Introduction
In recent years the problem of the origin and early evolution of life has gained enormous importance throughout the world. The early theories and current findings of scientists have laid special emphasis on the abiotic origin of molecular matrices such as nucleic acids and proteins. How far back these biopolymers and their monomers began to form and accumulate under prebiotic conditions has been speculated on, reviewed and experimented upon by scientists (Lemmon Reference Lemmon1970; Rao et al. Reference Rao, Odom and Oro1980; Ponnamperuma et al. Reference Ponnamperuma, Shimoyama and Friebele1982; Cairns-Smith & Hartman Reference Cairns-Smith and Hartman1986; Ferris et al. Reference Ferris, Hill, Liu and Orgel1996; Imai et al. Reference Imai, Honda, Hatori, Brack and Matsuno1999; Rode Reference Rode1999; Zaia Reference Zaia2004; Brack Reference Brack2007; Lambert Reference Lambert2008) during the last few decades, and results from research on abiogenesis of biomonomers from substances of the abiosphere have been promising. However, the complex prebiotic pathways of the formation and evolution of peptides on primitive Earth are not very clear. These reactions may proceed via amino acid dimerization, cyclization, sequence inversion and chain elongation. Moreover, some decomposition reactions such as hydrolysis, deamination and decarboxylation may also have proceeded.
It was postulated that life originated in the water of seas and oceans of the primitive Earth (Oparin Reference Oparin1924). For thousands of millions of years the organic compounds must have undergone structural changes and accumulated through time in the primeval young Earth era (Haldane Reference Haldane1933). It was emphasized that clays, minerals and silicates may have played a vital role in chemical evolution. The lagoons and pools along the oceans might be the probable sites for the concentration of biomonomers through wetting and drying processes. Thus, clays near the hydrosphere–lithosphere interface might have adsorbed key biomonomers on and between their silicate layers, thereby providing the high local concentrations of reactants needed to form certain biologically important macromolecules (Bernal Reference Bernal1951). Peptide-like polymers can be assumed to be among the first macromolecules to have played a significant role in chemical processes leading to the first proteins.
Earlier studies suggested that condensation reactions of precursors of proteins and nucleic acids proceeded under extreme natural fluctuating environments where rainstorms, flooding, dehydration and freezing occurred and thermal and/or solar ultraviolet (UV) flux played a significant role, giving rise to macromolecules by adsorption of monomers and their subsequent condensation (Lahav & Chang Reference Lahav and Chang1976; Paecht-Horowitz & Eirich Reference Paecht-Horowitz and Eirich1988). Thermal condensation by heating aspartic acid and glutamic acid with other amino acids over a temperature range of 180–200°C has been reported (Fox & Harada Reference Fox and Harada1960) and it has been shown that glutamic acid was relatively more effective in promoting the condensation reaction. Amino acid oligomerization in the absence of any condensing agent under drying and wetting cycles on cation-exchanged clays at temperatures below 100°C has been studied (Lahav et al. Reference Lahav, White and Chang1978; Lawless & Levi Reference Lawless and Levi1979). Condensing agents such as cyanamide, inorganic phosphates and some heterocyclic compounds have also been tested to obtain peptides in appreciable yield (Rohlfing & McAlhaney Reference Rohlfing and McAlhaney1976; Howker & Oro Reference Howker and Oro1981; Rishpon et al. Reference Rishpon, O'Hara, Lahav and Lawless1982) but their stability under prebiotic aqueous conditions seems very unlikely (Fox & Dose Reference Fox and Dose1977).
Salt-induced peptide formation and the effect of glycine in the dimerization of some other amino acids have been reported by earlier workers (Rode et al. Reference Rode, Son, Suwanachot and Bujdak1999; Suwannachot & Rode Reference Rode1999). The polymerization of glycine in sodium chloride solution containing Cu2+ ions at 70–100°C has been experimentally demonstrated (Schwendinger & Rode Reference Schwendinger and Rode1991; Plankenseiner et al. Reference Plankenseiner, Righi and Rode2002). Further, the formation of peptide-like polymers at temperatures >250°C has also been reported (Yanagawa et al. Reference Yanagawa, Kojima and Ito1985), but the oligomerization reaction at high temperature is questionable because it may decompose the reactants as well as products. A few studies (Ponnamperuma et al. Reference Ponnamperuma, Shimoyama and Friebele1982) have indicated that the zwiterionic form of amino acids adsorbed on the clay surface might undergo peptide formation when the clay–amino acid complex is heated in a temperature range of 140–244°C.
It has also been shown that clay, silica, alumina and many minerals concentrate amino acids and catalyse their condensation to peptides (Grim, Reference Grim1968; Lawless & Levi Reference Lawless and Levi1979; Ponnamperuma et al. Reference Ponnamperuma, Shimoyama and Friebele1982; Basiuk et al. Reference Basiuk, Gromovoy, Golovaty and Glukhoy1999; Basiuk & Sainz-Rojas Reference Basiuk and Sainz-Rojas2001; Matrajt & Blanot Reference Matrajt and Blanot2004; Zaia, Reference Zaia2004). However, in some previous investigations it has also been suggested that mineral catalysts are in general not really suitable for catalysing peptide formation from amino acids, but that they can exhibit their catalytic properties after the initial formation of small peptides which might have formed by some other mechanism. The role of clays and metal oxides was found mainly in the chain elongation of dipeptides rather than in the formation of peptides from amino acids (Bujdak et al. Reference Bujdak, Faybikova, Eder, Yongyai and Rode1995, Reference Bujdak, Remko and Rode2006; Bujdak & Rode Reference Bujdak and Rode1999, Reference Bujdak and Rode2004).
Although experiments concerning adsorption of amino acids and their short peptides on solid surfaces under prebiotic conditions have been carried out (Kalra et al. Reference Kalra, Pant, Pathak and Mehata2000, Reference Kalra, Pant, Pathak and Mehata2003; Meng et al. Reference Meng, Stevano and Lambert2004; Zaia, Reference Zaia2004; Whitehouse et al. Reference Whitehouse2005; Lambert Reference Lambert2008; de Paiva et al. Reference de Paiva, Morales and Diaz2008), the question of whether the nature or the composition of amino acids adsorbed on solid surfaces influences the formation of peptides/proteins of primitive living systems under thermal conditions is still to be explored in detail. Moreover, the combination of amino acids as reactants in prebiotic peptide formation has rarely been studied, although they possess catalytic properties. Therefore, in the present investigation we have chosen two different reaction systems consisting of glycine in combination with aspartic acid and glycine with valine as reactants in aqueous solution with a view to investigating the mutual catalysis of these amino acids in an enzyme-free prebiotic environment for the possible synthesis of peptides and the role of montmorillonite clay with and without divalent cations (Ca2+, Mg2+ and Ni2+) under the drying and wetting conditions presumed to be available near the lithosphere–hydrosphere boundary of the primitive sea. Thus, this could be an effective and alternative pathway of peptide bond formation during chemical evolution.
Materials and methods
Montmorillonite clay (E. Merck) was purified by sedimentation in water and the purity of a <2 μm sample was tested by X-ray diffraction measurement. Homoionic clays (Ca2+, Mg2+ and Ni2+) were prepared by the saturation method (Theng Reference Theng1974) with 50 ml of 1 M concentration of each metal chloride. The cation-exchanged clays thus prepared were repeatedly washed with double-distilled water and centrifuged for 10 minutes in Remi 3500 rpm until freed of chloride ion, and were used in all cases studied. All the chemicals including amino acids and reference oligopeptides were from Sigma Chemical Company.
Sterilized aqueous solutions of a mixture of glycine and L-aspartic acid as well as glycine and L-valine (25 ml, 0.1 M each) were taken separately in five Borosil glass reaction vessels (100 ml) fitted with air condensers and kept on heating plates at a temperature of 85°C±5°C. Evaporation cycles were performed by heating the reaction solution in the presence and absence of sodium montmorillonite clay with or without divalent cations, i.e. Ca2+, Mg2+ and Ni2+ (0.01 g each) for 8–10 hrs in each drying cycle till the last drop of reaction concentrate was evaporated. Sterilized water (20 ml) was then added to the reaction vessel to perform the next evaporation cycle. In every experiment samples of each reaction solution (10 ml) kept in Borosil glass containers (25 ml) heavily wrapped in black cloth and paper were kept separately as controls. Drying and wetting cycles were continued for varying periods up to 250 hrs and the reaction concentrates as well as the control solution were analysed periodically (50, 100, 150, 200 and 250 hrs) by paper chromatography (Hais & Macke Reference Hais and Macke1963) and high-performance liquid chromatography (HPLC) (Hancock & Harding Reference Hancock and Harding1982) methods. The reaction concentrates in the presence of clay with and without cations (Ca2+, Mg2+ and Ni2+) were shaken in aqueous CaCl2 solution to release the resulting peptides prior to analysis.
The preliminary analysis of samples of different reaction mixtures was carried out on Whatman No.1 filter paper using n-butanol:acetic acid:water (4:1:1 v/v) as the solvent system and ninhydrin as the colour-producing reagent. Shimadzu (SPD10A, UV-vis) HPLC apparatus having a C18 column with UV detector monitored at 210 nm was used to analyse the resulting peptides and other amino acids in all cases investigated. The mobile phase was 25% CH3CN:75% Na2HPO4, pH was adjusted to 2.5 with H3PO4 at 23–25°C and the flow rate was monitored at 1.0–1.2 ml min−1. The identity of reaction products was ascertain by Rf values, colour with ninhydrin, the HPLC retention time, UV (Jasco V-550 spectrophotometer) and infrared (IR) (Perkin-Elmer DX-II FT-IR spectrophotometer) spectra compared with authentic reference standards. The optical density of coloured spots was measured by using a MK III colorimeter monitored at 570 nm. The reaction yields were determined as a percentage of the reactants converted to the reaction products. The results of these investigations have been recorded in Tables 1 and 2 and illustrated in Figs 1–8, S1 and S2.

Fig. 1. Formation of peptides (% yield) from reaction system of glycine/aspartic acid and water as a function of heating time (hrs).

Fig. 2. High performance liquid chromatography chromatogram of reaction concentrate of glycine/aspartic acid and water heated for up to 250 hrs. The retention times 0.095, 1.095, 1.675, 2.923, 3.590, 4.513, 5.129 and 7.139 min correspond to Asp, (cGly)2, Gly, (Gly)2, (Gly)3, Gly-Asp, Ala and Lys, respectively.

Fig. 3. The infrared absorption spectrum of glycine/aspartic acid/water heated for up to 250 hrs shows absorption frequencies in regions of 3500–3450 cm−1 (+NH3 asym. stret.), 1750–1690 cm−1 (C=O stret. in CONH2), 1650–1550 cm−1 (asym. stret. of COO−), 1440–1360 cm−1 (COO− sym. stret.) and 1515–1490 cm−1 (C-N stret).

Fig. 4. Formation of peptides (% yield) from reaction system of glycine/aspartic acid and water in presence of montmorillonite clay incorporated with divalent cations.

Fig. 5. Formation of peptides (% yield) from reaction systems of glycine/valine and water as a function of heating time (hrs).

Fig. 6. High performance liquid chromatography chromatogram of reaction concentrate of glycine/valine and water heated for up to 150 hrs. The retention times 1.684, 2.120, 2.502, 2.913, 3.532, 4.012, 4.265, 5.495, 5.910 and 7.510 min correspond to Gly, (Gly)2, (Gly)3, (Gly)4, (Gly)5, Val, Val-Gly, Gly-Val, (Val)2 and Leu, respectively.

Fig. 7. The infrared absorption spectrum of glycine/valine and water system heated for up to 150 hrs shows absorption frequencies around 3350 cm−1 (N-H asym stret), 3170 cm−1 (N-H sym stret), 1640 cm−1 (C=O stret in CONH2) and 1425 cm−1 (C-N stret).

Fig. 8. Formation of peptides (% yield) from reaction system of glycine/valine and water in presence of montmorillonite clay incorporated with divalent cations.
Table 1. Percent yield of peptides formed from reaction system of glycine/aspartic acid/water

Table 2. Percent yield of peptides formed from reaction system of glycine/valine/water

* T, Trace amount
Results
Two parallel reactions, i.e. glycine and aspartic acid and glycine and valine in the presence and absence of montmorillonite clay with or without divalent cations (Ca2+, Mg2+ and Ni2+) under drying and wetting conditions were investigated for the possible formation of peptides. Chromatography of the reaction concentrate of glycine/aspartic acid with and without catalyst heated up to 250 hrs showed the formation of glycyl-aspartic acid (Gly-Asp), cyclic diglycine (cGly)2, triglycine (Gly)3 and diglycine (Gly)2 (Fig. S1). The linear and cyclic peptides of aspartic acid were not detected. Periodic analysis of the reaction concentrate of glycine and aspartic acid in the absence of clay heated up to 50 hrs showed the formation of (cGly)2 in an appreciable amount, (Gly)2 in significant quantity, (Gly)3 in moderate amount and Gly-Asp in detectable quantity. A remarkable increase in the quantity of Gly-Asp was noticed when the duration of heating was continued up to 100 hrs, while the amount of (cGly)2 was considerably decreased. A gradual increase in the quantity of (Gly)2 was observed on prolonging the effect of heat further. A slight increase in the amount of other peptides was observed throughout the course of heating up to 250 hrs. The variation in the amounts of resulting peptides with duration of heating is illustrated in Figs 1 and S1 and recorded in Table 1.
The results of above studies were further supported by the hydrolysis of the resulting glycine peptides with 6N HCl, affording glycine as the main product. The amounts of these peptides were determined by modified Biuret reaction based on the change in UV absorption spectra with absorption maximum at around 263 nm, which is attributed to the copper-ion complex formation of the peptide in alkaline medium. Peptides were also estimated colorimetrically at 570 nm. However, samples of the parallel-run control reaction system on chromatographic analysis showed no change. The samples of the reaction system heated up to 250 hrs on further HPLC analysis showed 12 peaks. Out of these, peaks corresponding to retention time of 0.095, 1.095, 1.675, 2.923, 3.590, 4.513, 5.129 and 7.139 min were tentatively identified as Asp, (cGly)2, Gly, (Gly)2, (Gly)3, Gly-Asp, Ala and Lys, respectively (Fig. 2).
The UV absorption spectrum of the sample showed the absorption maximum at around 220 nm (data not shown), indicating the formation of peptides. The IR absorption spectrum of a similar sample showed absorption frequencies in regions of 3500–3450 cm−1 (+NH3; asym. stret.), 1750–1690 cm−1 (C=O stret. in CONH2), 1650–1550 cm−1 (asym. stret. of COO−), 1440–1360 cm−1 (sym. stret. of COO−) and 1515–1490 cm−1 (C-N stret) (Fig. 3). The absorption frequency of C=O in a region of 1750–1690 cm−1 due to −CONH2 (amide group) clearly indicates the formation of peptides.
The reaction system consisting of glycine and aspartic acid under drying and wetting conditions in the presence of montmorillonite clay with or without metal cations heated up to 250 hrs upon chromatographic analysis showed formation of Gly-Asp, (Gly)3 and (Gly)2 along with residual reactants, while (Gly)3 was formed in better amounts as compared with Gly-Asp and (Gly)2. However, (cGly)2 was not formed at all.
The effect of wetting/drying cycles up to 250 hrs on a similar reaction system in the presence of calcium-exchanged clay showed the formation of an identical range of products. However, the quantity of (Gly)3 and (Gly)2 was slightly increased. On the other hand, in presence of magnesium-exchanged clay, the amount of Gly-Asp relatively increased while no remarkable change in the amount of (Gly)2 was observed. (Gly)3 was detected in a slightly lower amount. The results recorded in Table 1 clearly indicate that the yield of all the resulting peptides was relatively higher in the aqueous environment with nickel-incorporated clay (Fig. 4).
Another reaction system of glycine and valine was investigated under identical wetting and drying conditions to ascertain the formation of oligopeptides, and the results are recorded in Table 2 and illustrated in Figs. 5 and S2. The formation of linear peptides of glycine up to pentamer level (Gly)5 along with some homo- and hetero-peptides (Gly)4, (Gly)3, (Gly)2, Gly-Val, Val-Gly and (Val)2 was observed. Initially, the concentrate of the reaction system of glycine and valine heated up to 50 hrs on chromatography revealed divaline (Val)2 in good amount and valinyl-glycine (Val-Gly) in trace amount. The amount of Val-Gly was enhanced on heating the reaction mixture for up to 100 hrs, and the simultaneous formation of Gly2, Gly3 and Gly-Val was also observed. At 150 hrs of heating, several homo- and hetero-peptides (Gly)2 to (Gly)5 were detected along with Gly-Val, Val-Gly and (Val)2 in increased amount. Triglycine (Gly)3 was formed in appreciable amount on prolonging the duration of heating up to 200 hrs, while a remarkable decrease in the amount of (Gly)5 and (Gly)4 was noticed. A slight decrease in the amount of almost all the resulting oligopeptides was noticed on analysis of reaction concentrate at 250 hrs of heating. The HPLC analysis of the reaction concentrate (150 hrs) showed 10 peaks with retention times of 1.684, 2.120, 2.502, 2.913, 3.532, 4.012, 4.265, 5.495, 5.910 and 7.510 min, corresponding to Gly, (Gly)2, (Gly)3, (Gly)4, (Gly)5, Val, Val-Gly, Gly-Val, (Val)2 and Leu, respectively (Fig. 6).
The UV absorption spectrum of a similar solution with a band maximum at 220 nm (data not shown) and the IR absorption spectrum with absorption frequencies at around 3350 cm−1 (N-H asym stret), 3170 cm−1 (N-H sym. Stret), 1640 cm−1 (C=O stret of amide group) and 1425 cm−1 (C-N stretch) also indicate the formation of peptides (Fig. 7).
In the presence of montmorillonite clay with or without divalent cations (Ca2+, Mg2+ and Ni2+) small changes were observed in the formation of resulting peptides (see Table 2). Gly-Val and (Val)2 were formed in relatively better amounts whereas the quantity of other oligopeptides slightly decreased. However, (Gly)5 and Val-Gly were not formed at all, and a new peptide identified as (cGly)2 was formed.
It was noticed that presence of calcium-incorporated montmorillonite clay in the above reaction system slightly increased the quantity of (cGly)2, (Gly)2 and Gly-Val while the quantity of (Gly)4, (Gly)3 and (Val)2 relatively decreased. The presence of magnesium-incorporated clay showed the formation of an identical range of reaction products. The quantity of (Gly)3 and (Gly)2 increased whereas the quantity of (cGly)2, Gly-Val and (Val)2 was relatively decreased. When heating was carried out in presence of nickel-incorporated clay, the quantity of (Gly)3 was relatively increased (Fig. 8).
Discussion
Glycine when heated alone under anhydrous conditions at 85°C±5°C pyrolysed into a blackish-brown solid without the formation of peptides, while in aqueous solution (Gly)2 and (cGly)2 were formed under drying and wetting conditions in the same temperature range. It is interesting to note that in the glycine/aspartic acid system within a certain period of heating (50–100 hrs) the amount of (cGly)2 was remarkably decreased while an increase in the amount of (Gly)2 was noticed (Fig. 1). This may be due to either the ring opening of cyclic dipeptide of glycine or to the decomposition of the peptide bond of triglycine. A gradual increase in the amount of (Gly)2, (Gly)3 and Gly-Asp including (cGly)2 on prolonging the duration of heating may be accomplished by the reactivity of reactant amino acids and thermal stability of the oligopeptides.
The way in which the reactivity of reactants influences the formation of peptide bonds and decides the reaction pathways may be ascertained. Aspartic acid is a di-carboxylic amino acid with the terminal carboxylic end more reactive than the other containing the amino group in the α-position, and is able to activate the carboxylic end of glycine by anhydride formation. The activated glycine reacts with free glycine molecule to form (Gly)2 and finally (cGly)2 following molecular rearrangement (Fig. 9).

Fig. 9. Possible mechanism for the formation of peptides in the glycine/aspartic acid reaction system.
The hetero-peptide (Gly-Asp) was formed via condensation of the carboxyl end of glycine with the amino end of aspartic acid. However, aspartyl-glycine (Asp-Gly) could not be formed under the reaction conditions because the −COOH end near to the α-amino group in aspartic acid is less reactive (a weaker acidic site) in forming the amide bond with NH2– end of glycine. The various possible pathways for the formation of peptides have been summarized in Figs. 9 and 10.

Fig. 10. Possible pathways of glycine/aspartic acid reaction system.
Thus, aspartic acid initiated the formation of homopeptides of glycine up to trimer level (Gly)3 along with hetero-peptide (Gly-Asp). However, the dimer of aspartic acid (Asp)2 and cyclic aspartic acid (cAsp)2 were not formed. It appears that in aqueous medium the +NH3 end of the aspartic acid zwitterion with two electron-withdrawing carboxylate groups has more opportunity to combine with the COO− end of the glycine zwitterion to form Gly-Asp rather than linear (Asp)2 and cyclic (cAsp)2 di-aspartic acids.
The presence of montmorillonite clay accelerated the formation of (Gly)3 at the expense of (Gly)2 but completely inhibited the formation of (cGly)2. This was ascertained as being due to the esterification of the active site of the aspartic acid with the −OH group of silinol present in montmorillonite clay, thus leaving the glycine molecule for intermolecular condensation. The divalent cations incorporated on clay have, however, shown a more or less identical range of products. The order of their effectiveness towards chain elongation was found as follows:
On the other hand, the results of the glycine/valine system indicated that the neutral amino acid (valine) with a hydrophobic methyl group branching appears to accelerate the formation of glycine peptides up to pentamer level (Gly)5 along with formation of Gly-Val, Val-Gly and (Val)2. This may be ascribed to proper orientation of a branched chain isopropyl group attached to the α-carbon atom of valine, which may preferentially facilitate the smaller glycine zwitterion to interact through dipole–dipole interactions.
Among the hetero-peptides, Gly-Val was formed in greater amounts than Val-Gly. Due to the positive inductive effect of the isopropyl group, the electron density is increased on the carboxylate (COO−) end whereas the magnitude of the positive charge is decreased on the ammonium end of zwitterionic valine in the aqueous environment. In other words, the isopropyl group in valine increases the nucleophilicity of the ammonium group and decreases the electrophilicity of the carboxylate group (Fig. 11).
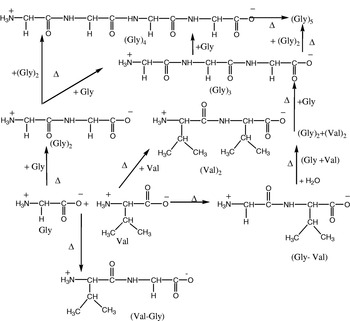
Fig. 11. Possible pathways of glycine/valine reaction system.
The presence of montmorillonite clay with or without divalent cations (Ca2+, Mg2+ and Ni2+) favoured intramolecular condensation of glycine resulting in the formation of (cGly)2, but retarded the formation of (Gly)5 and Val-Gly (see Fig. 8 and Table 2). The amount of homopeptide of glycine (Gly)4, (Gly)3 and (Gly)2 was decreased. It appears that montmorillonite clay with a silinol(-OH) layer in its structure has stabilized the reactant amino acids by selective adsorption on its surface according to their nature and size, hence facilitating cyclization of glycine into (cGly)2, as also suggested by Bujdak & Rode (Reference Bujdak and Rode1999).
The results of these studies have shown that a neutral amino acid (valine) in combination with glycine favours the formation of glycine peptides up to pentamer level rather than the acidic amino acid (aspartic acid) mixed with glycine. In the presence of clay with or without divalent cations, the glycine/aspartic acid system has shown efficiency for homo- and heteropeptide formation, while the glycine/valine system favoured cyclization of glycine. It is interesting to note that (Val)2 is not formed at all if valine is the only amino acid in the reaction system, but in the presence of glycine it is synthesized readily (50 hrs). The highest yield of Gly-Val and (Val)2 was found in presence of clay. Thus glycine appears to induce Gly-Val and (Val)2 formation in a mixed amino acid system.
All data indicate that the peptide formation depends on the duration of the heat effect, the nature of reactant amino acids and on montmorillonite clay incorporated with divalent cations under drying and wetting cycles, which might have been common phenomena in coastal regions of the primitive sea. Moreover, mixed amino acids taken as reactants have been experimentally proved as an effective and alternative system of peptide bond formation and chain elongation. It may be a possible pathway available in the prebiotic scenario, as the simultaneous presence of various amino acids is expected, rather than a single amino acid.
Acknowledgements
The authors thank the Head, Department of Chemistry and Physics for providing research facilities. One of the authors (MSM) is thankful to DST, New Delhi, for the young scientist fellowship.
















