Introduction
Hyper-arid environments are among the harshest to life, principally due to extremely low levels of rain or other forms of moisture. Some of the best known deserts of this class include the Atacama Desert along the Pacific coast of South America in Chile and Perú and the Antarctic Dry Valleys. Not counting in desert sips where the water table reaches the surface (McKay et al. Reference Mckay, Friedmann, Gomez-Silva, Caceres-Villanueva, Andersen and Landheim2003; Navarro-Gonzalez et al. Reference Navarro-Gonzalez2003; Conley et al. Reference Conley, Ishkhanova, Mckay and Cullings2006), the only functioning bio-systems reside in soils (Cowan et al. Reference Cowan, Russell, Mamais and Sheppard2002; Maier et al. Reference Maier, Drees, Neilson, Henderson, Quade and Betancourt2004; Drees et al. Reference Drees, Neilson, Betancourt, Quade, Henderson, Pryor and Maier2006; Smith et al. Reference Smith, Tow, Stafford, Cary and Cowan2006; Fletcher et al. Reference Fletcher, Conley, Valdivia-Silva, Perez-Montaño, Condori-Apaza, Kovacs, Glavin and Mckay2011), rock surfaces and cracks (Warren-Rhodes et al. Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gomez-Silva, Amundson, Friedmann and Mckay2006), and halite outcroppings (Wierzchos et al. Reference Wierzchos, Ascaso and Mckay2006; Davila et al. Reference Davila, Gomez-Silva, De Los Rios, Ascaso, Olivares, Mckay and Wierzchos2008). Important characteristics of hyper-arid deserts are that they typically have <1 mm of rain per year with sometimes decades in-between rain events (McKay, Reference Mckay2002, McKay et al. Reference Mckay, Friedmann, Gomez-Silva, Caceres-Villanueva, Andersen and Landheim2003; Warren-Rhodes et al. Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gomez-Silva, Amundson, Friedmann and Mckay2006); are devoid of visible primary producers; and have low organic content (Burkins et al. Reference Burkins, Virginia, Chamberlain and Wall2000, Reference Burkins, Virginia and Wall2001; Navarro-Gonzalez et al. Reference Navarro-Gonzalez2003; Ewing et al. Reference Ewing, Navarro-Gonzalez, Amundson, Wu and Mckay2004, Reference Ewing, Sutter, Owen, Nishiizumi, Sharp, Cliff, Perry, Dietrich, Mckay and Amundson2006; Valdivia-Silva et al. Reference Valdivia-Silva, Fletcher, Navarro-Gonzalez, Mckay, Perez-Montaño, Condori-Apaza and Conley2005, Reference Valdivia-Silva, Navarro-Gonzalez and Mckay2009, Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011; Warren-Rhodes et al. Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gomez-Silva, Amundson, Friedmann and Mckay2006).
Carbon is a key component of soil organic matter, and the measurement of organic carbon content provides an indication of the bio-activity and/or habitability of a soil environment; however, carbon forms a part of inorganic minerals, as well. The carbon minerals are principally in the form of the carbonates CaCO3 and MgCO3 with small additional quantities of carbon dioxide (CO2) and the bicarbonate HCO3−. The carbon associated with the organic material includes plant and animal residues at various stages of decomposition, cells and tissues of soil organisms, and substances synthesized by the soil population (Brady & Weil, Reference Brady and Weil2001). Typically in soils, the organic carbon can be further divided into two pools: a labile (or active) pool that is highly decomposable with turnover rates of a few months to a few years and a recalcitrant (or passive) pool that is stable and has long turnover rates of 20–40 years (Lucas, Reference Lucas2004). The passive pool is comprised of highly decomposed, chemically recalcitrant, humic compounds which could be physically protected as well as cellulose and plant detritus. The components of the active or labile pool, as described by Lucas (Reference Lucas2004) and Weil et al. (Reference Weil, Islam, Stine, Gruver and Samson-Liebig2003), include amino acids, simple sugars, polysaccharides, microbial synthesized bio-chemicals, exudates from plant roots and microbial biomass.
Quantification of low levels of soil organic matter is an important step in the understanding of the environment; however, most detailed methods of the quantification of organic matter in hyper-arid soils rely upon expensive equipment, which limits the capability to extensively sample and which can only be completed in the laboratory. A variety of laboratory methods have been utilized for the analysis of samples from this region. Navarro-Gonzalez et al. (Reference Navarro-Gonzalez2003) reported organic content from methods of Pyrolysis-Gas Chromatography-Mass Spectrometry (Pry-GC-MS) for 13 samples. Connon et al. (Reference Connon, Lester, Shafaat, Obenhuber and Ponce2007) and Lester et al. (Reference Lester, Satomi and Ponce2007) reported total organic carbon (TOC) by Elemental Analyser for two and three samples, respectively. Drees et al. (Reference Drees, Neilson, Betancourt, Quade, Henderson, Pryor and Maier2006) reported TOC for one sample by high-temperature combustion and a nitrogen/carbon/sulfur (NCS) analyser. Ewing et al. (Reference Ewing, Navarro-Gonzalez, Amundson, Wu and Mckay2004, Reference Ewing, Sutter, Owen, Nishiizumi, Sharp, Cliff, Perry, Dietrich, Mckay and Amundson2006) reported organic carbon (OC) for one sample (each study) by sealed tube combustion followed by cryogenic purification and manometric quantification. Navarro-Gonzalez et al. (Reference Navarro-Gonzalez2006) reported total organic matter (TOM) by thermal volatilization-GC-MS (TV-GC-MS) for one sample. Fletcher et al. (Reference Fletcher, Conley, Valdivia-Silva, Perez-Montaño, Condori-Apaza, Kovacs, Glavin and Mckay2011) reported OC by the Walkley–Black methods for five samples. Burkins et al. (Reference Burkins, Virginia, Chamberlain and Wall2000, Reference Burkins, Virginia and Wall2001) reported the most extensively using an Elemental Analyser with 41 samples from across seven sites in the Antarctic dry valleys.
Aside from the Burkins et al. work, very few samples have been analysed because of the cost and difficulty to collect and process samples through these various methods. All of these studies have demonstrated the need to have a field assay which could map soil organic matter concentrations while enabling real-time, strategic decision making to determine where to focus limited field time and resources as well as identifying the most interesting samples to send back for more extensive laboratory analyses.
In order to allow rapid, in-field deployment and analysis, we chose to modify an approach (Merck Chemical Company, Reference Merck Chemical Company1974) which utilizes acid hydrolysis combined with back titration of potassium permanganate (KMnO4) to determine the labile fraction of soil organic carbon, named here as labile organic carbon (LOC) (Weil et al. Reference Weil, Islam, Stine, Gruver and Samson-Liebig2003; Lucas, Reference Lucas2004). This method works well in hyper-arid soils, in particular, because there are no significant concentrations of recalcitrant forms of organic carbon (Ewing et al. Reference Ewing, Macalady, Warren-Rhodes, Mckay and Amundson2008; Valdivia-Silva et al. Reference Valdivia-Silva, Navarro-Gonzalez, Fletcher, Perez-Montano, Condori-Apaza and Mckay2012, Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011). This rapid, high-throughput method requires low-cost, portable equipment that can easily be deployed in the field as has been demonstrated by Valdivia-Silva et al. (Reference Valdivia-Silva, Fletcher, Navarro-Gonzalez, Mckay, Perez-Montaño, Condori-Apaza and Conley2005, Reference Valdivia-Silva, Navarro-Gonzalez and Mckay2009, Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011), Navarro-Gonzalez et al. (Reference Navarro-Gonzalez2006), and Fletcher et al. (Reference Fletcher, Valdivia-Silva, Perez-Montaño, Condori-Apaza, Conley and Mckay2012); however, it has not been previously described in detail. Here we present the details of a qualified method for the determination of low concentrations of LOC in hyper-arid soils including laboratory-based control tests and calibration curves; evaluation of samples from a variety of arid and hyper-arid locations; comparison against two thermal methods of calcination and pyrolysis; and in-field testing of the method.
Materials and methods
The following paragraphs outline the specific extraction and quantification protocol utilized in this study, the development of the calibration curve and the field experiments including sample collection. Also included are details on the two alternative protocols (calcination and Pyr–GC–MS), reagent standardization, control experiments (positive, negative, operator error and repeatability), the control of chlorides in the process and a description of the different laboratories used in this study.
LOC extraction and quantification protocols
The basis of our protocol was developed from the Merck Chemical Company, KGaA, laboratory manual (Merck Chemical Company, Reference Merck Chemical Company1974). A summary of the modified protocols used in our investigation are as follows: 1 g of sample was weighed (3–4 decimals of precision) and placed into a 15 ml Teflon centrifuge tube. Ten millilitres of 30% sulphuric acid was added to the tube and then sonicated for 5 min at ambient temperature. The tubes were then centrifuged for 15 min at 3500 rpm and the solution was decanted into a 25 ml Erlenmeyer flask. The flask was heated over a magnetic stirrer at approximately 80 °C for 3–5 min until condensation was present on the walls of the flask, and then titrated with KMnO4 (0.01 N) using a 25 ml burette (0.05 ml accuracy) until the liberated organic material was consumed as indicated by a slight shift in colour towards purple that was visible by the naked eye. Each repetition of a sample was analysed in triplicate with as many as three repetitions of each sample. The volume of permanganate solution consumed was converted into mmols of KMnO4. The converted value was then further adjusted with a calibration curve against an oxalic acid standard in order to determine the number of grams of LOC per gram of soil contained in the sample. These adjustments associate the amount of KMnO4 consumed directly to the LOC fraction and eliminate the errors associated with the consumption of the KMnO4 by other sources within the soil matrix such as chlorine, iron and non-labile organic matter.
Calibration curve
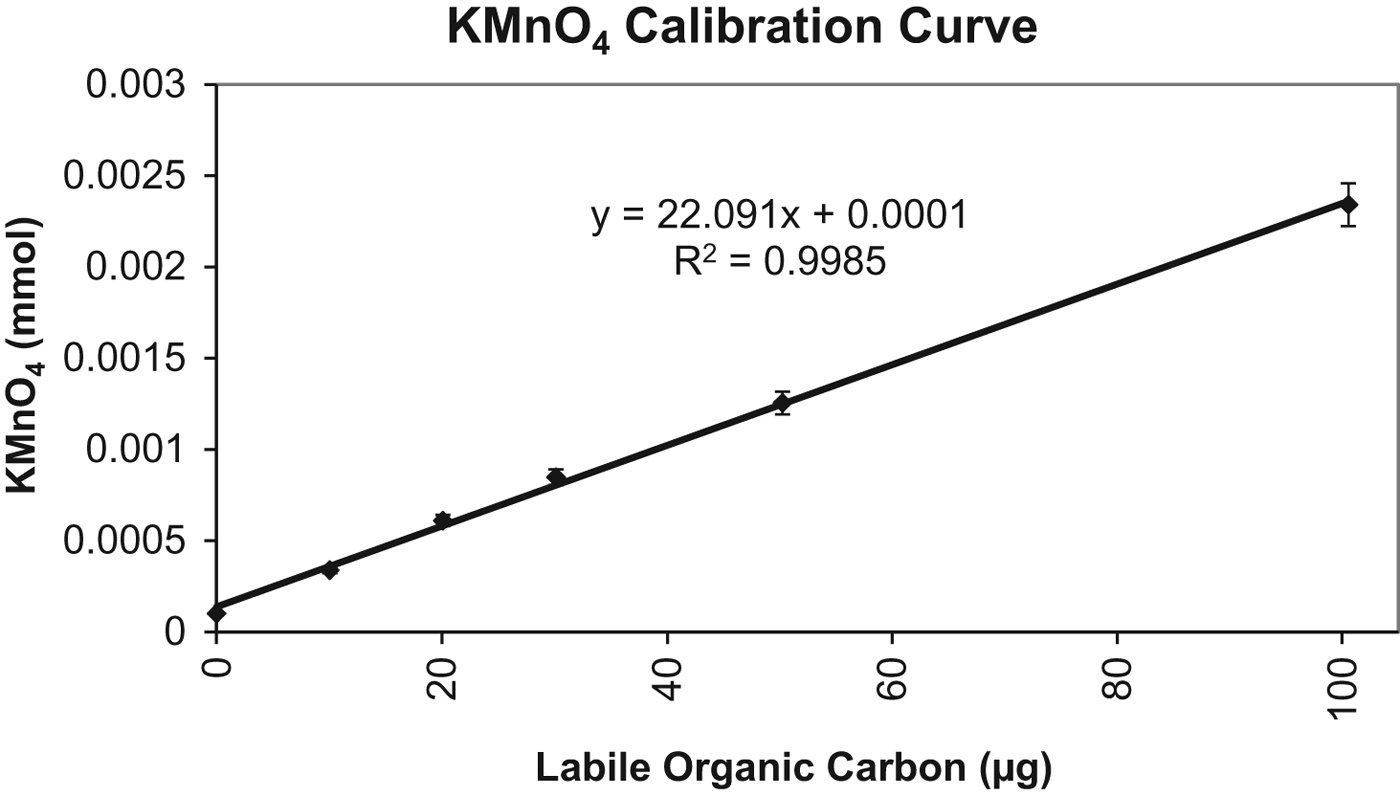
The calibration curve was developed by titrating a series of known concentrations of oxalic acid (prepared as was for the correction factor) at the following concentrations: 0, 10, 20, 30, 50 and 100 μg of organic carbon. The calibration curve and equation of the line are presented in Fig. 1.

Fig. 1. Potassium permanganate (KMnO4) calibration curve. The number of micrograms of LOC can be determined from the number of mmol of KMnO4 consumed during the reaction.
Field testing of environmental samples and sample collection
A mix of hyper-arid and arid samples from four different regions were collected and processed according to the described protocol (Table 1). Arid and hyper-arid regions were qualified by the use of the aridity index (AI), which is the relation between annual precipitation and evapotranspiration (Thornthwaite, Reference Thornthwaite1948; Middleton et al. Reference Middleton, Thomas and Programme1997), where an AI <0.05 is considered hyper-arid, and 0.05 up to 0.2 is arid. Hyper-arid samples were collected from the Atacama Desert at Yungay (McKay et al. Reference Mckay, Friedmann, Gomez-Silva, Caceres-Villanueva, Andersen and Landheim2003; Navarro-Gonzalez et al. Reference Navarro-Gonzalez2003), La Joya (Valdivia-Silva et al. Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011) and the Antarctic Dry Valleys. Arid samples were collected from Yungay and the Mojave Desert near the Zzyzxx Desert Research Station.
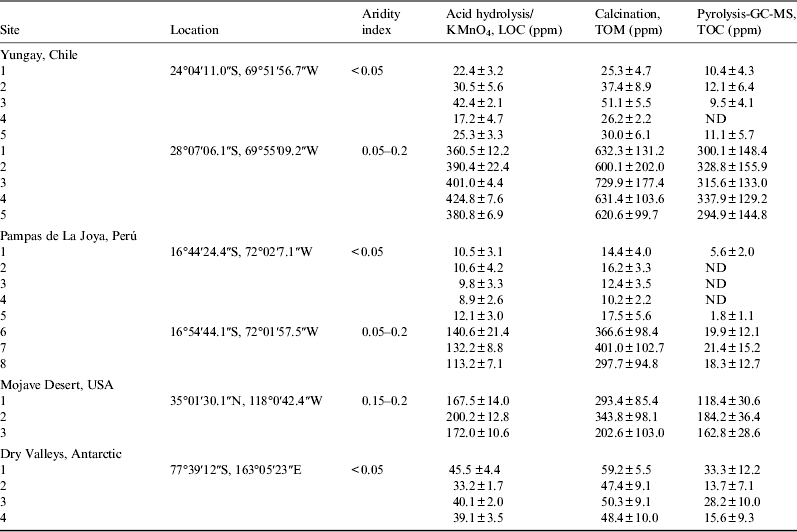
Table 1. Comparison of results for the three methods across a variety of arid and hyper-arid natural samples

One to three samples were taken at each location and each sample was sub-sampled at least three times with all values averaged for a single location. The samples were collected from 0 to 5 cm (maximum estimated) depth with sterile gloves and plastic spoons and transferred into sterile zip-lock bags or whirl-pak bags for storage and transport. The size of the samples ranged from 114 to 442 g with the average sample size collected as 313 g per bag. These samples were well mixed within the bag prior to analysis.
Standardization of the KMnO4 solution
Because of variations of the concentration of the prepared solution, a correction factor was determined by the titration of the prepared KMnO4 solution with a solution of oxalic acid of a known quantity. This was necessary because the prepared KMnO4 solution only has a life of about a week and the concentration of the solution degrades over time due to oxidation. This correction factor was determined at the beginning of each session of sample processing (or at least at the beginning of the work shift).
Control experiments
Negative and positive controls
Contamination of the process was tested using negative and positive controls with and without soil according to the described protocol. The first negative control tested the solution without the addition of any sample soil.
The second negative control test was accomplished with a soil sample that had first been washed free of all organic matter in a series of acid baths according to the following procedure. Six repetitions of 1 g of soil from two different samples (one that showed high levels of organics and one that showed low levels of organics) were measured out into separate 15 ml centrifuge tubes and treated with 1 ml of H2SO4/HCl in a mixture of 0.75/0.25 and left at room temperature for 12 h. Next, 9 ml of 30% sulphuric acid was added to the centrifuge tube which was then sonicated for 5 min and then centrifuged at 3500 rpm for 15 min. The supernatant was then decanted and discarded. Two millilitres of 100% nitric acid was then added to the centrifuge tube with the remaining sample and left at room temperature for 2 h. Eight millilitres of 97% H2SO4 were added to the centrifuge tube, which was then sonicated for 5 min and then centrifuged at 3500 rpm for 15 min. The supernatant was decanted and discarded. The remaining sample was then washed with 10 ml of distilled water (by adding 10 ml of distilled water and mixing with a glass stir rod), centrifuged at 3500 rpm for 15 min, with the supernatant decanted and discarded. This sample was washed with distilled water five times. Three repetitions of each sample were then titrated with the KMnO4 solution in order to show that the process had eliminated the organic material present in the sample while the remaining repetitions were saved for the second of the positive control tests.
The first positive control test was performed without soil and spiked 30% sulphuric acid in the first step of the extraction process with 0.5 ml of oxalic acid (as described in section 2.2). The second positive control test was to spike 0.5 ml of the same oxalic acid into the organic free sample as determined from the second negative control test, after the addition of the 30% sulphuric acid in the first step of the extraction process.
Repeatability and operator error
This was assessed by processing the same soil sample in three completely different batches, including the preparation of fresh solutions and determination of correction factors for each of the three batches. Each sample, as always, was processed with three replicates for each batch.
Control of reaction interferences by chlorides
The presence of chlorides was tested because the titration of KMnO4 can be influenced by the presence of non-organic chlorides, which consume additional KMnO4 (Vogel, Reference Vogel1989). A control test of the effect of chlorides was run by first spiking 4 mg of NaCl into two samples, one with a measured low organic content and one with a measured high organic content, and then running the normal permanganate titration as described. This was repeated with the addition of a silver nitrate (AgNO3) precipitation step in which three drops of 0.1 M AgNO3 was added in order to precipitate any chlorides present in the sample. These were added just after the addition of the 30% sulphuric acid in the first step of the extraction process and just prior to sonication and centrifugation.
Calcination and Pyr–GC–MS
The previously described protocol was compared against two additional methods (Table 1). The calcination technique indirectly detects the amount of TOM by weight loss. In short, 200–400 g of sample was heated to 80 °C for 6 h in order to eliminate the water content. The dried sample was weighed and then subjected to 500 °C for 24 h. The sample was weighed again and the difference indicated the content of TOM without water. The Pyr–GC–MS analysis of the samples was performed according to methods previously described (Navarro-Gonzalez et al. Reference Navarro-Gonzalez2003, Reference Navarro-Gonzalez, Iniguez, De La Rosa and Mckay2009; Valdivia-Silva et al. Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011). This method uses mass spectrometry (MS) to detect the ions that are released from organic molecules as they are broken by pyrolysis, which are then separated by gas chromatography (GC). The sum of the areas of these ions using some calibration patterns can give the TOC present in the sample. A variety of natural samples (Table 1) were taken and analysed from Yungay (Chile), Las Pampas de La Joya (Perú), the Mojave Desert (USA) and the Dry Valleys (Antarctica) in order to determine and compare the organic content. The samples from Yungay were processed in the field according to the protocol (see section 2.1), while the rest were processed using the same protocol in the laboratory. The results reported are the mean and standard deviation of five replicates for each sample and method. The protocol of this paper is reported in LOC, calcination is reported in TOM and Pyr–GC–MS is reported as TOC.
Laboratory set-up
Samples were processed in three locations: Field testing was done at the Yungay Desert Research Station of the University of Antofagasta, Chile, and at the Zzyzxx Desert Research Station in Mojave, USA. Samples from Perú were processed in the laboratories of the company, Wright S.A.C., located in Arequipa, Perú. Calcination and Pyr–GC–MS measurements were made in the laboratories of Química de Plasmas y Estudios Planetarios at the Universidad Nacional Autónoma de México.
Results
Calibration of the acid hydrolysis/KMnO4 method
The calibration curve is shown in Fig. 1 with an R 2=0.9985. The limit of sensitivity of this method as implemented was 10 μg of LOC. Natural samples from Perú were at the limits of sensitivity.
Negative and positive controls
The negative controls both resulted in a consumption of 13 μg of LOC. The positive controls resulted in 160 and 164 μg LOC, respectively, matching the calculated value for the amount of oxalic acid spiked into the tubes of 160 μg of LOC.
Repeatability and operator error
Results of each batch tested for repeatability and operator error was 201.3±9.6 μg LOC. The resulting error was about ±5%.
Tests for chlorides
Four samples from the Atacama were run with the AgNO3 precipitation step. The average value was −7.33±15.72% of the original value obtained for each sample after the AgNO3 precipitation step, indicating as much as a 23% error due to chlorides in these samples from the Atacama.
Organic matter content in sample soils
The organic carbon in hyper-arid soils evaluated by acid hydrolysis and permanganate titration technique, did not present significant differences with the values analysed by calcinations (Table 1). P values were 0.5, 0.2 and 0.17 for Yungay, Pampas de La Joya and Dry Valleys, respectively. The values of LOC ranged between 8.9±2.6 and 53 ppm for hyper-arid soils, whereas the values of TOM were between 10.2±2.2 and 66 ppm (LOC versus TOM shows no statistically significant difference with P≥0.17).
The content of organic carbon evaluated by any of the three techniques in arid environments (0.05>P/PET>0.2) showed higher values than the hyper-arid sites (P<0.001). The levels of LOC were significantly different from the TOM (P=0.02), demonstrating the presence of recalcitrant carbon in arid areas such as roots, different part of plants and organisms.
Discussion
Our method relies on a chemical extraction of the organic carbon and separation from the soil matrix which is then titrated with KMnO4 as a means to quantify the amount of organic carbon extracted during the acid hydrolysis steps. The total amount of KMnO4 consumed is the result of all components in the acid solution that could induce the reaction. This includes both inorganic and organic materials that are introduced into the reaction from either the base materials of the process itself (principally impurities in the water used for the preparation of the solution) or from the sample because it is possible that inorganic residues are suspended in the acid solution along with the extracted organic carbon. Because this is a cumulative consumption, the reported value for a sample can be calculated by subtracting the amount of consumption attributable to the impurities introduced by the protocol from the total amount measured in the titration. It has been directly correlated to organic carbon detected by Pyr–GC–MS (Valdivia-Silva et al. Reference Valdivia-Silva, Fletcher, Navarro-Gonzalez, Mckay, Perez-Montaño, Condori-Apaza and Conley2005, Reference Valdivia-Silva, Navarro-Gonzalez and Mckay2009, Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011). Importantly, the technique was capable of detecting very low levels of organics, which nearly reached the detection limits of the method. Values of carbon lesser than 8.9±2.6 ppm of LOC in Peruvian hyper-arid soils were detected in comparison to an absence of any signal of organics by Pyr–GC–MS. In addition, acid hydrolysis/permanganate only needs 1–2 g of sample during the analysis, in comparison with 200–400 g necessary by the calcination method, while still being capable of demonstrating the near absence of recalcitrant organic carbon in these types of soil (Ewing et al. Reference Ewing, Macalady, Warren-Rhodes, Mckay and Amundson2008; Valdivia-Silva et al. Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011, Reference Valdivia-Silva, Navarro-Gonzalez, Fletcher, Perez-Montano, Condori-Apaza and Mckay2012).
This approach can oxidize all organic carbon present in the soil, so the validity of the results is dependent upon the potential sources of organic carbon in the sample and the process designed to extract it.
In order to ensure that the specific protocol was designed to act principally on the LOC associated with hyper-arid soils, each of the two steps of acid hydrolysis and KMnO4 titration had to be carefully controlled in order to separate and quantify the LOC pool. The first step uses acid hydrolysis to separate the labile from the recalcitrant pools of soil organic carbon by a strong acid such as HCl, H2SO4 or HNO3 (Cheshire et al. Reference Cheshire, Mundie and Shepherd1969; Stout et al. Reference Stout, Goh, Rafter, Paul and Ladd1981; Leavitt et al. Reference Leavitt, Follett and Paul1996; Paul et al. Reference Paul, Morris, Conant and Plante2006). Specific acid hydrolysis protocols are compromises between maximum yields and quality (Oades et al. Reference Oades, Kirkman and Wagner1970), and the final protocol selected must consider the type of soil and the organic carbon fraction that is desired. In particular, concentrations of H2SO4 between 6 and 26 N have been used to separate the slow and fast pools of organic matter (Cheshire et al. Reference Cheshire, Mundie and Shepherd1969; Leavitt et al. Reference Leavitt, Follett and Paul1996; Rovira & Vallejo, Reference Rovira and Vallejo2000, Reference Rovira and Vallejo2002, Reference Rovira and Vallejo2007). While acid hydrolysis has been shown to remove proteins, nucleic acids, polysaccharides, carbohydrates and other fast pool organic carbon sources, it does not solubilize all of the cellulose or plant residues associated with the recalcitrant pool (Paul et al. Reference Paul, Follett, Leavitt, Halvorson, Peterson and Lyon1997).
Following centrifugation and removal of the soil matrix, the second step uses KMnO4 oxidation for the quantification of the organic matter. Organic matter can be oxidized by permanganate in an acid solution such as that extracted by acid hydrolysis (Vogel, Reference Vogel1978). It is well known that the action of the permanganate in an acid medium with organic compounds will oxidize a variety of functional organic groups like: Triple bonds, primary and secondary double-bonded alcohols, and ketones that are raised in the form of organic acids and which are finally converted in the process of oxide reduction in mineral CO2 (Gordon, Reference Gordon1951; Ladbury & Cullis, Reference Ladbury and Cullis1958; Siverman & Skoog, Reference Siverman and Skoog1963; Shaabani & Lee, Reference Shaabani and Lee2001; Lai & Lee, Reference Lai and Lee2002). In this case, sulphuric acid must be utilized for the acid hydrolysis as it is known that it has no action on the permanganate (Vogel, Reference Vogel1978). The interaction of the sulfuric acid with the complex organics forms ions of different composition such as C+, HSO4−, HSO3− or 2H2SO4. In this state, the organic carbon forms ions that permit the reaction with the permanganate (Sorokina et al. Reference Sorokina, Khaskov, Avdeev and Nikol'Skaya2005).
The best-known example of this is the Walkley–Black method which oxidizes soil organic matter directly in the soil using KMnO4 with a back-titration of FeSO4, and was developed in 1934 to quantify the amount of soil organic carbon (Walkley, Reference Walkley1947; Nelson & Summers, Reference Nelson, Summers and Sparks1996), and from which most variations of this approach have been developed (Paez-Osuna et al. Reference Paez-Osuna, Fong-Lee and Fernandez-Parez1984; Pauwels et al. Reference Pauwels, Wan Ranst, Verloo and Mvondoze1992; Blair et al. Reference Blair, Lefroy and Lisle1995; Haynes, Reference Haynes2000; Chan et al. Reference Chan, Bowman and Oates2001; Weil et al. Reference Weil, Islam, Stine, Gruver and Samson-Liebig2003; Lucas, Reference Lucas2004; Cabria et al. Reference Cabria, Bianchini and Mediavilla2005; Oyonarte et al. Reference Oyonarte, Mingorance, Durante, Pinero and Barahona2007). Several studies over the years have shown that the labile fraction is what is most readily oxidized in the Walkley–Black method (Lefroy et al. Reference Lefroy, Blair and Strong1993; Blair et al. Reference Blair, Lefroy and Lisle1995; Moody et al. Reference Moody, Yo and Aitken1997; Bell et al. Reference Bell, Moody, Yo and Connoly1999; Weil et al. Reference Weil, Islam, Stine, Gruver and Samson-Liebig2003; Lucas, Reference Lucas2004); however, the results of Tirol-Padre and Ladha (Reference Tirol-Padre and Ladha2004) indicated that only TOC could be attributed to these methods and that they did not correlate at all to the labile microbial biomass carbon. Lucas (Reference Lucas2004) noted that the concentration of the KMnO4 was critical to which fraction was oxidized where the lower concentration (0.02 M) as used by Weil et al. (Reference Weil, Islam, Stine, Gruver and Samson-Liebig2003) strongly correlated to the labile microbial biomass C, whereas the higher concentration (0.033 M) as used by Tirol-Padre and Ladha (Reference Tirol-Padre and Ladha2004) appears to have larger standard errors which reduce the correlation to microbial biomass C. However, in our protocol, the first step of acid hydrolysis and removal of the soil matrix by centrifugation avoids many of the complications associated with the traditional Walkley–Black method.
We designed our method utilizing these techniques in order to optimize the sensitivity to low concentrations of organic carbon in hyper-arid soils, in particular, and to avoid errors associated with impurities or organic carbon not directly from the sample itself which would induce consumption of the KMnO4. There were several specific considerations we had for the design of our protocol. Sonication was used to aid in the elimination of CO2 produced by action of the sulphuric acid on the mineral-based carbon. Heating of the acid solution containing the extracted organic carbon evaporates any remaining CO2 and O2. The temperature increase also favours the formation of acid permanganate HMnO4, a powerful oxidant, which is formed in the acid medium of the solution (Frigerio, Reference Frigerio1969; Rudakov & Lobachev, Reference Rudakov and Lobachev2000). In these conditions, the organic carbon contained in the solution reacts with the MnO4− anion forming Mn+2 and CO2 as final products. Our protocol uses 30% sulphuric acid for a short period of time at room temperature (∼20 °C), which oxidizes the functional carbon chains that are associated with the biological labile fraction. Other biological carbon associated with harder to oxidize components, such as plants and other detritus, are not oxidized as they are not exposed at a sufficiently high temperature (>80 °C), to a strong acid (>70%) or for a long-enough time (1–24 h) (Stout et al. Reference Stout, Goh, Rafter, Paul and Ladd1981; Paul et al. Reference Paul, Follett, Leavitt, Halvorson, Peterson and Lyon1997; Rovira & Vallejo, Reference Rovira and Vallejo2000). In hyper-arid samples where there are no plants or other detritus, there is no need to increase any of these factors in order to extract fractions of organic carbon from these harder to oxidize sources.
The limit of detection and the sensitivity of the method are central to the usefulness of this method. Because the concentrations of organic materials in hyper-arid soils are very low, it is important for any method to be able to detect at lower concentrations than the expected local concentrations. Several controls, as described in the methods and results, were developed in order to ensure that the reported results represent the actual concentrations.
Two alternative thermal methods, calcination and Pyr–GC–MS, were used to independently assess the accuracy of this method. The few differences between acid hydrolysis/permanganate and calcinations are due to other atoms such as N, O, S and H, which are present in organic molecules and which are consumed during the thermal process. The absence of significant differences when compared with the calcination technique corroborates the negligible presence of recalcitrant carbon in this type of soil (Ewing et al. Reference Ewing, Macalady, Warren-Rhodes, Mckay and Amundson2008). The higher values of TOC found in arid soils, using the calcination technique, are due to the presence of remains of plants and large quantities of recalcitrant carbon that permanganate cannot destroy.
On the other hand, the Pyr–GC–MS method appears to underestimate values of organic carbon in hyper-arid soils, when it was compared with the values of the other two methods (P<0.001). The results here corroborate previous studies that have demonstrated the oxidation of organic molecules during thermal analyses due to the presence and possible accumulation of minerals and/or oxidants in the soil matrix of these extreme dry environments, which transform the major percentage of organic molecules into CO2 (Navarro-Gonzalez et al. Reference Navarro-Gonzalez2006, Reference Navarro-Gonzalez, Vargas, De La Rosa, Raga and Mckay2010; Valdivia-Silva et al. Reference Valdivia-Silva, Navarro-Gonzalez and Mckay2009, Reference Valdivia-Silva, Navarro-González, Ortega-Gutierrez, Fletcher, Perez-Montaño, Condori-Apaza and Mckay2011). Indeed, a previous study using Pyr–GC–MS has shown no reliable detection of organics in agricultural soils when the level of organics were below 50,000 ppm C or in the presence of iron oxides (Schulten and Leinweber, Reference Schulten and Leinweber1993).
Conclusions
In this study, our objective was to design a rapid, portable field method for the quantification of LOC in hyper-arid soils. Previous studies were only able to analyse a few samples from each site due to the difficulty of processing samples with expensive and non-portable laboratory instrument. Field testing of natural environmental samples with this protocol was successfully completed allowing the rapid and inexpensive collection of data which increased the understanding of the role of organic materials in hyper-arid environments. While this method should not be considered as a replacement for laboratory analyses, it has the advantage of being able to quickly survey large areas in order to determine the distribution of low and high values of organic materials. This makes it extremely valuable as a real-time, strategic decision-making tool to optimize limited field time and analyses and as a means of planning for more detailed and expensive laboratory analyses.
Acknowledgements
We would like to thank Dr Benito Gomez for many years of support and access to the Yungay Desert Research Station, Chile; to Antonio Ballón, for his help in the collection of samples, and to two anonymous reviewers whose suggestions greatly improved this manuscript. Acknowledgement is given to the NASA ASTEP programme for providing partial funds in support of this research work.