Introduction
Space flights are associated with short-term hypergravity load (STHL) at spaceship launch and landing. The effects of hypergravity are being studied mainly at a whole-organism level. As many other stressors, hypergravity influences physiological homoeostasis and affects neuroendocrine (Cananäu et al. Reference Cananäu, Groza, Albu, Dragomir, Petrescu and Zaharia1975; Groza et al. Reference Groza, Carmaciu, Nicolescu, Cananau, Vrancianu and Bobic1976) and immune (Erofeeva et al. Reference Erofeeva, Krasnov and Sapin2003; Grigorenko et al. Reference Grigorenko, Krasnov and Sapin2003) systems, psychophysiological state (Schneider et al. Reference Schneider, Guardiera, Kleinert, Steinbacher, Abel, Carnahan and Strüder2008), memory performance (Levin et al. Reference Levin, Andersson and Karlsson2007) and behaviour (Santucci et al. Reference Santucci, Corazzi, Francia, Antonelli, Aloe and Alleva2000). One of the most critical effects of the hypergravity in vertebrates is elevation of arterial pressure that in turn considerably influences other physiological parameters. Little is known about the effects of hypergravity at the cellular level. Few works report hypergravity-induced alteration of cell ultrastructure (Belàk et al. Reference Belák, Kocisová and Bod'a1977; Nikogosova et al. Reference Nikogosova, Kaĭfadzhian, Barinian and Akopian1991; Bózner et al. Reference Bózner, Boda, Dostál, Matĕjková and Devecka1993) or protein metabolism (Mednieks et al. Reference Mednieks, Hand and Grindeland1998).
In the present study, DNA- and RNA-synthesizing activities of the atrial cells and behaviour of the atrial granular cells (GCs) in the snail Achatina achatina were examined at different time points after STHL. Furthermore, the strong reaction of neuroendocrine and immune systems was revealed, which suggested a stressful effect of STHL on the snail.
Snails have an open vascular system in which numerous haemocytes freely circulate in haemolymph. Thus, this model allows examining the direct action of hypergravity covered in vertebrates by consequences of blood flow disturbances. The heart complex in A. achatina has a ventricle and an atrium enclosed in a pericardium. The wall of the atrium consists of a single-layered epicardium facing the pericardial cavity, myocardium and an uncontinuous layer of endothelial cells, which lines the heart lumen. The muscle fibres are arranged as trabeculae running across the lumen. Abundant large GCs are located on the surface of muscles under endothelial cells and contact tightly with nerve endings. In previous studies, we have shown that the granules of these cells contain wide variety of bioactive substances (Martynova et al. Reference Martynova, Krylova and Bystrova2004, Reference Martynova, Bystrova, Shabelnikov, Margulis and Prokofjeva2007; Shabelnikov et al. Reference Shabelnikov, Bystrova and Martynova2008; Bystrova et al. Reference Bystrova, Shabelnikov and Martynova2013), are very sensitive and answer with degranulation to different kind of stress-induced factors such as electrical stimulation of the heart nerve (Shabelnikov et al. Reference Shabelnikov, Bystrova, Ivanov, Margulis and Martynova2009) or the injection of some neuromediators (our unpublished data). The high stress-reactivity of the atrial GCs and their functional analogy to vertebrate mast cells has determined our choice of snail atrium as an object of study. Moreover, molluscs are among the animal models employed in the spaceflight examinations (Gorgiladze et al. Reference Gorgiladze, Korotkova, Kuznetsova, Mukhamedieva, Begrov and Pepeliaev2010; Balaban et al. Reference Balaban2011). The hypergravity experience at spaceship launch may significantly affect the results of tests conducted in a space station.
Materials and methods
Animals and experimental design
The population of giant African pulmonate snail, A. achatina (Gastropoda; Pulmonata), was maintained for several years in the laboratory at constant temperature (25 ± 1 °C) under an artificial lighting regiment (light: 12 h; dark: 12 h) on a herbivorous diet. In experiments, adult animals (15 ± 2 mm shell length, and a body weight in the range of 15–20 g and age 4–5 months) were used.
Centrifugation-stress treatment was performed on the centrifuge 5810 R (Eppendorf). The snails were subjected for 15 min to 14 g acceleration gravity force (300 rpm). In preliminary tests, it was found that these conditions give the most pronounced reaction of the atrial GCs with an absolute survival of snails.
High-performance liquid chromatography (HPLC) analysis
Haemolymph samples were taken with syringe from the pulmonary vein. The catecholamines including dopamine, noradrenaline and adrenaline were measured before and 20 min, 24 h and 72 h after centrifugation using HPLC with amperometric detection. Four snails were taken per each time point. 3–5 ml collected haemolymph with 1 ml anticoagulant (60 mM EDTA; 61 mM NaCl; 3.3 mM KCl – all from Sigma; pH 7.4) was centrifuged at 950 g for 10 min in order to obtain cell-free plasma, which was then stored at −20 °C until assayed for catecholamines. HPLC analysis was performed as described elsewhere (Kartsova et al. Reference Kartsova, Sidorova, Kazakov, Bessonova and Yashin2004) within a week of receipt of the samples. Before analysis, the samples were subjected to purification on activated aluminium oxide. The catecholamines were separated on an Ascentic C18 column (4.6 × 250 mm) with a mobile phase consisted of 30 mM KH2PO4, 1.44 mM sodium octylsulphonate, 40 mM monochloroacetic acid and acetonitrile (pH 3) at a flow rate of 1 ml min−1. We used a Waters 590 liquid chromatograph with an amperometric detector (NPO Khimatomatika, Moscow, Russia) with a glassy-carbon working electrode (+0.85 V). The tests were repeated for three times.
Haemocyte counting
To determine the total haemocyte quantification, haemolymph samples were taken with syringe from the pulmonary vein of control snails and the snails 20 min, 2 h and 24 h after centrifugation. Eight animals were taken for each time point. Haemolymph solution was mixed with the anticoagulant (60 mM EDTA; 61 mM NaCl; 3.3 mM KCl; pH 7.4) at a ratio of 1 : 3. Haemocytes were counted using a Fuchs – Rosenthal counting chamber and a phase-contrast light microscope (×40). Results were expressed as mean±SE of cells number per mm3 of haemolymph sample.
Haemocyte proliferation
The effect of stress on haemocyte proliferation was assessed by tritiated thymidine uptake assays and labelled cell counts. Tritiated thymidine ([3H]thymidine; State Institute for Applied Chemistry, St. Petersburg, Russia) at a dose of 5 μCi g−1 of body weight was injected into the snail body cavity before (n = 3) and 1 h after (n = 3) centrifugation. The samples of haemolymph were taken 1 h after isotope injection. The haemocyte smears were made, fixed in 4% paraformaldehyde, and processed for autoradiography. The preparations were exposed for 3 weeks, developed and stained with haematoxylin – eosin. The number of labelled and unllabeled cells was determined in radioautographs; at least 1000 haemocytes were analysed for each snail.
Electron microscopy (EM)
The atria were rapidly removed and fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, containing 2% sucrose, for 1.5 h at 4 °C. The tissue was then post-fixed in 1% osmium tetroxide in the cacodylate buffer for 1 h, dehydrated and embedded in a mixture of Araldite and Epon. Ultrathin sections were cut with a diamond knife using an LKB-Ultratome, stained with uranyl acetate and lead citrate and examined in a Zeiss Lbra 120 electron microscope operated at 80 kV.
Autoradiography
Autoradiographic analysis of DNA synthesis in the snail atrial cells was conducted at 2 h, 1 day, 2 days, 5 days and 10 days, and RNA synthesis at 2 h, 5 days and 10 days after STHL. Tritiated thymidine ([3H]thymidine) or tritiated uridine ([3H]uridine; State Institute for Applied Chemistry, St. Petersburg, Russia) with a specific activity of 16 Ci μmole−1 was injected at a dose of 5 μCi g−1 of body weight into the body cavity of snail 1 h prior to tissue fixation. Untreated control animals were also injected with isotopes. Three snails per group were used. The heart tissue was fixed and processed for light microscopic (LM) and EM autoradiography. For LM autoradiography, semi-thin sections (1–2 μm in thickness) were placed on microscopic slides, coved with light-sensitive emulsion and exposed for 1 month at 4 °C. After development, the autoradiograms were stained with toluidine blue, and photographed using a light microscope LSMS PASCAL (Zeiss) with the digital camera Leica DFC 420C. The percentage of [3H]uridine- or [3H]thymidine-labelled epicardial, endothelial or GCs from the total number of these cells were calculated. Cells were considered [3H]thymidine- or [3H]uridine-positive when they contained a minimum of five discrete silver grains. Over 500 cells from each animal were examined. EM autographs were prepared by the method of Larra & Droz (Reference Larra and Droz1970) with an exposure time of about 5 months. After development, the sections were doubly stained with uranyl acetate and lead citrate.
Statistical analysis
All data were expressed as mean±SE. The values obtained were compared by a Student's t-test.
Results
Stress-response of the nerve-endocrine system
Catecholamine levels in snail haemolymph before and after different time periods after STHL were measured by HPLC (Fig. 1). Basal values averaged 1201.4 pg ml−1 for dopamine, 289.9 pg ml−1 for noradrenaline and 855.8 pg ml−1 for adrenaline. Dopamine was the most abundant catecholamine found in the snail haemolymph. Immediately after STHL, the content of noradrenaline in the snail haemolymph was elevated, while the level of adrenaline was lowered. Although there was a trend towards an increase in dopamine concentration, it was not statistically significant at the 0.05 level. Within a day after STHL, circulating levels of all three catecholamines returned to control values.

Fig. 1. Levels of catecholamines in snail haemolymph before and 20 min, 24 and 72 h after STHL measured by HPLC. Each point represents the mean from three separate experiments. Vertical bars are 95% confidence limits.
Stress-response of the immune system
Haemolymph of the snail A. achatina contain only one morphological type of circulating haemocytes – agranular blast-like cells. Data presented in Fig. 2 establish that immediately after STHL, the number of haemocytes increased almost 10-fold in comparison with that in untreated snails (P < 0.05), and maintained at high level at least for 2 h. Between 2 and 24 h after STHL, the haemocyte count decreased until the physiological level.

Fig. 2. Circulating haemocyte counts in control and rotated snails 20 min, 2 and 24 h after STHL. Vertical bars are 95% confidence limits.
Proliferative activity of haemocytes was evaluated by the autoradiography. Under physiological condition, only a small proportion of haemocytes incorporated DNA precursor comprising 0.47 ± 0.03% of the total number of haemocytes. One hour after STHL, the proportion of haemocytes labelled by 3HT showed a statistically significant increase to 4.10 ± 1.27%.
Stress-response of the atrial endothelial and pericardial cells
The effect of STHL on DNA and RNA synthesis in snail atrial cells was assayed based on the biosynthetic incorporation of [3H]thymidine and [3H]uridine into newly replicating DNA or transcribed RNA, respectively.
DNA synthesis
According to LM autoradiography, before and immediately after STHL, no atrial cells exhibited labelling with [3H]thymidine. [3H]thymidine-positive cells were observed among the atrial endothelial cells 1, 2, 5 and 10 days after STHL (Table 1). The highest index of DNA-synthesizing endothelial cells was observed at post-stress day 5. Nuclei of the pericardial or muscle cells did not incorporate the isotope at any post-stress stage.
Table 1. Percentages of [3H]thymidine-labelled endothelial cells in the snail atrium at different time after STHL
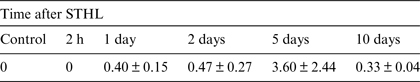
Notes: In all cases [3H]thymidine was injected 2 h prior to fixation. Each value is displayed as mean ± standard error (n = 3).
RNA synthesis
LM autoradiography analysis has showed that 2 h after STHL, the number of [3H]uridine-labelled both epicardial and endothelial cells decreased as compared with the control level, then increased significantly at post-stress day 5, and dropped to the basal level by post-stress day 10 (Table 2). A short pulse of [3H]uridine (1 h) resulted in localization of silver grains mostly over the nuclei, the cytoplasm being without labelling. In atrium of both control and rotated snails only rare poorly [3H]uridine-labelled myonuclei were seen.
Table 2. Percentages of [3H]uridine-labelled cells in the snail atrium at different time after STHL

Notes: In all cases [3H]uridine was injected 2 h prior to fixation. Each value is displayed as mean ± standard error (n = 3).
Stress-response of the atrial GCs
The state of atrial GC population has been traced over a month after STHL. The numerous GCs filled by membrane-bound granules were available in the atrium of control snails (Figs. 3 and 4). Immediately after STHL, many GCs showed progressive morphological phases of degranulation which was accompanied by the signs of surface activation – active shedding of the plasma membrane. Some GCs underwent rapid cytoplasm fragmentation (Fig. 5). After detachment of cytoplasm fragments, the GCs maintained the plasma membrane integrity, and endured as small cells with high nuclear – cytoplasmic ratio (Fig. 6). These small cells devoid of granules could hardly be detected by LM but were readily identifiable in the ultrathin sections on the basis of several typical characteristics: they localized beneath the endothelial cells, formed tight contacts with the nerve endings, showed surface shedding, and often possessed nuclear roundish osmiophilic structures with sharply defined contours (120–150 nm in diameter). All these features together or separately enabled us to distinguish between devoid of granules GCs and endothelial cells. At longer time intervals (5–10 days), the number of the degranulated GCs was markedly increased. Then, 1 month after STHL, which was the last term we investigated in the experiments, the GC population became morphologically identical to those in control snails, both in terms of the number and the appearance of individual GCs.

Fig. 3. Low magnification light micrograph of a semi-thin section from atrium of a normal snail. Note the GCs (arrows) located on the surface of the muscle fibres (m) under endothelial cells (arrowheads). L, heart lumen. Stained with toluidine blue. Bar: 20 μm.

Fig. 4. Electron micrograph of a GC in the atrium of unrotated snail. Cell is tightly filled with granules (g). Arrow shows shedding of plasma membrane. e, endothelial cell; n, nucleus of GC; L, heart lumen. Bar: 2 μm.

Fig. 5. Electron micrograph demonstrating cytoplasm fragmentation of a GC (2 h after STHL). Note active shedding of plasma membrane. The cell cytoplasm is almost completely fragmented but its nucleus (n) remains intact and the thin rim of cytoplasm surrounding the nucleus is maintained. Arrowheads point to the process of endothelial cell. L, heart lumen; m, muscle fibre. Bar: 2 μm.

Fig. 6. Electron micrograph showing a completely degranulated GC. Noteworthy here is a roundish osmiophylic structure (arrow) in nucleoplasm. m, muscle cells; n, nucleus of GC. Bar: 2 μm.
DNA synthesis
S een on the LM autographs, the occasional small [3H]thymidine-labelled cells located upon muscle fibres and under endothelial cells might be assumed to be degranulated GCs (Fig. 7). However, the small diameter and absence of granules did not permit to ascertain unequivocally whether the labelled nucleus belonged to the degranulated GC or to the endothelial cell. As the number of [3H]thymidine-labelled cells in atrium increased progressively after stress attaining a maximum at post-stress day 5, we have examined atrial tissue at this time point by EM autoradiography. On EM autographs, some [3H]thymidine-labelled cells were identified as completely degranulated GCs upon the criteria listed above (Fig. 8). Mature GCs did not incorporate [3H]thymidine.

Fig. 7. LM autoradiograph of a semi-thin section from atrium of a snail at the 5th day after STHL. Arrow points to the small devoid of granules [3H]thymidine-labelled cell, that most probably is degranulated GC. The assumption is based on its localization adjusting to muscle cells (m), and beneath endothelial cell (arrowhead). Isotope was injected 2 h prior to fixation. Stained with toluidine blue. Bar: 5 μm.

Fig. 8. Electron microscopic autoradiograph of [3H]thymidine-labelled cell devoid of granules which can be identified as degranulated GCs due to localization under endothelial cell (e) and the occurrence of osmiophylic structure in nucleoplasm (framed region). Five days after STHL; [3H]thymidine was injected 2 h prior to fixation. Insert: framed region of Fig. 8 at higher magnification. Bar: 2 μm.
RNA synthesis
LM autoradiography for RNA synthesis has revealed that in control snails 56.4 ± 5.6% (SD = 13.3) GCs showed transcription activity. Two hours after STHL, only single-labelled GCs were detected. EM autoradiography has revealed RNA synthesizing activity in both mature and totally degranulated GCs. The cells with [3H]uridine-labelled and -unlabelled nuclei did not show any notable differences in ultrastructure. At days 5 and 10, only a few mature GCs were seen; no [3H]uridine-labelled GCs among them were detected.
Discussion
Our investigation of STHL action on snail has demonstrated rapid and relatively short-term reactions at the organism level and long-lasting processes at the cellular level. The following sequence of events takes place after STHL. Immediately after stress-action there were alterations in the endocrine system involving increase of the noradrenaline and dopamine levels and decrease of the adrenaline level, as well as in the immune system including an increase of both the number and mitotic activity of haemocytes. STHL also caused quick decrease in the number of RNA-synthesizing atrial endothelial and epicardial cells and degranulation of the atrial GCs. One day after STHL all three catecholamines and a number of haemocytes in haemolymph have returned to their control levels. At this time, a few DNA-synthesizing endothelial cells appeared in the atrium. Fifth day was marked by significant increase in indices of DNA-synthesizing endothelial cells and RNA-synthesizing both endothelial and epicardial cells. Almost all GCs have been degranulated. Then, by the day 10 the index of DNA-synthesizing endothelial cells decreased to that at the day 2, and the indices of RNA-synthesizing endothelial and epicardial cells decreased to control values. However, the GC population at this time has not yet returned to its normal state; most GCs remained to be degranulated. One month after STHL the number and appearance of the GCs in the snail atrium became visually indistinguishable from those in pre-stress animals. Although the atrial epicardial, endothelial and GCs clearly reacted on stress, the atrial muscle cells did not. One may speculate that working cells of the contractile organ are less sensitive to and more protected against stressful actions.
Whether the changes in replicative and transcriptional activity of the atrial cells are under the direct influence of the mechanical forces generated by gravitation overload or they are regulated indirectly via neuroendocrine and/or immune system remains unclear. Although the regulatory role of catecholamine hormones is generally recognized, the immediate action of hypergravity cannot be excluded. Physical stimuli were shown to exert influence on transcription and cell proliferation through a direct modulation of transcription factor activity (Mendez & Janmey Reference Mendez and Janmey2012).
Interrelation of neuroendocrine and immune systems during the reaction to STHL should also be examined. In molluscs, as in other invertebrates and vertebrates, close bi-directional interactions occur between the neuroendocrine and immune systems (Ottaviani Reference Ottaviani2006). Our data have revealed a rapid increase in the number of haemocytes in haemolymph, which can be explained by their redistribution from tissues into circulation and, at least in part, by their enhanced proliferative activity. Activation of haemocytes can be directly related to the concurrent catecholamine increase. Involvement of these cells in the secretion of catecholamines by stressing conditions has been shown in the sea mussel (Cao et al. Reference Cao, Ramos-Martínez and Barcia2007). On the other hand, the behaviour of immune cells in molluscs is modulated by neurohormones (Zhou et al. Reference Zhou, Wang, Yang, Zhang, Kong, Wang, Qiu and Song2011a).
In invertebrates, the mechanisms of stress-reaction have many common features with those seen in vertebrates. Thus, involvement of neuroendocrine and immune systems in stress-response in molluscs is well established. Changes in circulating catecholamines were demonstrated in molluscs exposed to mechanical disturbance, elevated temperature, altered salinity and air exposure (Lacoste et al. Reference Lacoste, Malham, Cueff and Poulet2001; Chen et al. Reference Chen, Yang, Xu, Wang and Liu2008) or by bacteria challenge (Zhou et al. Reference Zhou, Wang, Shi, Zhang, Gao, Wang, Kong, Qiu and Song2011b). The data obtained in our and the cited works are contradictory. In response to stressors, the adrenaline and noradrenaline concentrations increased and dopamine concentration decreased in the haemolymph of the scallop Chlamys farreri (Chen et al. Reference Chen, Yang, Xu, Wang and Liu2008), whereas both circulating noradrenaline and dopamine increased in the oyster Crassostrea gigas (Lacoste et al. Reference Lacoste, Malham, Cueff and Poulet2001). As we have revealed STHL induced the increase in noradrenaline and dopamine concentrations and decrease in the adrenaline level in the haemolymph of the snail Achatia achatina. Whether species differences, strength of stress-action, experimental design or something else are responsible for these variations is unclear.
Altered numbers of circulating haemocytes, which is considered to mediate immunological activities at invertebrates, were also described in molluscs subjected to stress. Some studies have shown an increase in the circulating haemocytes (Malagoli et al. Reference Malagoli, Casarini, Sacchi and Ottaviani2007; Roberts et al. Reference Roberts, Sunila and Wikfors2012), whereas the others – a decrease (Malham et al. Reference Malham, Lacoste, Gélébart, Cueff and Poulet2003; Aladaileh et al. Reference Aladaileh, Nair and Raftos2008), which are considered as immunostimulation or immunosuppression, respectively. STHL, as we have observed, acted as immunostimulator.
Particular attention in this work has been given to the atrial GCs. As we have shown earlier, granules of the atrial GCs contain natriuretic peptide ANP (Martynova et al. Reference Martynova, Krylova and Bystrova2004), Hsp70 (Martynova et al. Reference Martynova, Bystrova, Shabelnikov, Margulis and Prokofjeva2007, substance P and FMRFamide (Shabelnikov et al. Reference Shabelnikov, Bystrova and Martynova2008), histamine and serotonin (Bystrova et al. Reference Bystrova, Shabelnikov and Martynova2013). All these peptides possess a wide spectrum of functions and are implicated in a broad range of physiological processes. Their rapid release into haemolymph by GC degranulation undoubtedly contributes to the early general stress-response.
A striking similarity between the snail atrial GCs and vertebrate mast cells in morphological features, the proteins their granules contain, the tight contacts they form with nerve endings, was already discussed in our other articles (Shabelnikov et al. Reference Shabelnikov, Bystrova and Martynova2009; Bystrova et al. Reference Bystrova, Shabelnikov and Martynova2013). Here we would like to mention the capacity of mast cells for recycling after complete degranulation (Burwen Reference Burwen1982; Nielsen & Clausen Reference Nielsen and Clausen1982). As we have shown, the snail atrial GCs are also long-living cells and their population is able to self-renewal. Likeness once again appears in the influence of gravitational stress (both hypergravity and weightlessness) on mast cells revealed in rodents (Stauber et al. Reference Stauber, Fritz, Burkovskaya and Ilyina-Kakueva1993; Antonelli et al. Reference Antonelli, Santucci, Amendola, Triaca, Corazzi, Francia, Fiore, Alleva and Aloe2002). All these similarities allow exploiting the snail atrial GCs as appropriate model for mast cell testing. Sensitivity of mast cells to any stressors and their active role in stress-related physiology are well known. We may postulate that mast cell population in astronauts also undergoes strong disturbances following launch and landing STHL. Prolonged altered state of mast cell population might seriously affect its ability to respond effectively to new challenges.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research [grant number 12-04-00146] and the granting programme Molecular and Cell Biology of Russian Academy of Sciences.












