rate ranging from 22.1 to 56.6% (Fig. 6, black trace, Table 1). Further, the dissolution of peptides from the montmorillonite projectiles in the presence of blank aerogel was also tested to see if the methylated silica matrix of aerogel possesses affinity towards certain peptides released from the montmorillonite surface (Fig. 6, blue trace, Table 1). All 16 peaks were detected in the presence of both montmorillonite and aerogel with a diverse recovery rate ranging from 8.5 to 36.8%. However, when we compared the recovery rate of rock projectile alone versus rock projectile in the presence of a blank aerogel sample, results indicated higher recovery of peptides featuring acidic amino acids (Asp and Glu)-bearing tripeptides with lower mobility in electrophoretic experiments (>62.5%) compared with faster migrating peptide (<50.9%; Table 1). This could be due to ionic interactions between silanol moieties of the aerogel and peptides (based on the electrophoretic results, the degree of dissociation of longer migrating species such as Tyr-Glu-Tyr and Tyr-Asp-Tyr was expected to be lower compared with other peptides). Evidence of accretion of compressed aerogel on captured projectiles (Hörz and Zolensky, Reference Hörz and Zolensky2000) may also enhance such selective liquid extraction. Furthermore, extraction from blank aerogel showed a clean CZE profile under UV spectra, suggesting no contamination above the detection limit (Fig. 6, green trace). Extraction of peptides from the aerogel sample with projectiles bearing all 17 tripeptides resulted in several weak signals featuring mobility similar to Tyr-Glu-Tyr diastereomers, but we were unable to resolve the peaks to provide clear answer (denoted by an asterisk in Fig. 6, violet trace). Because most of the tripeptides were below detection limit (40 ng ml−1 per peptide = 0.22% recovery), the peptide recovery rate to reach the detection limit after the impact was predicted to range from 8.0 to 34.5% under our HIE conditions (Fig. 7). Thermal alteration is considered to be the main cause of change in organic contents due to the impact shock heating reaching up to a few thousand degrees Celsius (Hörz et al., Reference Hörz, Cintala, See and Messenger2009). The general track model implies that organic molecules deposited on the impact track will be exposed to high temperature flash heating (Domínguez, Reference Domínguez2009), while HIE with hydrated mineral grains showed that the mineralogical thermal alteration only occur on the projectile surface while interior remains unaltered with just 150 nm below the rim (Okudaira et al., Reference Okudaira, Noguchi, Nakamura, Sugita, Sekine and Yano2004). Hence, the recovery rate of the organic molecules depends on their localization within the projectile (surface or interior) as well as the thermal history of the projectile immediately after the impact.
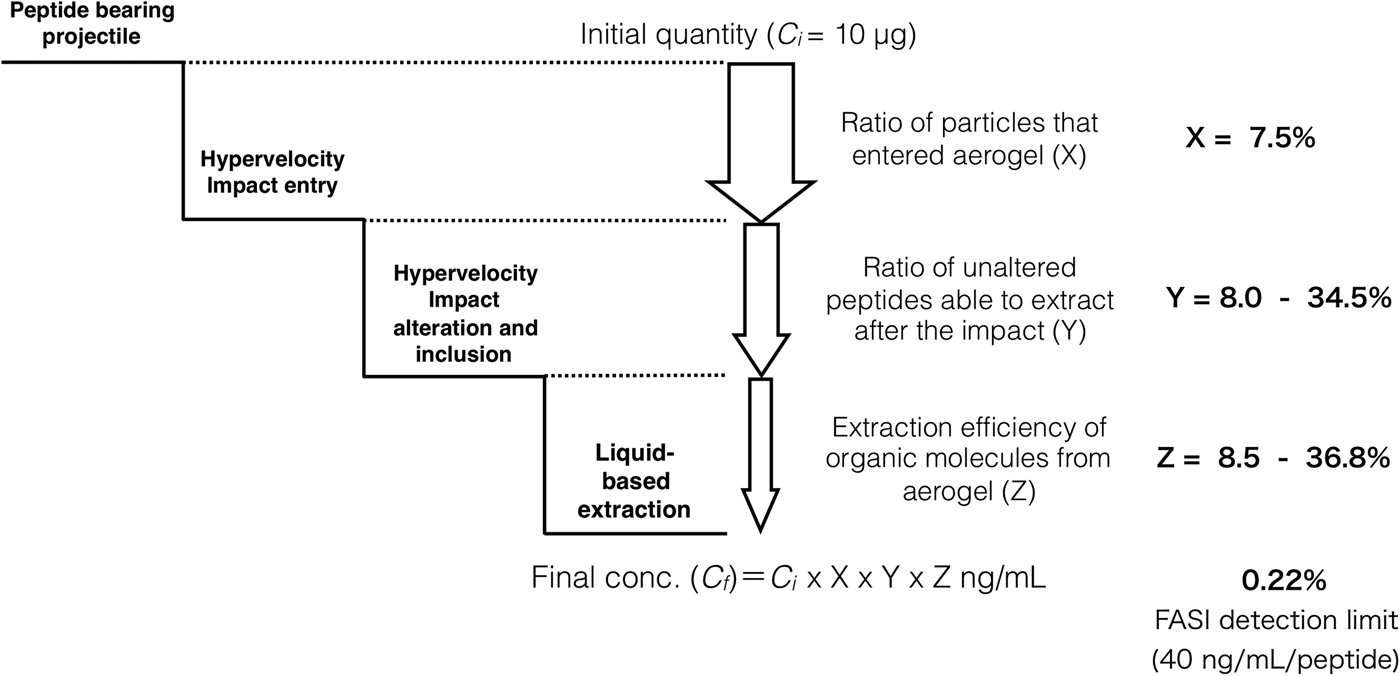
Fig. 7. A schematic representation of organics’ extraction flowchart for simulated flyby sample capture and analysis. Initial peptide input (10 µg) undergoes stepwise reduction in quantity due to the ratio of particle entry into the aerogel (X), hypervelocity impact alteration/isolation (Y) and liquid-based extraction efficiency (Z). Particle entry rate X: 7.5% is estimated from the number of impact holes from shot 1. Liquid-based extraction efficiency under the presence of aerogel Z: 8.5–36.8% is estimated based on Table 1. Accordingly, impact alteration/isolation rate Y: 8.0–34.5% was estimated given the FASI detection limit of 40 ng ml−1 per peptide.
Table 1. Summary of the quantitative data obtained from capillary electrophoresis analysis of peptide samples
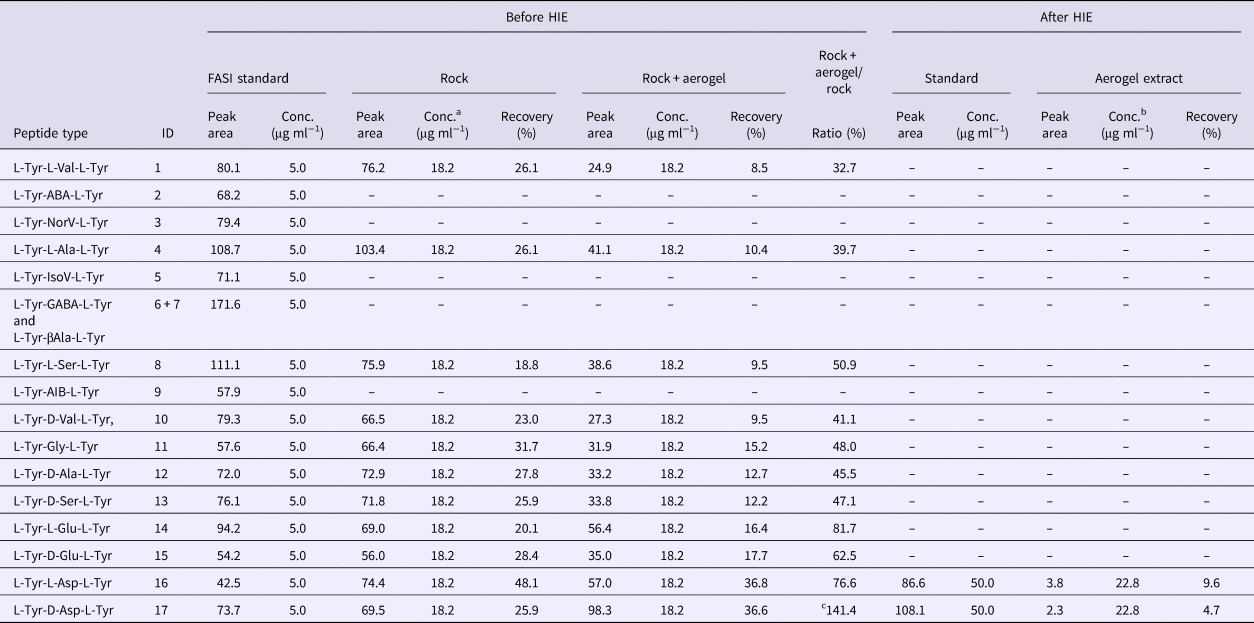
a 10 mg rock = 10 µg L + D peptide mix = 0.91 µg/50 µl = 18.2 µg ml−1 per peptide.
b 10 mg rock = 10 µg L + D peptide mix = 0.91 µg/40 µl = 22.8 µg ml−1 per peptide.
c Value should not exceed 100%, reference purpose only.
To assess this possibility, we further examined extracts from the second aerogel sample shot with 11 L + D tripeptides. The majority of the tripeptide peaks was undetectable; however, two peaks were detected using hydrodynamic injection (Fig. 8, black trace). These peak signals showed identical mobility to that of Tyr-Asp-Tyr diastereomer standards under the same CZE conditions (Fig. 8, black trace) with a recovery rate of 9.6 and 4.7%, respectively (Table 1) indicating extractable peptides survived the impact velocity of 5.61 km s−1 in montmorillonite particles. Why only the anionic peptides were extracted could be partly explained by the high liquid extraction efficiency of Tyr-Asp-Tyr tripeptides (Table 1), but it does not explain why Tyr-Glu-Tyr tripeptide peaks were not observed. Together, these findings indicate a need for better characterization of sorptive properties of target organic molecules in accordance with the chemical properties of aerogel and the presence of other solid materials such as minerals and salts.
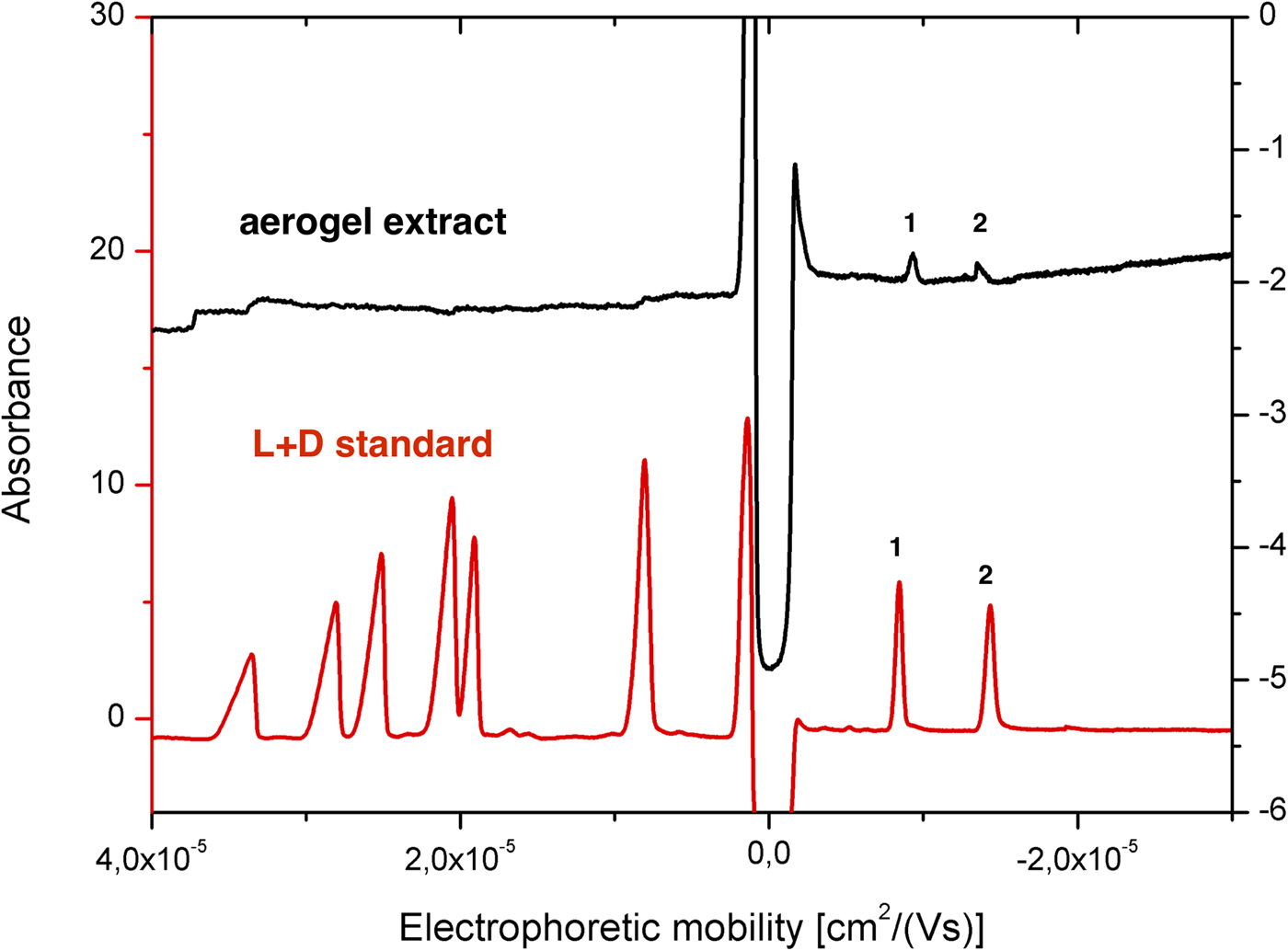
Fig. 8. CZE analysis of extract of aerogel sample obtained in HIE (black) and standard mixture of canonical L + D tripeptides (red). Abbreviations: (1) L-Tyr-L-Asp-L-Tyr and (2) L-Tyr-D-Asp-L-Tyr. Background electrolyte was composed of 50 mM ammonium acetate (pH 3.65). The injection was performed hydrodynamically (8 s, 50 mbar). Other conditions can be found in the ‘Materials and methods’ section.
Conclusion
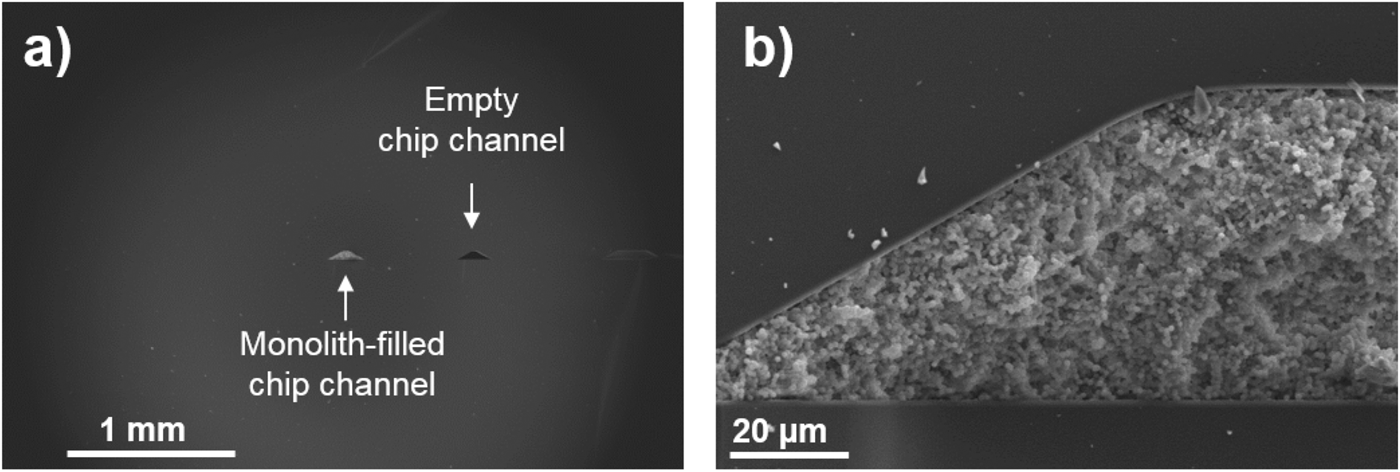
We have provided evidence that CEC and CZE are suitable to separate various simple organic compounds including some of which are found in extraterrestrial environments and are also relevant to terrestrial life. Electromigration techniques can be further applied to separate and detect potential biosignatures including extraterrestrial polymers, as well as simpler organics including amino acids, amines, aliphatic and aromatic hydrocarbons, sugars, phenols, alcohols, nucleobase, carboxylic acids, sulphonic acids and more. While analytical detection limit shown in this work is still 100-fold poorer than what would be expected for the actual trace biomarker detection (100 pg ml−1), these CZE and CEC approaches have the benefits of miniaturization and versatility to be coupled to high-resolution MS or coupled to fluorophore to further lower the detection limit. For example, we show that our generic poly(N-acryloxysuccinimide-co-ethylene glycol dimethacrylate) monolith synthesis protocol can be transferred to a microfluidic chip to further support the application of capability of the monolithic CEC technology for future spaceflight missions (Fig. 9).
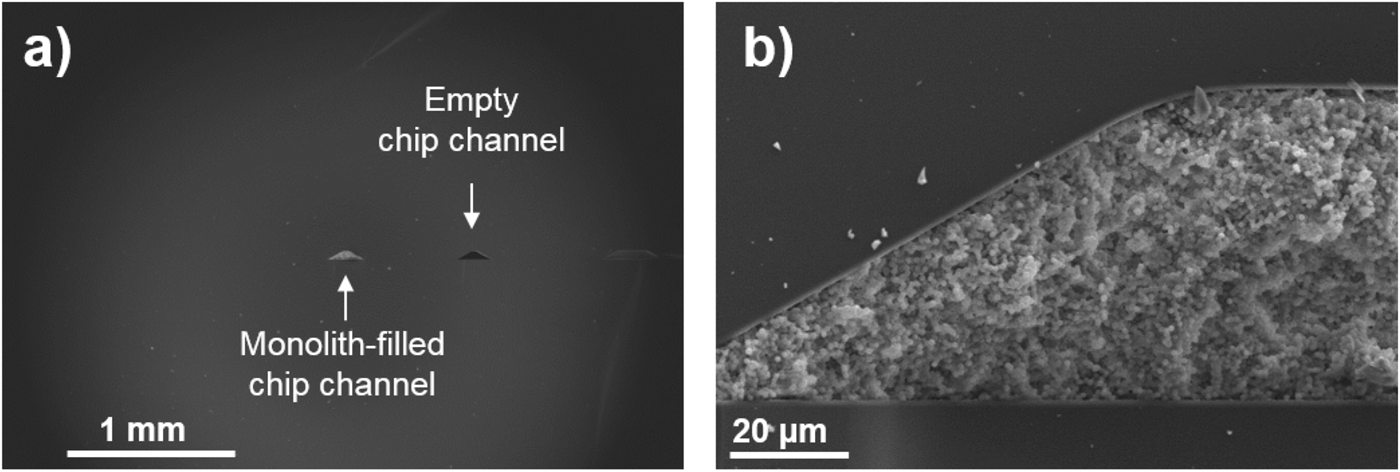
Fig. 9. Scanning electron microscopy images showing cross-section views of (a) monolith-filled and empty chip channels. Panel (b) shows a magnification of a monolith-filled chip channel where a macroporous structure consisting of small globuli fused together characteristic of the monolithic morphology can be easily recognized. An intimate contact between the organic monolith and inorganic chip wall is seen confirming the success of the silanization pretreatment of the chip wall providing strong anchoring of the monolith.
We have also performed HIE and liquid-based extraction using tripeptides as a biosignature simulant to validate the feasibility of sample capture and analysis for the future astrobiology-oriented flyby mission to the icy moons. We deduced recovery rate of the tripeptides in each step of the HIE (Fig. 7) and found that extraction efficiency varies up to 4-fold among peptide samples under the presence of montmorillonite and aerogel. Hence even with a 90% peptide loss after the hypervelocity impact, anionic peptides could be technically detectable due to their efficient liquid extraction, which in part explains the recovery of Y-D-Y diastereomers (Fig. 8). Although, it is important to note that the clay mineral projectile used in this study is not relevant to the actual constituents of the plumes of Enceladus (ice particles and salt crystals) and thus results needs to be treated as a reference to the future impact experiment. Hence, clear separation of diastereomeric tripeptides using CZE is promising for further evaluation as a potential biosignature for life detection in space. For future work, both the physical and chemical adsorption effect of peptides and other organics on the aerogel, needs to be evaluated quantitatively to fully assess the practicality of aerogel for future flight missions. Thus, extending the use of aerogel as a capture media for HIE, then soaking the entire aerogel to perform liquid-based extraction, separation and detection of target organic molecules will support future missions involved in the search for extraterrestrial life, which is essential for answering basic astrobiology question: are we alone in the universe?
Author ORCIDs
Kosuke Fujishima, 0000-0002-8844-812X
Acknowledgements
We are grateful to the NASA Ames Research Center Innovation fund for support. K.F. was supported by the ELSI Origins Network (EON), through a grant from the John Templeton Foundation. The impact experiments led by H.Y. were supported by S. Hasegawa and A. Suzuki of ISAS/JAXA as a collaborative programme with its Hypervelocity Impact Facility. H.Y. is thankful for the project grant of the Astrobiology Center Program of National Institutes of Natural Sciences (NINS). Finally, we thank the reviewer for valuable comments and constructive criticisms for improving this manuscript. We are also thankful for the discussion regarding the interpretation of the HIE result with Wataru Takahagi, Sota Numaho and Kaito Seo from Keio university astrobiology group.