The task of surviving radiation under simulation experiments
Extremophilic organisms can survive and proliferate in non-mesophilic conditions such as extreme temperatures, salinity, pressure, pH, desiccation and radiation (Rothschild & Mancinelli Reference Rothschild and Mancinelli2001). They are thus useful for a wide range of research areas, from basic microbiology to the development of industrial technology. The poly-extremophilic bacteria Deinococcus radiodurans is one of the most radiation-resistant organisms yet discovered, capable of surviving acute doses of ionizing radiation (IR) exceeding 15 kGy (Daly et al. Reference Daly, Ouyang, Fuchs and Minton1994) and growing under chronically delivered gamma radiation (60 Gy h−1) (Daly Reference Daly2000). The molecular mechanisms underlying extreme radiation resistance in D. radiodurans have been the subject of basic research for decades (e.g. Makarova et al. Reference Makarova, Aravind, Wolf, Tatusov, Minton, Koonin and Daly2001; Cox & Battista Reference Cox and Battista2005), but now it is conceived that its resistance arises from the summing of anti-oxidant activities (Blasius et al. Reference Blasius, Sommer and Hübscher2008; Daly et al. Reference Daly, Gaidamakova, Matrosova, Kiang, Fukumoto, Lee, Wehr, Viteri, Berlett and Levine2010).
The resistance of D. radiodurans also applies to ultraviolet radiation, specifically to shortwave ultraviolet, with the dose leaving ∼1/3 survivors (LD37) approaching 600 J m−2 (Battista Reference Battista1997). Nevertheless, its extremely efficient repair of critically lethal double strand breaks (DSB) resealing thousands of DSB (Zahradka et al. Reference Zahradka, Slade, Bailone, Sommer, Averbeck, Petranovic, Lindner and Radman2006) is assumed to be on the basis of its radio-resistance. Other anti-oxidant functions, if overcome by excessive oxidation, render cells much more sensitive to damage (Daly et al. Reference Daly2007). If cellular post-IR recovery relies on the activity of rescue proteins, one mechanism described to prevent IR-induced protein oxidation is the accumulation of Mn+2 complexes in resistant organisms, decreasing intracellular concentration of damaging reactive oxygen species (ROS) formed during irradiation (Daly Reference Daly2009).
Extremophilic micro-organisms have been largely tested to experimentally measure the limits for life to exist in order to assist the search for life in the Universe. The Solar System has a number of interesting astrobiological locations – ranging from planet Mars to the moons Europa, Titan and Enceladus.
The panspermia hypothesizing one origin of life on Earth
The panspermia hypothesis (Arrhenius Reference Arrhenius1903) (‘seeds everywhere’) suggests that life could be spread everywhere. In this hypothesis, it is assumed that spreading life in habitable places would depend on three steps: (i) the escape step, i.e., ejection of contaminated planetary material towards space, normally caused by a large impact on the parent planet; (ii) the journey in space through time scales comparable with those experienced by the Martian meteorites (estimated as 1–15 million years) and (iii) the landing process in a manner to afford non-destructive deposition of the biological material on a recipient planet (Horneck et al. Reference Horneck, Mileikowsky, Melosh, Wilson, Cuccinota, Gladman, Horneck and Baumstark-Khan2003). In the last decade, many experiments have been used to evaluate micro-organism survival in Earth-orbit conditions and also different species of micro-organisms have been utilized for that (e.g. Horneck et al. Reference Horneck, Klaus and Mancinelli2010; Olsson-Francis & Cockell Reference Olsson-Francis and Cockell2010).
The first step of the panspermia hypothesis has already been tested by exposing spores of Bacillus subtilis, cells of Chroococcidiopsis and thalli and ascocarps of the lichen Xanthoria elegans to strong shock pressures ranging from 5 to 40 GPa. Their results support the hypothesis that biological material could be successfully ejected from planets in a way that seeding of early Earth might have occurred (Horneck et al. Reference Horneck2008).
For the second step, one of the many possible mechanisms for panspermia is called lithopanspermia. It was initially suggested as a mechanism where meteorites passing through the Earth's low-orbit in a shallow angle allow them to collect viable micro-organisms present in the upper atmosphere (Nicholson Reference Nicholson2009) and meteorites would serve as vehicles transferring life forms throughout space. Calculations by Mileikowsky et al. (Reference Mileikowsky, Cucinotta, Wilson, Gladman, Horneck, Lindegren, Melosh, Rickman, Valtonen and Zheng2000) have predicted that any rock-captured micro-organisms such as D. radiodurans and Bacillus sp. should be shielded against space radiation inside rocks in the order of meter size at least, to keep a viable minimal population during time ranges suitable to afford Mars–Earth interplanetary travel (about 1 million years). The main constraint concerning lithopanspermia is the secondary radiation effect caused by charged particles which enhance energy absorption by a massive rock fragment. The step of long-term survival in space awaited more concrete results to reinforce the panspermia hypothesis (see below).
Microbial survival in the Martian environment had been a somewhat similar question pursued by many authors. Specific conditions making an impact on the survival of micro-organisms in Martian-simulated environments were already tested such as dehydration, exposure to gases and ultraviolet. Dose et al. (Reference Dose, Bieger-Dose, Dillmann, Gill, Kerz, Klein, Meinert, Nawroth, Risi and Stridde1995) revealed that spores, fungal conidia or vegetative cells (D. radiodurans) exposed to space conditions (vacuum and unshielded Solar radiation) as cells monolayers were shown to be extremely sensitive; on the other hand, biological material assembled in multilayers or macroscopic clusters can resist for months or years, even if exposed to full Solar light. Top cell layers are supposed to become inactivated, while keeping cached cells shielded from ultraviolet damage and also partially from dehydration. Osman et al. (Reference Osman, Peeters, La Duc, Mancinelli, Ehrenfreund and Venkateswaran2008) observed significant survival of spore-forming bacteria after irradiation with full spectrum Martian ultraviolet irradiation if shielded by soil particles (<60 mm) from the Atacama Desert. In addition, Pogoda de la Vega et al. (Reference Pogoda de la Vega, Rettberg and Reitz2007) have found that even soil nanoparticles can afford survival of D. radiodurans upon ultraviolet irradiation under simulated Martian conditions.
The third and decisive step, re-entry on a habitable body, was investigated by Cockell et al. (Reference Cockell2007), who exposed an endolithic photosynthetic organism Chroococcidiopsis sp. in the ESA's STONE experiment. Inoculated into a gneissic rock sample, this cyanobacterial sample did not resist speedy re-entrance into the Earth's atmosphere since extreme heating reached 5 mm deep in the rock.
Brazilian experimental setup to test a novel concept in the panspermia scenario
Current lithopanspermia models admit the possibility of interplanetary transport of endolithic microbes between donor and recipient telluric planets (Nicholson et al. Reference Nicholson, Schuerger and Setlow2005). The planned experimental setup designed by our team envisaged the ‘n = 1’ perspective, i.e. if such a process might ever have occurred, it took place in our own Solar System.
Our group's goal was set on performing simulation experiments concerning the critical step of microbial resistance to long-term exposure to extraterrestrial-simulated conditions. A simulated source of Solar radiation present in interplanetary space was used in the Brazilian Synchrotron Light Laboratory (LNLS) toroidal grating monochromator (TGM) beamline (Cavasso Filho et al. Reference Cavasso Filho, Homen, Fonseca and Naves de Brito2007). The beam was focused on samples inside a vacuum chamber with a spectral region comprising from infrared (IR) to vacuum-ultraviolet (VUV) (photon flux of ∼1014 s−1 between 0.1 and 21.6 eV range). The non-spore forming radio-resistant bacteria D. radiodurans was prepared as a deeply dehydrated cell powder and exposed to increasing doses of VUV radiation mimicking the Solar spectrum. If trapped into a porous carbon tape and shielded by cell multilayers surrounded by organic matrix material, D. radiodurans was shown to survive doses equivalent to tens of days exposure to unfiltered VUV at 1 AU (Paulino-Lima et al. Reference Paulino-Lima, Pilling, Janot-Pacheco, de Brito, Barbosa, Leitão and Lage2010b).
In the frame of an astrobiological perspective, this data can be interpreted as one possible context for incoming microparticulate, extremophile-contaminated material to disperse on top atmospheric layers. As such, alien cells would remain viably suspended in Earth's orbit for sufficient periods of time before landing on the planet surface at any point in Earth's history.
In another experimental setup, cells of D. radiodurans were exposed to different sources of simulated charged particles found in Solar wind. Naked cells or cells mixed with dust grains (basalt or sandstone) were exposed to electrons, protons and carbon ions aiming at determining the probability of cell survival under Solar wind particles bombardment. The results indicate that low-energy particle radiation (2–4 keV), typically present in the slow component of Solar wind had no effect on dehydrated cells, even if exposed to equivalent energies accumulated after some 1000 years at 1 AU. Carbon ions of higher energy (200 keV) found in Solar flares would inactivate 90% of exposed cells. These results show that Solar wind charged particles are relatively less damaging compared with the highly deleterious effects of VUV radiation, and organisms protected by dust grains from ultraviolet radiation would also be protected from charged particles (Paulino-Lima et al. Reference Paulino-Lima2011).
Following the study on radio-resistant micro-organisms in the context of panspermia, the salt-resistant haloarchaea Natrialba magadii and Haloferax volcanii were exposed to the same VUV TGM beamline, dehydrated and under vacuum. N. magadii was remarkably resistant to high vacuum with a survival fraction larger than the one of D. radiodurans, differently from the survival observed for H. volcanii, which was much more sensitive. Radiation resistance profiles were similar for both haloarchaea and D. radiodurans for VUV doses up to 150 J m−2. For higher doses, there was a significant drop in survival of both haloarchaea, and H. volcanii was shown to be completely killed by any further dose increment. Survival for D. radiodurans was 1% after exposure to the higher VUV dose (1.35 kJ m−2), whereas N. magadii survival approached some 0.1%, yet a remarkable score for this organism. Such survival fractions are discussed regarding the possibility of interplanetary transfer to Earth of viable salt-loving micro-organisms from particular extraterrestrial salty environments such as planet Mars and Jupiter's moon Europa. This is the first work reporting survival of haloarchaea under simulated interplanetary conditions (Abrevaya et al. Reference Abrevaya, Paulino-Lima, Galante, Rodrigues, Mauas, Corton and Lage2011).
Carbonaceous composition and surface porosity of the substrate carbon tape on which cells were deposited for VUV irradiation were indeed similar to real samples of micrometeorites found in the Concordia station, Antarctica (Maurette Reference Maurette1998) (Fig. 1). Bacterial survival was similarly high against simulated Solar wind bombardment when spread on silicate microparticles (Paulino-Lima et al. Reference Paulino-Lima2011). By providing better shielding for micro-organisms, carbonaceous or silicate cosmic dust particles should be effective in transferring living matter in interplanetary space. Such micron-sized material should also provide a minimal impact upon atmosphere re-entry, allowing micro-organisms to reach the planetary surface (Coulson Reference Coulson2004). However, our key contribution in respect of microbial survival in the panspermia context was to broaden the idea that microbial-harbouring cosmic dust could spread microbial life anywhere.
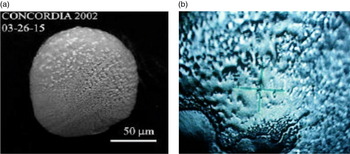
Fig. 1. (a) Morphologic comparison between surfaces of two Concordia 2002 micrometeorites (Antartica), and (b) of the carbon tape on which bacterial powder was layered on for irradiation at the toroidal grating monochromator (TGM) beamline (micrometeorite photos shown with permission of M. Maurette).
Peering Earth extreme environments in search of novel extremophiles
The Antarctic region is usually remembered for its extreme environmental conditions such as low temperature, low-nutrient availability, high-ultraviolet radiation flux, freeze and thawing cycles and long Sun-shaded periods. The Antarctic environment offers unique opportunities for research concerning microbial diversity and it is one of the untouched, least characterized environments available for research on Earth. Its many ecologically interesting environments include permanently frozen soil (permafrost), mineral soils, frozen lakes, snow and glacial ice. Micro-organisms sampled from these peculiar fields can help unveil how broad the limits for life to exist can be, in a particularly interesting extraterrestrial analogue environment.
Antarctic bacteria have been used as model organisms in the study of extremophilic biology. One particular goal that has been followed by our group is screening for ultraviolet resistance among these micro-organisms. Remarkably, Antarctic bacteria were able to survive high-ionizing radiation doses (Dartnell et al. Reference Dartnell, Hunter, Lovell, Coates and Ward2010), and high-ultraviolet doses, although the doses necessary to sterilize Antarctic bacterial samples were effectively cited in only one Ukranian paper abstract (Romanovskaya et al. Reference Romanovskaya, Tashirev, Shilin, Chernaya, Rokitko and Levishko2011). Ultraviolet radiation resistance has thus been used to select micro-organisms to be subjected to future extraterrestrial conditions simulation experiments.
In a new project developed by our team, novel bacterial isolates from Antarctic soils were analysed. Besides ultraviolet screening, these bacteria were also characterized by their ability to produce biotechnologically interesting enzymes with potential industrial use. This approach was set with a view to streamline astrobiology and biotechnology for more robust bioprocesses.
Another astrobiologically interesting environment under bio-prospection is the Atacama Desert in Chile, which is considered similar to Mars due to parameters such as extremely low humidity and intense Solar ultraviolet radiation (Navarro-Gonzalez et al. Reference Navarro-Gonzalez2003; Connon et al. Reference Connon, Lester, Shafaat, Obenhuber and Ponce2007). Its location comprises the hyper-arid desert of Peru, Chile. The Chilean portion extends between 1°N and 37°S, incorporating the arc of Peru, northern Chile and parts of the western Andes cordillera (Hartley et al. Reference Hartley, Chong, Houston and Mather2005). The nearly absent clouds contribute to the occurrence of a yearly intense flux of Solar radiation over the entire Atacama. Altogether, in the Atacama environment many Martian features are cast. In the case of Mars, high levels of poorly filtered ultraviolet radiation reach the surface (Schuerger et al. Reference Schuerger, Mancinelli, Kern, Rothschild and McKay2003), contrary to what occurs on Earth, where wavelengths below 300 nm are atmospherically attenuated. As such, any putative micro-organism present on the Martian surface would be subjected to constant exposure to high fluxes of highly deleterious ultraviolet light (Cockell Reference Cockell2001).
In a parallel set of experiments, procedures were developed for isolation of ultraviolet-resistant micro-organisms of soil from the Atacama Desert. As for the Antarctic project, the goal of this project was to isolate novel ultraviolet-resistant micro-organisms to subject them to future experimental simulations. Among some 30 richly pigmented Atacama isolates, a particular one was shown to be highly ultraviolet resistant, although not to the same extent as D. radiodurans (Paulino-Lima et al., Reference Paulino-Lima, Azua-Bustos, Vicuña, González-Silva, Salas, Teixeira, Rosado, Leitao and Lage2012).
The screening for novel extremophile species has been incorporated into the group as a task to investigate surviving strategies under extraterrestrial conditions. In the near future, a new chamber under construction in the Astrobiology Laboratory in São Paulo-Brazil will allow deeper investigation of such mechanisms, besides raising clues for future advise on interplanetary protection procedures.
Deinococcus radiodurans surprises again
In another branch of applied research, D. radiodurans was shown to be a prospective candidate for bio-remediation of radioactive environmental waste sites, based on its ability to grow and functionally express cloned genes during exposure to chronic gamma irradiation (Brim et al. Reference Brim, McFarlan, Fredrickson, Minton, Zhai, Wackett and Daly2000, Reference Brim, Osborne, Kostandarithes, Fredrickson, Wackett and Daly2006). For example, a clone of D. radiodurans was engineered to completely degrade aromatic hydrocarbons (e.g. toluene) (Lange et al. Reference Lange, Wackett, Minton and Daly1998) and to reduce toxic metals (e.g. Cr6+, Hg2+) in the presence of 137Cs (Brim et al. Reference Brim, Osborne, Kostandarithes, Fredrickson, Wackett and Daly2006).
There are hydrocarbon-rich reservoirs in other bodies of the Solar System. Titan, Saturn's largest natural satellite, is one of them and has potential microbial-habitable properties (Niemann et al. Reference Niemann2005). The presence of methane and other hydrocarbons in its dense atmosphere (Atreya et al. Reference Atreyaa, Adamsa, Niemannb, Demick-Montelarab, Owenc, Fulchignonid, Ferrie and Wilson2006) attenuates surface reaching ultraviolet radiation. Ultraviolet radiation from the Sun decomposes reactive hydrocarbons in the upper atmosphere and the residuals precipitate over the surface, creating lakes and rivers of hydrocarbon by-products (Perron et al. Reference Perron, Lamb, Koven, Fung, Yager and Ádámkovics2006; Mitri et al. Reference Mitri, Showman, Lunine and Lorenz2007; Stofan et al. Reference Stofan2007; Lorenz et al. Reference Lorenz2008).
In this field of research, we are currently investigating if intrinsically biological properties of the poly-extremophilic bacterium D. radiodurans could enable it to survive (and even metabolize) complex organic mixtures, avoiding the need for genetic engineering techniques. D. radiodurans was seen to tolerate complex hydrocarbon mixtures. Microbial growth was observed after incubation with such mixtures (Dalmaso et al. Reference Dalmaso, Paulino-Lima and Lage2011).The results obtained in this study demonstrate the proliferation ability of D. radiodurans in a metabolic carbon-unvailable environment, with a high concentration of organic solvents. Moreover, these results suggest the potential use of a new class of micro-organisms in the biodegradation and bioremediation of contaminated areas.
Apart from the interesting perspective for application of a naturally resistant micro-organism in bioremediation procedures, our initial positive results also suggest that an extremophilic micro-organism such as D. radiodurans could proliferate in hydrocarbon-rich sites such as the surface of Titan, with implications for astrobiological research. Such outcomes support the notion that carbon-based life might dwell on alternative solvent ponds elsewhere.
Concluding remarks
Previous experiments performed by Dose et al. (Reference Dose, Bieger-Dose, Dillmann, Gill, Kerz, Klein, Meinert, Nawroth, Risi and Stridde1995), Osman et al. (Reference Osman, Peeters, La Duc, Mancinelli, Ehrenfreund and Venkateswaran2008) and Pogoda de la Vega et al. (Reference Pogoda de la Vega, Rettberg and Reitz2007) have shed light on biological conditions affording a minimal survival against simulation Mars conditions. Our contribution in the field envisaged a broader concept in terms of microbial survival in extraterrestrial environments. We have shown that D. radiodurans resists simulated Solar radiation whenever shielded by carbon or silicate grains.
The main challenges concerning extraterrestrial environments are the bystander effects due to absorption of high-energy charged (HZE) particles, showering secondary electrons, shock waves and additional thermophysical events (Paulino-Lima et al. Reference Paulino-Lima2011). Large rock fragments can indeed accumulate higher doses during a long-term stand in space. Interestingly, microparticles could hypothetically afford a better shielding for micro-organisms in the transfer of living matter across interplanetary space. Therefore, hypothetical microbes within particles would be mostly inactivated by direct hits. Interplanetary travel on hypothetic micro/dust particles should also be advantageous because of the minimal shock impact upon atmosphere re-entry, allowing nomad micro-organisms to reach the planetary surface. Experimental evidence shows that small particles containing bacteria can survive the temperature regimens imposed during re-entry into Earth's lower atmosphere (Coulson Reference Coulson2004).
The collection of results obtained by our team (see summarized data in Table 1) has joined the concept of cells multilayer shielding rescued inside dust microparticulate material, similar to existing carbon or silicate cosmic dust. Such a micro-environment seems to escape radiation direct hits, while in long-term standing in space, and also does not appear to suffer from re-entry ablation on top of an atmosphere-containing recipient planet. If, after all the gathered evidence, the panspermia hypothesis does not hold valid due to the really harsh extraterrestrial environment, at least our dusty Earth-borne contaminated particles may be widespread in our Galaxy, inseminating other putatively flourishing bodies with extremophilic life.
Table 1. Summary of results observed under extraterrestrial-simulated environments

Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Pesquisas e Desenvolvimento Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Laboratório Nacional de Luz Síncrotron (LNLS-CNPEM) for research Grants and fellowships. This project was also funded by the Ministry of Science, Technology & Innovation and CNPq joint program of National Institutes of Science and Technology (INCT), INEspaço.




