Introduction
Geological records indicate that life has been present on Earth for at least 3.5 Gyr, and life probably began as early as 3.8 billion years ago (Ga) (Nisbet Reference Nisbet1987; Nisbet & Fowler Reference Nisbet and Fowler1996). Right after the Moon-forming impact that occurred at about 4.5 Ga (Valley et al. Reference Valley2014), the surface of Earth turned into a magma ocean that was unsuitable for life to start. However, there were critical steps for the early Earth to evolve from a hell of fire to a habitable planet in the Hadean eon. Unfortunately, because of the extensive geological reworking, there is almost no petrologic record for this piece of history (e.g. Valley et al. Reference Valley2014). Current knowledge on Earth's Hadean environment suggests that the proto-atmosphere formed a few million years after the Moon-forming impact, was composed of a few hundred bars of water steam, 100–200 bars of CO2 and small amount of CO, H2, etc. (Condie Reference Condie1980; Walker Reference Walker1985; Liu Reference Liu2004; Zahnle Reference Zahnle2006; Zahnle et al. Reference Zahnle, Arndt, Cockell, Halliday, Nisbet, Selsis and Sleep2007; Sleep Reference Sleep2010). The continuous cooling of the Earth's surface had led to the condensation of the atmospheric water steam and subsequently the formation of the earliest ocean (Martin et al. Reference Martin, Albarede, Claeys, Gargaud, Marty, Morbidelli and Pinti2006) before 4.4 Ga (Wilde et al. Reference Wilde, Valley, Peck and Graham2001; Valley et al. Reference Valley2014). The early interactions between lithosphere, atmosphere and ocean of the Earth not only changed the physicochemical condition of the Earth's surface, but also initiated the prebiotic chemistry towards life. To understand these early planetary processes, most studies have focused on the Earth with a hydrosphere because life was emerged from aqueous environment (Russell et al. Reference Russell, Hall and Turner1989, Reference Russell, Daniel, Hall and Sherringham1994; Fruth-Green et al. Reference Fruth-Green, Connolly, Plas, Kelley and Grobety2004; Holm et al. Reference Holm, Dumont, Ivarsson and Konn2006; Schulte et al. Reference Schulte, Blake, Hoehler and Mccollom2006; Martin & Russell Reference Martin and Russell2007). Serpentinization in the hydrothermal environment is then suggested to be one important process due to its highly chemically active mineral assemblages and catalysed synthesis of simple organic molecules (Russell et al. Reference Russell, Hall and Martin2010). However, before the formation of the earliest ocean, the supercritical H2O–CO2 proto-atmosphere might have reacted with the newly solidified crust and these direct interactions should be different from the water–rock interactions after the formation of the ocean. This direct atmosphere–rock interaction could have significant impact on the chemistries of the early atmosphere and the crust.
Sepentinization can produce Fe- and Ni-bearing sulphides, oxides and native metal alloys that could play critical roles in catalysing the reduction of primordial CO2 to organic molecules, and further the formation of biopolymers (Yoshida et al. Reference Yoshida, Nishizawa, Tabata, Abe, Kodama, Tsuji and Tamaura1993; Horita & Berndt Reference Horita and Berndt1999; Chen & Bahnemann Reference Chen and Bahnemann2000; Fu et al. Reference Fu, Lollar, Horita, Lacrampe-Couloume and Seyfried2007; Pizzarello Reference Pizzarello2012; McCollom & Seewald Reference McCollom and Seewald2013). As an important secondary mineral, magnetite in such processes has been experimentally investigated by many previous studies (Berndt et al. Reference Berndt, Allen and Seyfried1996; Seyfried et al. Reference Seyfried, Foustoukos and Fu2007; Marcaillou et al. Reference Marcaillou, Munoz, Vidal, Parra and Harfouche2011). These studies mainly paid attention to the detection of magnetite to constrain its formation conditions during serpentinization, but overlooked its crystal habits, which is critical in catalysing the organosynthesis. Because of the low productivity, Seyfried et al. (Reference Seyfried, Foustoukos and Fu2007) only detected magnetite in the run product using vibrating sample magnetometer. Marcaillou et al. (Reference Marcaillou, Munoz, Vidal, Parra and Harfouche2011) identified magnetite using X-ray diffraction (XRD) in the product of hydrothermal alteration of peridotite. Magnetite crystals were only directly observed by Normand et al. (Reference Normand, Williams-Jones, Martin and Vali2002) and McCollom & Seewald (Reference McCollom and Seewald2013) that showed octahedral shapes with the size of a few to tens micrometres. These limited observations are insufficient for the understanding of its catalytic property and geochemical fates.
In the present study, we conducted batch experiments to simulate the interaction between the crust and proto-atmosphere at the earliest evolution stage of the Earth. Followed by our experiments, we examined the formation of secondary minerals especially magnetite to gain some new insights into the direct interactions between atmosphere and lithosphere and their influence on the mineral evolution and the prebiotic chemical evolution on the early Earth.
Materials and methods
Distilled deionized (DD) water was used as the starting liquid phase to avoid the participation of alkaline in reactions occurred in the batch experiments. Since CO2 cannot be directly added into the Au capsule, Ag2C2O4 was used as the source of CO2 in the batch experiments. Fresh Ag2C2O4 was prepared by the aqueous reaction between H2C2O4 and excess AgNO3 (equation 1). The precipitated Ag2C2O4 was centrifuged and washed by DD water for at least three times to completely remove AgNO3. Once the autoclave is heated, Ag2C2O4 breaks down to pure Ag and CO2 (equation 2).
As the reduced chemical species (for example, CO, CH4 and H2) in the proto-atmosphere only constituted very small fractions of Earth's earliest atmosphere (Condie Reference Condie1980; Walker Reference Walker1985; Liu Reference Liu2004; Zahnle Reference Zahnle2006; Zahnle et al. Reference Zahnle, Arndt, Cockell, Halliday, Nisbet, Selsis and Sleep2007; Sleep Reference Sleep2010), they would not play substantial roles in the interaction between the proto-atmosphere and the ultramafic crust. Therefore, the H2O–CO2 system was used to represent the proto-atmosphere. Fresh komatiite from Zimbabwe with typical spinifex texture and characterized by high Mg content, high Fe2+/ΣFe and relatively high content of Ni and Cr (Nisbet et al. Reference Nisbet1987; Berry et al. Reference Berry, Danyushevsky, O'Neill, Newville and Sutton2008) was used to represent the earliest crustal ultramafic rocks interacting with the proto-atmosphere. The value of Fe2+/ΣFe in komatiite was measured by the 57Fe Mössbauer spectroscopy with a 25mCi 57Co/Pb source at the University of Hong Kong. The finely ground komatiite was prepared as absorbent with relative thicknesses of ~5 mg Fe cm−2. Spectra were collected at room temperature in transmission mode with a constant acceleration mode. The velocity scale was calibrated relative to 25 μm α-Fe at room temperature. Lorentzian doublets were used for fitting the areas of sub-spectra.
The experiments were carried out at Hydrothermal Laboratory at Guangzhou Institute of Geochemistry, Chinese Academy of Sciences. All materials were weighted and loaded into a flexible Au capsule. Then the Au capsule was put in Ar atmosphere and sealed by a welding machine to protect the materials from being oxidized. The water–rock ratio was adjusted to approximately 2 : 1 and Ag2C2O4 was restricted to a suitable amount to avoid the explosion of the sealed Au capsule. The sealed Au capsule was then put into a steel alloy autoclave. The pressure inside the autoclave was monitored by a barometer and temperature was controlled by a digital temperature controller. Batch Experiments were carried out by heating the autoclave to a constant temperature under 500 bar pressure for at least 2 weeks. The temperature varied from 200 to 450°C with an increment of 50°C. At the end of experiment, the autoclave was quenched to room temperature in a few seconds with ice water. Blank experiment loaded with pure Ag2C2O4 and water was also conducted to examine the compositions of the gas phase from the decomposing of Ag2C2O4. The experimental conditions were summarized in Table 1.
Table 1. Summary of the initial experimental conditions for the CO2–H2O–komatiite batch experiments

The morphology of solid products after experiments was observed by a Hitachi S-4800 field-emission scanning electron microscope (SEM) at the University of Hong Kong. The accelerating voltage was set to 3–5 kV so as to take SEM images with fine surface structures. Energy-dispersive X-ray spectroscopic (EDS) measurements were also carried out at 20 kV to measure the chemical compositions. However, the EDS analyses could only provide limited information because the secondary minerals were always adhered to the host rock that contributed strong background signals. A FEI Tecnai G2 20 S-TWIN scanning transmission electron microscope (STEM) was used to characterize the crystallographic structures of secondary minerals. Specimens were loaded on copper grids with carbon-coated formvar and examined in the normal STEM mode to obtain bright images using an accelerating voltage of 200 kV. Detailed structure information of minerals was obtained by selected area electron diffraction (SAED) patterns and high-resolution transmission electron microscopy (HRTEM) micrographs.
The volatile components in the Au capsule were analysed by an Agilent 6890 N gas chromatograph (GC) connected with a gas-collection system. Firstly, the sealed Au capsule was cleaned and put into the gas-collection system. After the whole system was evacuated, the Au capsule was pierced and the sealed volatile components would fulfil the gas-collection system. The valve between the gas-collection system and 6890 N GC was then opened to allow gases to get into the GC. The organic and inorganic gas components were analysed in an automatically controlled procedure.
Results and discussion
Mössbauer spectroscopy and Fe2+/ΣFe of komatiite
Since komatiite used in this study is consisted of multiple types of minerals/materials, the site distribution of Fe2+ and Fe3+ can hardly be assigned accurately. Therefore, the spectrum was fitted with two Fe2+ doublets and one Fe3+ doublet (Fig. 1). Doublet with quadrupole splitting (QS)=0.49 mm s−1 and isomer shift (IS)=0.24 mm s−1 was assigned to paramagnetic Fe3+ in silicates; while the doublet with QS=2.73 mm s−1, and IS=1.09 mm s−1 and doublet with QS=2.12 mm s−1 and IS=1.09 mm s−1 were both assigned to paramagnetic Fe2+ in silicates. The Fe2+/ΣFe ratio of komatiite was 0.80 based on the fitting of the spectra.

Fig. 1. Mössbauer spectrum of komatiite powder.
Nucleation of magnetite
The SEM observation showed abundant clay minerals and carbonates stack on the initial komatiite in the run-products (Fig. 2). Clay minerals can be found in all experiments with different temperatures, whereas carbonates can only be found in experiments with temperature below 400°C. The EDS analyses showed that the formed phyllosilicates containing less amount of Fe than those in olivine and glass matrix of the original komatiite. This result indicated that a portion of the Fe released from the glass matrix and the fayalite component of olivine was sequestered by other secondary minerals. As described by many previous studies, serpentinization of ultramafic rocks is always coupled with the production of H2 (Barnes et al. Reference Barnes, Sheppard, Gude, Rapp and Oneil1972; Moody Reference Moody1976; Frost Reference Frost1985; Berndt et al. Reference Berndt, Allen and Seyfried1996; Marcaillou et al. Reference Marcaillou, Munoz, Vidal, Parra and Harfouche2011) owing to the oxidation of Fe(II) on the lattice of minerals to Fe(III) by water. H2 was detected in all experiments with variable concentrations (from tens to hundreds ppm). The oxidation of Fe(II) activated from silicates would probably lead to the nucleation of magnetite and it has been reported in several previous serpentization experiments (Berndt et al. Reference Berndt, Allen and Seyfried1996; Seyfried et al. Reference Seyfried, Foustoukos and Fu2007; Marcaillou et al. Reference Marcaillou, Munoz, Vidal, Parra and Harfouche2011). However, magnetite was not observed in our experiments carried out between 200 and 400°C. The only experiment in which magnetite was observed was carried out at 450°C. This temperature is higher than that of previous serpentinization experiments (mostly between 200 and 300°C) in which magnetite was reported (Berndt et al. Reference Berndt, Allen and Seyfried1996; Seyfried et al. Reference Seyfried, Foustoukos and Fu2007; Marcaillou et al. Reference Marcaillou, Munoz, Vidal, Parra and Harfouche2011). One possible reason for the lack of magnetite in our lower temperature experiments (conducted between 200 and 400°C) is the relatively short run duration compared with previous experiments (up to several thousand hours). As shown by Marcaillou et al. (Reference Marcaillou, Munoz, Vidal, Parra and Harfouche2011), magnetite can only be detected in the latest stages of hydrothermal alteration of peridotite at 300°C/300 bar after 70 days. The thermodynamic modelling (Klein et al. Reference Klein, Bach, Jons, McCollom, Moskowitz and Berquo2009) have showed that both the partitioning and the oxidation state of iron are sensitive to temperature and water/rock ratio, while the formation of magnetite depends on the availability of an external source of silica at higher temperature. With low temperature and low water/rock ratio, Fe3+ is allowed to partition into silicates that may also account for the lack of magnetite under these conditions. For example, Seyfried et al. (Reference Seyfried, Foustoukos and Fu2007) carried out the serpentinization experiment at 200°C and 500 bar; likewise, their results showed the production of H2 but little magnetite. Another possible reason for the difference in the magnetite formation conditions between our experiments and previous studies is the activity of CO2. The fugacity–fugacity diagram of the Fe–C–O–H system shows that siderite is the most stable phase under relatively reduced condition with high CO2 fugacity under reduced condition and pH values >5 (Fig. 3). In our experiments, the high CO2 fugacity may result in the incorporation of Fe into carbonates while the prevention of magnetite crystallization. The EDS analysis of the carbonates formed in our experiments also confirmed the incorporation of iron into the carbonates (Fig. 2).

Fig. 2. SEM image of komatiite before (A) and after (B) the experiment. The observed typical secondary minerals are phyllosilicate and carbonate (300°C).

Fig. 3. The influence of CO2 and O2 fugacity to the stabilities of iron species in P–T condition similar to our experiments. The diagram was calculated with Geochemists Workbench (Bethke Reference Bethke1996) with the Lawrence Livermore National Laboratory database.
The crystallography of magnetite
Magnetite produced at 450°C was characterized by TEM (Figs 4 and 5). Unlike the conventional octahedral crystal habits that have been commonly observed in previous experiments (McCollom & Seewald Reference McCollom and Seewald2013), magnetite crystals produced in this study were hexagonal plates of a few hundred nanometres (Fig. 5). The EDS analyses showed that the plate-like magnetite nanocrystals also contained small amount of Ni and Cr (~7 wt% Ni and 1 wt% Cr). SAED analysis of the crystals (Fig. 4(b)) yields Miller indices (hkl) of (220), (311), (440) and (511) that confirmed the electron diffraction pattern of magnetite (Table 2). The TEM images showed porous structures on magnetite crystals (Figs 4 and 5). The pores on the thin magnetite crystals revealed that the crystals were thin hexagonal nanocrystals rather than the projection of octahedral magnetite crystals. HRTEM images showing exactly the same set of lattice fringes at the opposite sides of one pore on the magnetite crystals (Fig. 4(d)) suggested that the porous structure was produced after the crystallization of magnetite. The measured spacing of the crystallographic planes was 0.29 nm (Fig. 4(d)), equals to the d-space of (220) planes of magnetite crystals (PDF800389), implying that these magnetite nanocrystals grew along the [110] direction.

Fig. 4. TEM characterization of Fe-oxide found in higher temperature experiments (450°C). Panel (B) shows the SAED pattern of this mineral from (A), which indicates that the secondary mineral is magnetite. (D) The HRTEM image of selected part of (C) showing the same interplanar spacings at both sides of one pore.

Fig. 5. TEM image of plate-like magnetite nanocrystals. (A), (B), (C) and (D) show the hexagonal crystal shape of magnetite nanocrystals.
Table 2. Electron diffraction data for magnetite crystals
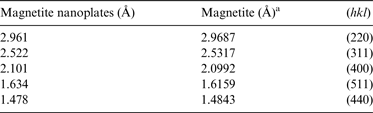
a PDF: 86–1326.
Various experimental studies have showed that magnetite can act as catalyst in abiotic synthesis of small organic molecules such as hydrocarbons, amino acids and acetic acid (Chen & Bahnemann Reference Chen and Bahnemann2000; Fu et al. Reference Fu, Lollar, Horita, Lacrampe-Couloume and Seyfried2007; Pizzarello Reference Pizzarello2012). All these reactions are heterogeneous catalyses in which the total surface area of catalyst has a critical effect on the reaction rate because it determines the availability of catalytic sites (Swathi & Sebastian Reference Swathi and Sebastian2008). Taken Fischer–Tropsch synthesis of methane as an example, the catalytic reaction happens on the surface of catalyst (magnetite) in high-H2 environments in which iron carbides are formed (Satterfield et al. Reference Satterfield, Hanlon, Tung, Zou and Papaefthymiou1986a, Reference Satterfield, Hanlon, Tung, Zou and Papaefthymioub; Lee et al. Reference Lee, Lee and Chang1990) that can act as reaction intermediates. Compared with magnetite observed in the serpentinization experiments, smaller size and the porous structure of magnetite platelets observed in this study make them have lager specific surface area that favours the catalysis of abiotic synthesis.
In our experiment, nickel and chromium were the other two transition metals found incorporated in magnetite, implying its potential in catalysing multiple types of simple organics. These two metals have been demonstrated be important in abiotic synthesis of hydrocarbons. Foustoukos & Seyfried (Reference Foustoukos and Seyfried2004) suggest that chromite (FeCr2O4) was an efficient catalyst for Fischer-Tropsch type (FTT) synthesis of longer chain hydrocarbons. Horita & Berndt (Reference Horita and Berndt1999) reported the excellent catalytic capability of awaruite (a Ni–Fe alloy) in the conversion from CO2 to CH4. As in the original komatiite, Ni and Cr are minor components with the concentrations of several thousand ppm. Although Ni–Fe-alloys, Ni/Cr oxides and sulphides were not observed in our experiments due probably to the short run duration and lack of sulphur compound in the komatiite, geological observation and thermodynamic calculation (Klein & Bach Reference Klein and Bach2009; McCollom & Seewald Reference McCollom and Seewald2013) consistently demonstrate that these minerals could form under hydrothermal conditions. Together with magnetite, they constitute the common accessory minerals in serpentiniation in the hydrothermal systems (McCollom & Seewald Reference McCollom and Seewald2013).
Implication for the organosynthesis of the prebiotic evolution on the early Earth
It has been proposed previously that alkaline hydrothermal vent is a favourite site for the origin and early evolution of life because the physicochemical conditions favour the prebiotic chemical processes (Russell et al. Reference Russell, Hall and Turner1989, Reference Russell, Daniel, Hall and Sherringham1994, Reference Russell, Hall and Martin2010; Fruth-Green et al. Reference Fruth-Green, Connolly, Plas, Kelley and Grobety2004; Sleep et al. Reference Sleep, Meibom, Fridriksson, Coleman and Bird2004, Reference Sleep, Bird and Pope2011; Holm et al. Reference Holm, Dumont, Ivarsson and Konn2006; Schulte et al. Reference Schulte, Blake, Hoehler and Mccollom2006; Martin & Russell Reference Martin and Russell2007). Experimental simulations and examinations of geological samples (Berndt et al. Reference Berndt, Allen and Seyfried1996; McCollom et al. Reference McCollom, Seewald and Simoneit2001; McCollom & Seewald Reference McCollom and Seewald2003a, Reference McCollom and Seewaldb, Reference McCollom and Seewald2004, Reference McCollom and Seewald2007; Seewald et al. Reference Seewald, Zolotov and McCollom2006; Seyfried et al. Reference Seyfried, Foustoukos and Fu2007) have showed that methane, short hydrocarbons and minor formate, methanol and trace acetate (these may also be the by-products of microbial activity) can be produced by serpentinization in the hydrothermal systems. In addition, laboratory experiments (Horita & Berndt Reference Horita and Berndt1999; Chen & Bahnemann Reference Chen and Bahnemann2000; Foustoukos & Seyfried Reference Foustoukos and Seyfried2004) have showed that many of these organic compounds can be produced with Fe-, Cr- and Ni-bearing oxides, sulphides as catalysts.
According to the results of our batch experiments, magnetite can be produced quickly at a temperature of 450°C. As the physicochemical conditions we chose for the experiments are similar to the Hadean Earth with H2O steam and high CO2 in the atmosphere, thus the formed magnetite could accumulate before the formation of the earliest ocean by 4.4 Ga (Wilde et al. Reference Wilde, Valley, Peck and Graham2001; Valley et al. Reference Valley2014). Being so, reactions associated with the abiotic synthesis of acetate or other simple organic compounds from CO2, as catalysed by Fe and Ni oxide/sulphide minerals, might have already happened at an earlier time before the emergence of the earliest ocean. Meanwhile, the abiotic synthesis of organic molecules would occur widespread since the global occurrence of the reaction between the ultramafic crust and the CO2–H2O dominant atmosphere. Although the surface temperature was too high for the organic molecules to be preserved long enough, they may gradually accumulate along with the catalytic secondary minerals on the early Earth and led to the origin of life after the cooling of the Earth's surface (Holm & Andersson Reference Holm, Andersson and Brack1998; Martin & Russell Reference Martin and Russell2007).
Conclusion remarks
Experiments simulating the interaction between the rock crust and atmosphere on the Hadean Earth were performed using komatiite, H2O and CO2 from 200 to 450°C to investigate the production of secondary minerals. The results showed the secondary minerals are mainly phyllosilicates and carbonates. The formation of magnetite was only confirmed in experiment carried out at 450°C. Particularly, magnetite crystals observed in this study is hexagonal plates of a few hundred nanometres. This is the first report and characterization of plate-like magnetite nanocrystals in water–rock experiments. The porous structures on magnetite crystals are very likely the result of post-crystallization erosion. The detection of magnetite in our experiments suggests a general formation and accumulation of magnetite nanoparticle on Earth’ surface before the formation of its earliest ocean. With these magnetite nanoparticles and the other catalytic minerals, the prebiotic chemical evolution could start early and extensively than previously thought.
Acknowledgements
We thank Professor Weidong Sun and Dr. Xing Ding for their advices on our experiments and the support from the Hydrothermal Laboratory at Guangzhou Institute of Geochemistry, Chinese Academy of Sciences. This study was financially supported by Research Grants Council of Hong Kong (HKU703911P).









