Introduction
Lava tubes and caves are common features in terrestrial basaltic settings. Stable conditions in these environments lead to the formation and preservation of secondary mineral deposits, which may harbour chemical compounds produced by living organisms or derived from other biogenic organic compounds reflective of the processes by which the minerals formed or of any biological activity in the environment (such biotic chemicals will be referred to as bio/organic compounds). Understanding the abiotic and biotic physiochemical processes in terrestrial analogues may provide valuable insight into the occurrence of life on Mars (Marlow et al. Reference Marlow, Martins and Sephton2011). Lava tubes and caves on Mars would represent ideal locations to search for past evidence of aqueous and biological activity because these subsurface environments would offer protection from radiolytic degradation and diurnal temperature fluctuations (Boston et al. Reference Boston2001; Boston Reference Boston2010; Léveillé & Datta Reference Léveillé and Datta2010). Owing to the thin atmosphere and weak magnetic field, the upper few metres of the Martian surface are constantly bombarded with ultraviolet radiation, gamma-rays and high-energy galactic cosmic particles (Benner et al. Reference Benner, Devine, Matveeva and Powell2000; Badhwar Reference Badhwar2004; Martinez-Frias et al. Reference Martinez-Frias, Amaral and Vazquez2006; Parnell et al. Reference Parnell2007). As the Martian surface is considered an inhospitable environment for life, the Martian subsurface may provide more favourable physiochemical conditions for growth and preservation of bio/organic compounds (Boston et al. Reference Boston2001). Thus, the Martian subsurface, including caves, may offer the most stable environment to harbour life and the best chance to observe evidence of extinct or even extant life (Northup et al. Reference Northup, Melim, Spilde, Hathaway, Garcia, Moya, Stone, Boston, Dapkevicius and Riquelme2011).
Owing to the weaker Martian gravity allowing for less gravitation collapse, lava tubes and caves are widely considered to be more common features on Mars than their occurrence in terrestrial basaltic environments (Léveillé & Datta Reference Léveillé and Datta2010). Indeed, Mars lava tubes and related features have been observed by Martian orbiters (Wyrick et al. Reference Wyrick, Ferrill, Morris, Colton and Sims2004; Léveillé & Datta Reference Léveillé and Datta2010). Imagery from Mars Odyssey, Mars Global Surveyor, Mars Express and Mars Reconnaissance Orbiters have shown features due to the presence of ‘skylights’ that originate from the partial collapse of lava channels and pit ceilings (Wyrick et al. Reference Wyrick, Ferrill, Morris, Colton and Sims2004; Keszthelyi et al. Reference Keszthelyi, Jaeger, McEwen, Tornabene, Beyer, Dundas and Milazzo2008; Léveillé & Datta Reference Léveillé and Datta2010). At visible and infrared wavelengths, the Thermal Emission Imaging System aboard the Mars Odyssey Orbiter revealed cave openings measuring between 100 and 250 m in diameter (Cushing et al. Reference Cushing, Titus, Wynne and Christensen2007). These caves are located in the Tharsis shield volcano region, which is considered to be morphologically and topographically similar to the basaltic shields of the Eastern Snake River Plain in Idaho, USA (Sakimoto et al. Reference Sakimoto, Gregg, Hughes and Chadwick2003).
Craters of the Moon National Monument and Preserve (COM) are located along the northern flank of the Eastern Snake River Plain in southern Idaho. It is composed of more than 60 individual Holocene-aged basaltic flows (Kuntz et al. Reference Kuntz, Champion, Spiker and Lefebvre1986, Reference Kuntz, Covington, Schorr, Link, Kuntz and Platt1992; Reid Reference Reid1995). COM basalts are enriched in Si, Fe and alkali elements relative to basaltic flows elsewhere in the Eastern Snake River Plain (Hughes et al. Reference Hughes, Smith, Hackett, Anderson, Hughes and Thackray1999). These differences in elemental composition putatively reflect larger degrees of crustal assimilation and/or fractionation (Leeman et al. Reference Leeman, Vitaliano and Prinz1976). Mineralogical and bulk chemical comparisons between COM and Martian basalts show similar compositions, especially in their high Fe concentrations (Hughes et al. Reference Hughes, Smith, Hackett, Anderson, Hughes and Thackray1999; Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). However, COM basalts are slightly higher in Al, P, Ti, Na, and K and lower in alkali-earth elements and S than their Martian counterparts (Kuntz et al. Reference Kuntz, Champion, Spiker and Lefebvre1986; Hughes et al. Reference Hughes, Smith, Hackett, Anderson, Hughes and Thackray1999; Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Despite these differences, COM basalts are still closer in chemical composition to Martian basalts than other frequently used terrestrial analogues, which suffer similar limitations and also have much lower Fe concentrations. In addition to a potentially similar basaltic chemical composition between COM and Mars, COM also has an extensive array of lava tubes and caves similar to those observed on Mars. Of these subsurface features at COM, several locales have secondary mineral deposits that have been identified as hydrous and anhydrous Na-sulphate species (Stearns Reference Stearns1963; Peck Reference Peck1974; Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012).
The importance of Na-sulphate minerals has been recognized by the astrobiological community since their discovery in several planetary bodies throughout the solar system (Hall & Hamilton Reference Hall and Hamilton2008). They are a major surficial component on the icy moon of Europa (McCord et al. Reference McCord1998, Reference McCord1999; Kargel et al. Reference Kargel, Kaye, Head, Marion, Sassen, Crowly, Ballesteros, Grant and Hogenboom2000; Fanale et al. Reference Fanale, Li, De Carlo, Farley, Sharma, Horton and Granahan2001; Zolotov & Shock Reference Zolotov and Shock2001), and visible and infrared spectroscopic investigations have suggested that they may be present in the Martian regolith (Zhu et al. Reference Zhu, Xie, Guan and Smith2006; Kargel et al. Reference Kargel, Furfaro, Prieto-Ballesteros, Rodriguez, Montgomery, Gillespie, Marion and Wood2007; Mangold et al. Reference Mangold, Gendrin, Gondet, LeMouelic, Quantin, Ansan, Bibring, Langevin, Masson and Neukum2008). Moore et al. (Reference Moore, Bullock, Newsom and Nelson2010) have suggested that Na-sulphate was likely precipitated on Mars in the past when the atmosphere was more acidic. In addition, anhydrous Na-sulphate (thenardite) is a proven host mineral in direct detection of bio/organic compounds using laser desorption Fourier transform ion cyclotron resonance mass spectrometry (LD-FTICR-MS) (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008, Reference Richardson, Hinman and Scott2009). The role of microbial activity in mineralization of Na-sulphates in the basaltic subsurface of COM and the ability of these minerals to assist in direct detection of bio/organic compounds has implications for the search for life on Mars, especially considering the potential future use of laser desorption mass spectrometry instruments that could search for similar bio/organic compounds on various space missions (Evans-Nguyen et al. Reference Evans-Nguyen, Becker, Doroschenko and Cotter2008; Becker et al. Reference Becker, Cornish, Antione, Cotter, Evans-Nugyen, Doroschenko, Goesmann, Raulin and Ehrenfreund2009; Kolleck et al. Reference Kolleck, Büttner, Ernst, Hülsenbusch, Lang, Marwah, Mebben, Priehs, Kracht and Neumann2010; Richardson & Becker Reference Richardson and Becker2010; Scott et al. Reference Scott, Beardsley, Groenewold, Lammert, Lee, McJunkin, Ritchie, Almirall and Becker2012).
Several past hypotheses have tried to explain the unusual occurrence of secondary sulphate deposits within the basaltic subsurface of COM. Stearns (Reference Stearns1963) suggested a fumarolic origin, which might explain the isolated jarosite deposits found in two spatter cones at COM, but fails to explain the formation of more soluble and low-temperature secondary minerals (i.e. mirabilite, thenardite and burkeite). Peck (Reference Peck1974) hypothesized that the deposits were formed through groundwater infiltration followed by subsequent deposition in open cavities on the cave floors. A combination of fumarolic activity and subsequent groundwater leaching and redeposition was introduced as a third possible scenario for mineral precipitation (Karlo et al. Reference Karlo, Jorgenson and Shineldecker1980); however, this hypothesis still suffers the same limitations as its predecessors. None of these hypotheses were further explored using detailed analytical techniques or subsequent field observations.
In our previous work, we have characterized the COM basalts and secondary Na-sulphate deposits with field observations along with Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), LD-FTICR-MS, electron probe microanalysis, X-ray fluorescence and geochemical modelling exercises (Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Based on the characterization and geochemical modelling, an abiotic explanation for formation of thenardite from leaching of basalt by rainwater and subsequent precipitation was presented. Geochemical modelling based on leaching water collected near the COM secondary deposits and bulk composition of the host basalt showed that calcite and sodium heptahydrate (NaSO4·7H2O) are the only species that reach saturation (Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). In cold dry environments, such as the surface of Mars, heptahydrate should be the stable species but can quickly dehydrate or hydrate upon slight changes in temperature and water vapour pressure to form thenardite or mirabilite, respectively (Hall & Hamilton Reference Hall and Hamilton2008).
In this work, we extend our characterization to explore potential biotic influences in the formation of secondary Na-sulphate deposits found within the basaltic subsurface of COM by further analysis with scanning electron microscopy (SEM), XRD, sulphur isotope ratio mass spectrometry (IRMS), LD-FTICR-MS and FTIR.
Materials and methods
Mineral collection
Secondary deposit samples were collected biannually between June 2006 and October 2008 as detailed in Richardson et al. (Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Samples taken from the cave floors were collected from the interior of the deposits to limit any possible mammalian influence on the samples. The precipitates were carefully transferred to glass scintillation vials with conical polymer liners to ensure that no contaminants were introduced. Samples were then transferred to sealed storage containers in order to maintain ambient humidity and temperature during transport and storage. Owing to safety regulations, COM park officials did not grant access to the two spatter cones, Crystal Pit and Snow Cone Pit. As a result, all samples from these spatter cones were obtained through subsampling from the mineral archive at COM Park Headquarters. The Crystal Pit and Snow Cone Pit samples were originally collected by the Environmental Science and Research Foundation.
SEM with energy dispersive X-ray (EDX)
High resolution SEM was performed using a Hitachi S-4700 Field Emission SEM with EDX (Gresham Sirius 30) capabilities for elemental analysis. Samples were sputter coated with carbon multiple times to provide sufficient conduction. Data were analysed with Quartz XOne software.
X-ray diffraction
XRD was performed using a PANalytical X'Pert PRO XRD (Almelo, The Netherlands). Samples were prepared by grinding and then random-mounted on glass slides. Data were collected using X'Pert Data Collector software and analysed using X'Pert HighScore Plus. Diffraction patterns were identified by comparison with published mineral patterns from the International Center for Diffraction Data (ICDD).
Isotope ratio mass spectrometry
Evidence of sulphur fractionation between the secondary deposits and the host basalts was obtained using a continuous flow stable IRMS by DPRA-Zymax Industries (Escondido, CA). Eleven secondary mineral samples, from the three sampling locations, were analysed and compared with two host basalts collected from the Wilderness Caves area and on the flank of Crystal Pit Spatter Cone (Fig. 1(a)). Sulphur isotope ratios are reported in the conventional δ-notation, expressed as ‰ (error is ±0.2‰) deviation relative to Vienna Canyon Diablo Troilite (VCDT).

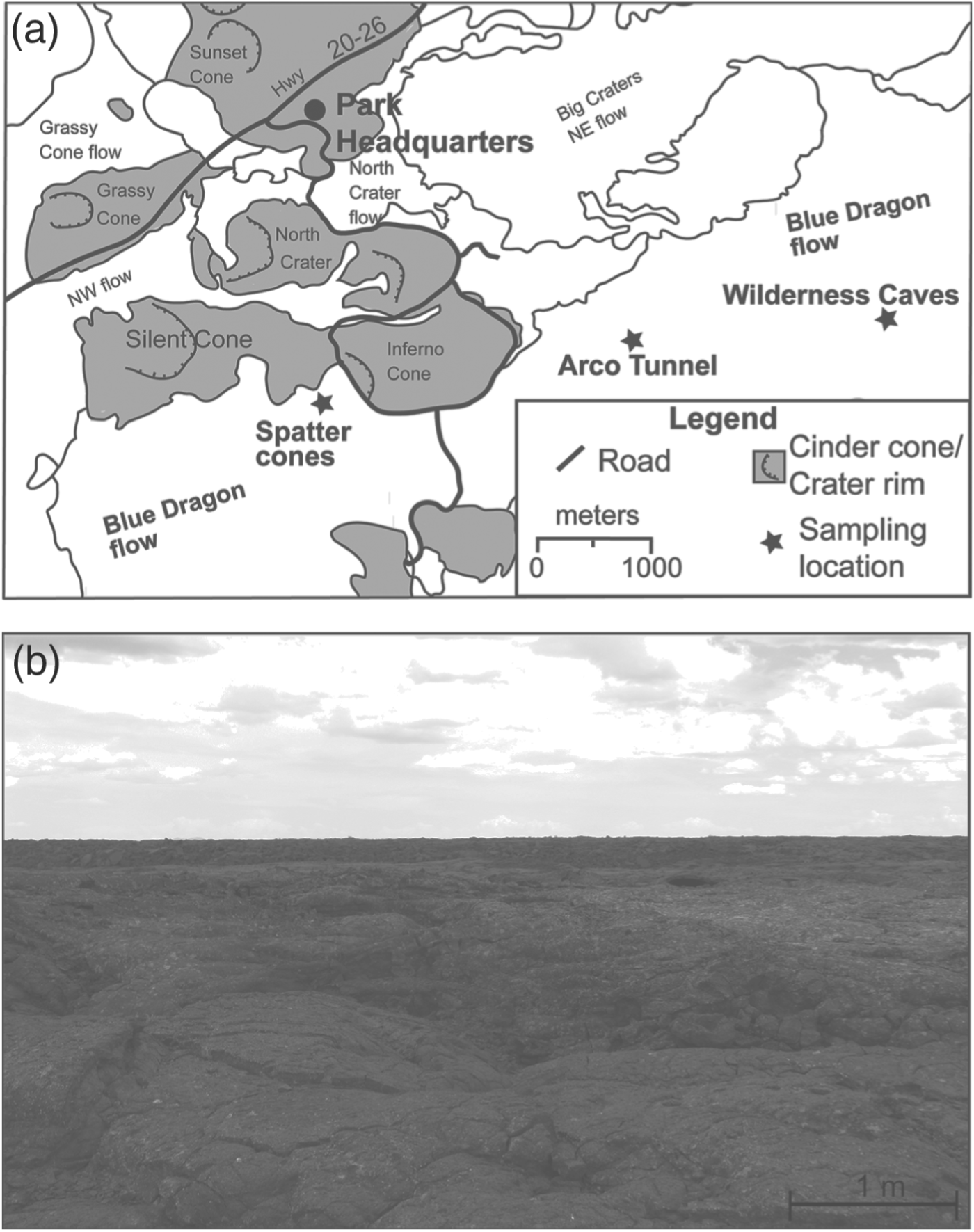
Fig. 1. (a) Map of COM lava field showing cave locations in Blue Dragon flow. Map modified from Kuntz (Reference Kuntz1989) with permission of American Geophysical Union. (b) Photograph of the COM surface terrain.
Fourier transform ion cyclotron resonance mass spectrometry
Spectra of the secondary sulphate deposits were obtained using a laboratory-built imaging LD-FTICR-MS (McJunkin et al. Reference McJunkin, Tremblay and Scott2002; Scott & Tremblay Reference Scott and Tremblay2002; Scott et al. Reference Scott, McJunkin and Tremblay2003) equipped with a 7T Oxford (Oxford, England) superconducting magnet. Instrument parameters have been previously described (Yan et al. Reference Yan, Stoner, Kotler, Hinman and Scott2007a, Reference Yan, Stoner and Scottb; Kotler et al. Reference Kotler, Hinman, Yan, Stoner and Scott2008; Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008). All spectra were acquired from single laser shots. Peak identification was accomplished using the Odyssey Interpreter Software (Thermo-Finnigan FT/MS, Bremen, Germany) and following a systematic approach outlined by Kotler et al. (Reference Kotler, Hinman, Yan, Stoner and Scott2008).
Fourier transform infrared spectroscopy
Attenuated total reflectance Fourier transform infrared spectroscopy spectra were obtained using a Thermo Nicolet Nexus 670 FTIR spectrometer (Madison, WI). Spectra were collected using an average of 100 scans with a resolution of 4 cm−1. All spectra were measured in absorbance between 2500 and 500 cm−1.
Results and discussion
Description of subsurface features at COM
Of the 60 basaltic flows that compose COM, the young Blue Dragon flow (∼2.1 ka) contains the majority of accessible lava tubes and caves. This high abundance of lava tubes and caves is because of the young age of the flow, as it has had less time to undergo gravitational collapse. As a result, the Blue Dragon flow hosts a majority of the secondary sulphate deposits found at COM; this is probably the result of its morphological characteristics rather than any chemical differences from adjacent COM flows (Stout et al. Reference Stout, Nicholls and Kuntz1994; Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012).
Three locations within the Blue Dragon flow (Wilderness Caves, Arco Tunnel and Spatter Cones) were chosen for mineral sampling based on mineral occurrence, mineral abundance, cave accessibility and lack of public access. The extent of the Blue Dragon flow and cave locations used in this study is shown in Fig. 1(a). A representative photograph (Fig. 1(b)) shows the absence of surface vegetation at the Blue Dragon flow. The Wilderness Caves area is a collection of roughly six small lava tubes and caves covering an area ∼0.13 km2. Approximately 2 km to the west of the Wilderness Caves area is the branching lava tunnel of Arco Tunnel. Arco Tunnel is composed of a series of connected lava tunnels that extend for over 1 km. The final sampling location comes from the two hollow magma chambers located within adjacent spatter cones (Crystal Pit and Snow Cone Pit). These spatter cones are approximately two and half kilometres west of Arco Tunnel on the western boundary of the Blue Dragon flow. These magma chambers are only accessible through narrow <20 m vertical throats (Peck Reference Peck1974). The hollow magma chambers of Crystal and Snow Cone Pit are only two of three such formations accessible in North America known to the authors because similar magma chambers tend to be inaccessible due to lava infill, weathering and/or ice plugs.
Description of secondary deposits
The secondary Na-sulphate minerals were found intermittently dispersed as localized, efflorescent, white and powdery deposits in small cavities on the ceilings and walls as well as mounds on the floors and are described in detail in Richardson et al. (Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012); thus, only a brief summary is provided here. The secondary deposits appear to be seasonal because the size and amount of each mineral deposit varied on a semi-annual basis. The depths of floor deposits ranged between 1 and 10 cm (Fig. 2) and are not considered a direct weathering product of the host basalts because the authigenic mineral-cave floor interface was quite sharp with no mineralogical or gradient into the underlying basalt. Secondary deposits were also found infilling small cavities and cracks on cave ceilings and walls. No correlation was observed between the ceiling and floor deposits.

Fig. 2. Photograph of thenardite deposit in a wall cavity inside Arco Tunnel.
Figure 3(a) shows an SEM image of a secondary deposit. The deposits are primarily composed of small crystals with a few larger, needle-like crystals. Given that organic film fragments have been detected on other evaporite minerals (Galeev et al. Reference Galeev, Vinokurov, Mouraviev and Osin2009), SEM images were acquired of the COM thernadite samples; however, no discernible features suggestive of organic film were observed. The EDX spectrum of the elements present is provided in Fig. 3(b) and shows the presence of Na, S, O, Ca and C. XRD analysis of the sample showed that it is predominantly thenardite when compared with published ICDD data (Fig. 3(c)). The carbon present in the EDX spectrum is probably due to the presence of Na-carbonate species such as burkeite (Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012).

Fig. 3. (a) SEM image of efflorescent secondary mineral deposit. (b) EDX spectrum showing elements associated with deposit. (c) Background corrected XRD pattern of secondary mineral demonstrating that it is primarily thenardite.
Sulphate formation
The secondary sulphate deposits probably form in part from the primary weathering of sulphidic minerals from accessory pyrite and possibly from basaltic glass from the overlying host basalt. Sulphur concentration in the Blue Dragon flow was reported to be ∼0.06 wt% (Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). The specific factors controlling the kinetics and mechanisms of sulphide oxidation to sulphate are still poorly understood. Oxidation of pyrite may be oxidized by either molecular oxygen or ferric iron and is typically very slow under abiotic conditions. However, micro-organisms can accelerate this process by at least six orders of magnitude (Sampson et al. Reference Sampson, Phillips and Ball2000; Pisapia et al. Reference Pisapia, Chaussidon, Mustin and Humbert2007). Pyrite oxidation is complicated and involves many intermediate sulphur species with oxidation states between −I and +VI (McGuire et al. Reference McGuire, Edwards, Banfield and Hamers2001; Heidel & Tichomirowa Reference Heidel and Tichomirowa2011). The equations below summarize the general oxidation mechanism of pyrite without intermediate reactions (Singer & Stumm Reference Singer and Stumm1970):
Sulphur isotope analysis was performed to further explore the transformation of pyrite and help determine potential involvement of microbes in formation of the deposits by identifying preferential fractionation of sulphur isotopes.
Sulphur fractionation
Sulphur isotopic systems have been suggested as a potential means for searching for signs of life on Mars by Parnell et al. (Reference Parnell, Boyce, Osinski, Izawa, Banerjee, Flemming and Lee2012), especially sulphates that are prolific on the Martian surface. The oxidation of sulphidic minerals to sulphate through a series of intermediate species represents an important energy-yielding pathway for endolithic micro-organisms. Iron and sulphur species are two of the most prevalent redox active elements, acting as an important metabolic energy source for sulphur oxidizing organisms (Balci et al. Reference Balci, Shanks, Mayer and Mandernack2007; Philippot et al. Reference Philippot, Van Zuilen, Lepot, Thomazo, Farquhar and Van Kranendonk2007; Wacey et al. Reference Wacey, Saunders, Brasier and Kilburn2011). As FeS2 is one of the most abundant metal sulphide minerals in basaltic rocks, it is considered the main source of reduced sulphur in COM basalts. Although sulphur isotopes have been used to track bio-oxidation in many settings (Peterson Reference Peterson1999; Bottrell & Newton Reference Bottrell and Newton2006; Peters et al. Reference Peters, Strauss and Farquhar2009, Reference Peters, Strauss, Farquhar, Ockert, Eickmann and Jost2010; Zerkle et al. Reference Zerkle, Farquhar, Johnston, Cox and Canfield2009; Takai & Nakamura Reference Takai and Nakamura2011), little is known about the direct oxidation pathways and mechanism of bio-oxidation of sulphidic minerals in basalts. Analysis of ∂34S fractionation values between the host basalt and the secondary deposits values was performed in hopes it would give an insight into the oxidation pathways and the role of micro-organisms in formation of the deposits.
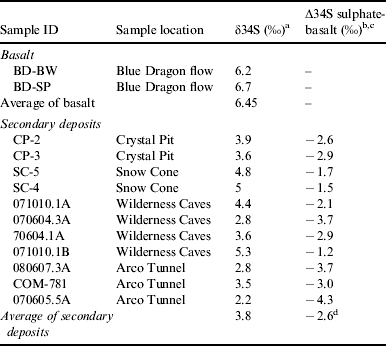
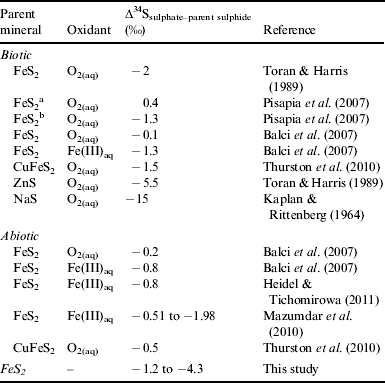
Sulphur fractionation values between the secondary sulphate deposits and the host basalt (∆34SSO4-b) are shown in Table 1. The largest ∆34SSO4-b depletion was −4.3‰, with an average depletion of −2.6‰ (n=12, 1σ). No correlation with the cave location or within individual caves was observed regarding ∂34S values. This lack of spatial correlation may imply that similar bio-oxidation pathways occur throughout the sampling area. These small but significant differences may imply biological oxidation as abiotic sulphide oxidation fractionation values typically are unidirectional and are generally indistinguishable from their parent mineral (Rye et al. Reference Rye, Bethke and Wasserman1992; Taylor & Wheeler Reference Taylor, Wheeler, Alpers and Blowes1994; Lefticariu et al. Reference Lefticariu, Pratt and Ripley2006; Balci et al. Reference Balci, Shanks, Mayer and Mandernack2007; Zerkle et al. Reference Zerkle, Farquhar, Johnston, Cox and Canfield2009) Oxidation is likely to proceed by biotic mechanisms because the fractionation observed in the COM samples (Table 1) exceeds reported abiotic values (Table 2). Previous biotic oxidation experiments of sulphidic minerals have yielded slightly more depleted ∂34S fractionation values than the abiotic values and reflect the ∆34SSO4-b values observed from the COM secondary deposits in a better manner.
Table 1. Sulphur isotopic values of secondary deposits and host basalts

a ±0.2‰ error.
b δ34S=[(34S/32S)sample/(34S/32S)VCDT]×1000.
c Average of basalt δ34S values used.
d Standard deviation=1.0.
Table 2. Literature compilation of biotic and abiotic sulphur fractionation for oxidation of sulphidic minerals

a Non-stoichiometric pathway.
b Stoichiometric pathway.
Bio-oxidation of pyrite involves several intermediate species, such as polythionates (SxOy2−) and elemental sulphur. During bio-oxidation, disproportionation micro-organisms utilize these intermediate compounds as both electron donors and acceptors and show no preferential uptake between the sulphur isotopes. This results in largely negligible ∂34S values between sulphate and sulphide (Habicht et al. Reference Habicht, Canfield and Rethmeier1998; Smock et al. Reference Smock, Bottcher and Cypionka1998; Bottcher et al. Reference Bottcher, Thamdrup and Vennemann2001; Finster Reference Finster2008). The presence of disproportionating bacteria may cause ∂34S values from the secondary deposits to remain near the host basaltic values. Depletion in ∆34SSO4-b may imply biological activity, however, it is possible that these fractionation values may be due to stoichiometric (breaking of ionic S-FeS2 bonds) abiotic oxidation. More extensive experiments would be required to elucidate the exact role of microbial activity and detailed bio-oxidation pathways involved in the formation of secondary sulphate deposits.
Presence of bio/organic compounds
Biological activity associated with mineral deposits can be inferred through various methods, including chemical or isotopic signatures, which can be preserved in the minerals (Boston et al. Reference Boston2001). The secondary deposits at COM were investigated for chemical and isotopic biological signatures, which could suggest direct or indirect biological involvement in mineralization of the Na-sulphate deposits. The presence of bio/organic compounds in the secondary minerals was determined using geomatrix-assisted laser desorption/ionization (GALDI) (Yan et al. Reference Yan, Stoner, Kotler, Hinman and Scott2007a). This technique uses a mineral matrix to assist in desorption and ionization of bio/organic compounds with little to no sample preparation. The ability of minerals to facilitate in the desorption and ionization of bio/organic compounds is a primary focus in previous studies of GALDI-FTICR-MS as a viable technique for bio/organic compound detection (Yan et al. Reference Yan, Stoner, Kotler, Hinman and Scott2007a, Reference Yan, Stoner and Scottb; Kotler et al. Reference Kotler, Hinman, Yan, Stoner and Scott2008; Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008, Reference Richardson, Hinman and Scott2009). Previous studies using synthetic and natural thenardite have demonstrated the ability of thenardite to assist in the ionization and detection of bio/organic compounds down to detection levels of 3 ppt (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008, Reference Richardson, Hinman and Scott2009).
To assist in identification of any associated bio/organic peaks in the mass spectra from the COM secondary deposits, the spectra were compared with a suite of FTICR-MS standard spectra of inorganic thenardite, Na-carbonate (trona, natron), and physical combinations between these Na-sulphate and Na-carbonate minerals (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008, Reference Richardson, Hinman and Scott2009, Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). All spectra of the standard Na-sulphate and Na-carbonate minerals had peaks with mass defects (i.e. the non-integer value after the decimal point) suggestive of inorganic constituents. Mass defects must be a linear sum of the mass defects of individual elemental components. Common non-hydrogen elements associated with bio/organic compounds have mass defects near 0.00 u (e.g. 12C at 12.000 u, 16O at 15.995 u). Hydrogen (at 1.008 u) tends to dominate the mass defects of bio/organic compounds because there are usually twice as many hydrogen atoms as other elements. Such distinction between an inorganic and organic ion based on the peak's mass defect is easily achieved when the ion has a mass-to-charge (m/z) ratio less than 400, because inorganic ions tend to have mass defects that are near to or would round up to next nominal integer mass, whereas bio/organic compounds tend to have mass defects that would round down to next integer mass. As the m/z rises above ∼400, the trends in mass defects for bio/organic and inorganic ions tend to shift (e.g. organic ions accumulate enough hydrogen atoms causing the mass defect to appear to indicate inorganic elements as the m/z value approaches the next integer).
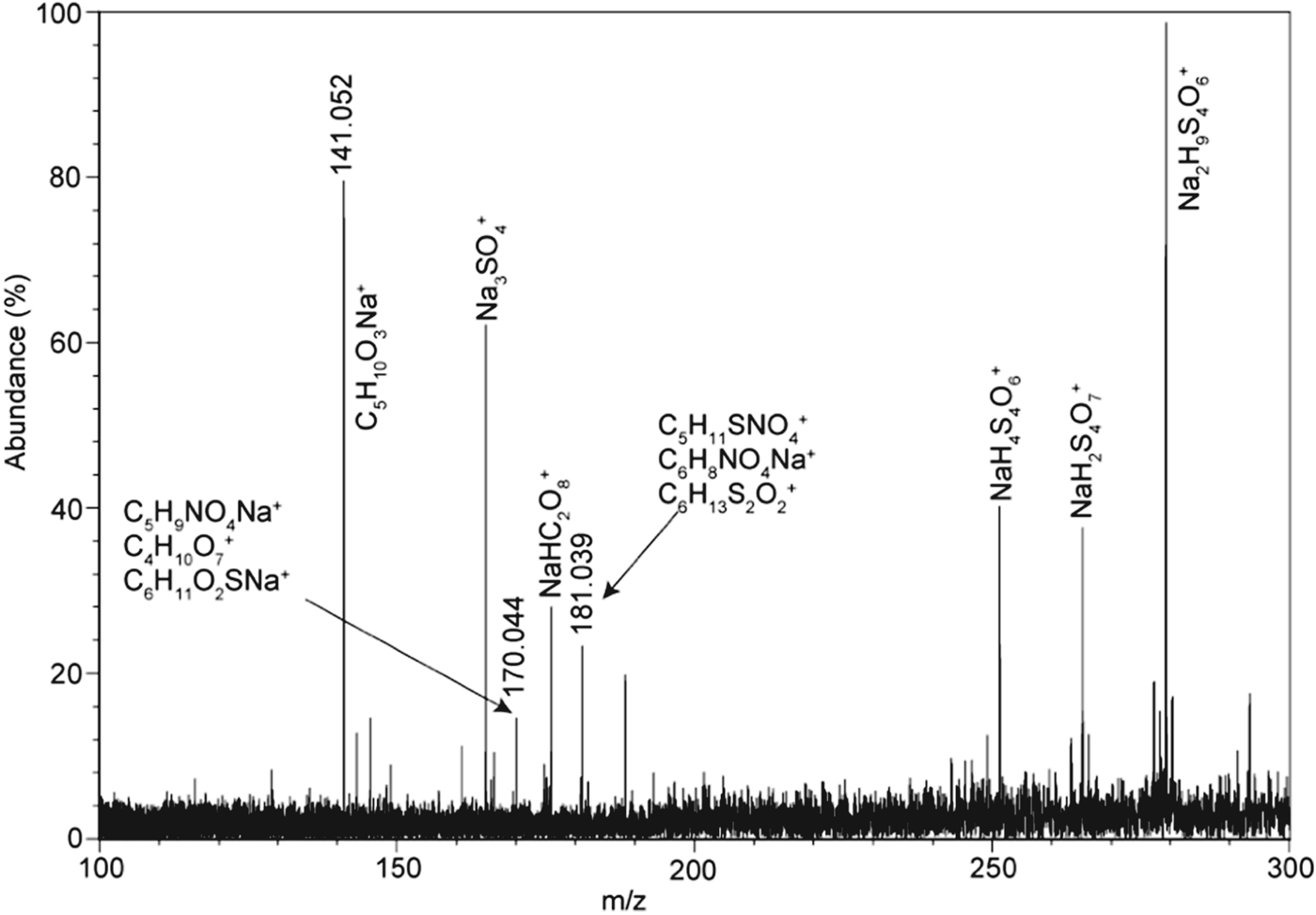
Figure 4(a) shows a two-dimensional LD-FTICR-MS map where each circle represents a spectrum obtained from a single laser shot. The spectra represented by the open circles in the map only had peaks indicative of inorganic species. The coloured or shaded circles indicate the occurrence of peaks suggestive of bio/organic compounds. The heterogeneous distribution of inorganic and organic peaks in the map is common to all COM secondary deposits analysed. A representative negative mode spectrum from a secondary deposit collected from a wall cavity in Arco Tunnel is shown in Fig. 4(b). The inorganic peaks observed in the spectrum have been reported in previous studies of Na-sulphate spectra (Poels et al. Reference Poels, Van Vaeck and Gijbels1998; Van Vaeck et al. Reference Van Vaeck, Adriaens and Adams1998; Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008, Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Peaks at m/z 183.081 and 339.040 (Figs. 4(c) and (d), respectively) have mass defects suggestive of bio/organic compounds. These peaks are related to bio/organic compounds because of the (1) absence of the peaks in synthetic inorganic Na-sulphate and Na-carbonate standards, (2) mass defects suggestive of bio/organic elements, and (3) isotopic distributions that correspond to the theoretical isotopic distribution of suggestive bio/organic formulas. The bio/organic related peaks are an example of complex cluster ions, similar to that reported for glycine with jarosite (Kotler et al. Reference Kotler, Hinman, Yan, Stoner and Scott2008) and stearic acid with thenardite (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008). Using a systematic procedure based on the mass defects and isotopic distributions outlined by Kotler et al. (Reference Kotler, Hinman, Yan, Stoner and Scott2008), the most probably composition for the peaks at m/z 183 and 339 are C8H16SONa− and C10H20S3O5Na−, respectively. The occurrence of these complex cluster ions is not unusual, as cluster ions often form because of complex reactions in the laser desorption plume or in the gas phase (Karas et al. Reference Karas, Bachmann and Hillenkamp1985; Knochenmuss et al. Reference Knochenmuss, Dubois, Dale and Zenobi1996; Budimir et al. Reference Budimir, Blais, Fournier and Tabet2007). These reactions lead to ions larger than the expected molecular ion due to formation of adducts from the addition of matrix components and/or analyte species (Knochenmuss et al. Reference Knochenmuss, Dubois, Dale and Zenobi1996; Karas & Kruger Reference Karas and Kruger2000; Ham et al. Reference Ham, Durham and Scott2003; Budimir et al. Reference Budimir, Blais, Fournier and Tabet2007). The exact identification of the original bio/organic compound was unobtainable as it is quite difficult to ascertain without undertaking a systematic experiment using a variety of bio/organic compound/thenardite combinations. Such a systematic experiment was conducted to identify that the peak at m/z 390.260 observed in evaporitic thenardite samples from Searles Lake, CA originated from the presence of stearic acid (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008), which was also observed in the COM samples (Table 3).

Fig. 4. (a) LD-FTICR-MS chemical pseudo-image showing heterogeneity of chemical composition within the sample. Colored circles indicate areas with potential organic compounds: green for m/z 183, orange for m/z 339 and black for a mix of m/z 183 and 339. Blank circles are locations with peaks indicative of inorganic only compounds. (b) Negative mode laser desorption ion FTICR-MS spectrum of secondary sulphate deposits. Peaks at (c) m/z 183 and (d) m/z 339 have mass defects and isotopic distributions suggestive of bio/organic cluster ions.
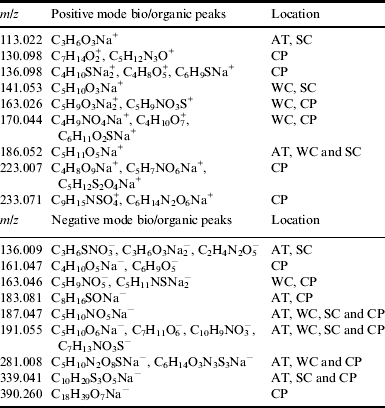
Table 3. Suggestive chemical formulae for bio/organic peaks associated with the secondary sulphate deposits observed in the LD-FTICR-MS spectra

AT, Arco Tunnel; WC, Wilderness Caves; SC, Snow Cone Pit; CP, Crystal Pit.
A representative positive mode spectrum (Fig. 5) shows several high mass peaks, most of which are easily identified as inorganic based on their mass defects (values were generally >0.5 u), their occurrence in the inorganic standard spectra and inorganic peaks (such as m/z 165 and 181) that have been previously reported in Na2SO4 spectra (Poels et al. Reference Poels, Van Vaeck and Gijbels1998; Van Vaeck et al. Reference Van Vaeck, Adriaens and Adams1998; Ignatova et al. Reference Ignatova, Van Vaeck, Gijbels and Adams2003; Richardson et al. Reference Richardson, Hinman and Scott2009). Close inspection revealed that the peaks at m/z 141.052 and 170.044 have mass defects suggestive of bio/organic compounds and are absent in the spectra of the inorganic standards. Peak identification in the positive spectra was more difficult because the minor isotopic peaks were less distinct because of poor signal-to-noise (i.e. low abundance). However, peak identification was obtained by comparing the observed mass defects with theoretical mass defects. Using this method, the peak at m/z 141.052 was found to have only one likely composition with a theoretical weight of 141.053 u, corresponding to a formula of C5H10O3Na+. Under the FTICR-MS parameters used, the mass accuracy is ∼0.003 u with a resolution of 10 000. Precise identification of the peak at m/z 170.044 was unobtainable because several likely formulae could account for the mass defect. However, this peak is considered to be from a bio/organic compound because of its mass defect and its absence in the inorganic Na-sulphate and Na-carbonate spectra. The formula C5H10O3Na+ could correspond to a small molecular or fragment ion similar in structure to diethyl carbonate, ethyl lactate, or hydroxyvaleric acid that have a C=O group as a potential site for cation attachment of Na+.

Fig. 5. Positive mode LD-FTICR-MS spectrum of a secondary sulphate deposit from a floor deposit within a cave in the Wilderness Caves area.
A number of complex bio/organic or organic cluster ions are seen in the positive and negative spectra of the secondary deposits. A comprehensive list of these positive and negative mode peaks and their suggestive chemical formulae is shown in Table 3. Approximately 30% of these suggestive formulas had C : H : O ratios that resemble ratios observed in lipids, sugars, and/or amino acids. Unfortunately, many of the suggestive formulas indicate that the ions may result from gas-phase reactions in the laser desorption or ablation plume. These gas-phase reactions can lead to unusual chemical formulae. Previous FTICR-MS studies have yielded similar gas-phase reactions with glycine mixed with jarosite (Kotler et al. Reference Kotler, Hinman, Yan, Stoner and Scott2008) and stearic acid with thenardite (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008).
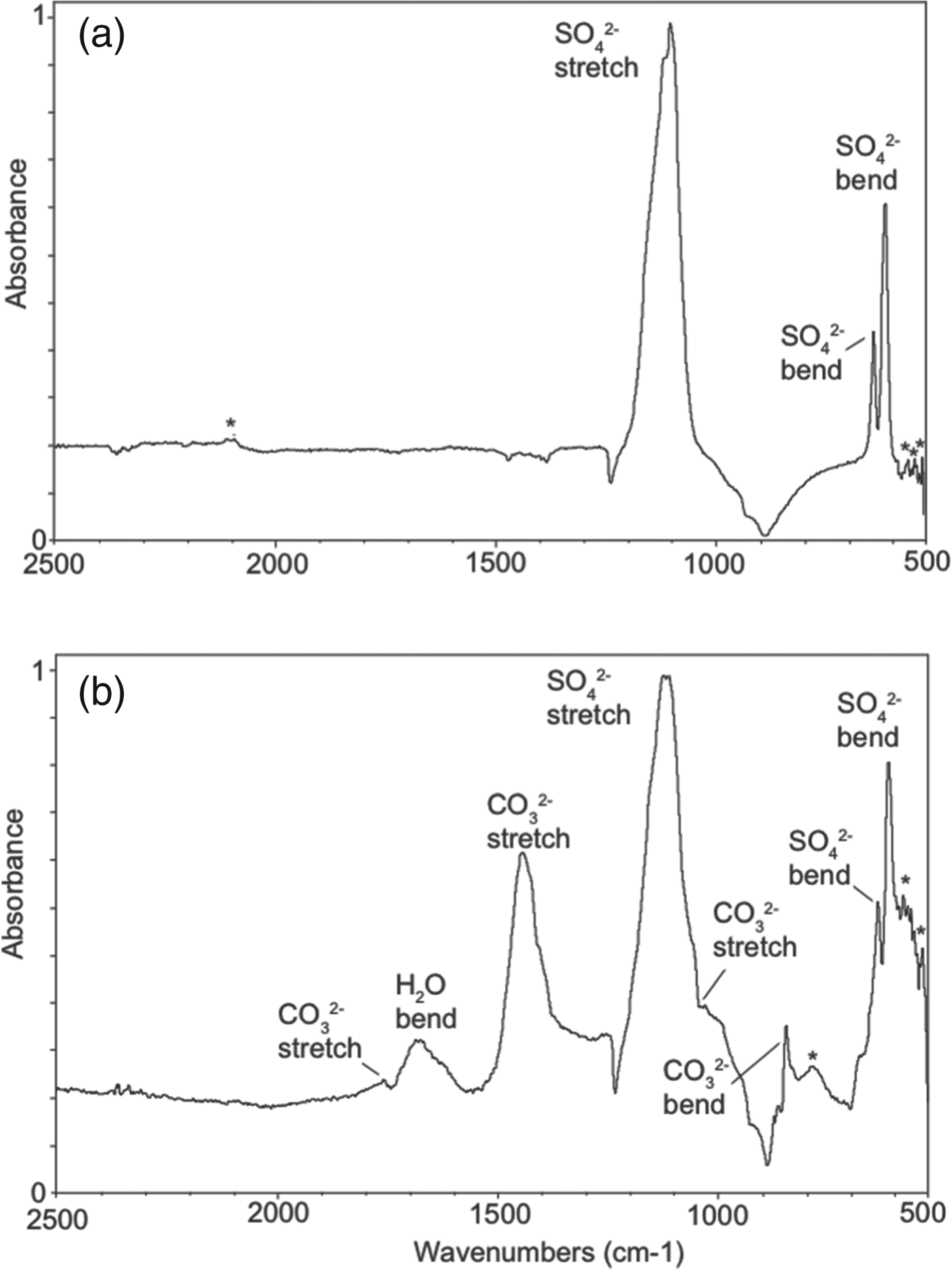
Additional evidence for associated bio/organic compounds in the secondary deposits was gathered using FTIR techniques because Bullen et al. (Reference Bullen, Oehrle, Bennett, Taylor and Barton2008) have shown that FTIR can detect low levels of metabolic compounds on minerals associated with caves. Even though FTIR does not typically have the sensitivity (∼0.1 wt%) (Materials Characterization Lab 2012) that is possible with LD-FTICR-MS (i.e. 3 ppt) (Richardson et al. Reference Richardson, Hinman, McJunkin, Kotler and Scott2008), several bands were observed that support the presence of organic compounds. Representative FTIR spectra are shown in Fig. 6. The inorganic bands in Fig. 6(a) are representative of thenardite; a more detailed description of these bands can be found in Richardson et al. (Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Evidence of organic-related bands can be seen at 559, 545 and 536 cm−1, which are the characteristics of stretching and rocking of singly bonded carbon compounds (Smith Reference Smith1999). In Fig. 6(b), the prominent bands result from a combination of sulphate and carbonate oxyanions (Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Similar to Fig. 4(a), several bands could represent organic compounds. The bands at 536 and 580 cm−1 are representative of singly bonded organic compounds (Smith Reference Smith1999). In addition, the band at 785 cm−1 is reminiscent of a C–H stretch associated with aromatic organic compounds (Coates Reference Coates and Meyers2000).

Fig. 6. Representative FTIR spectra of secondary deposits. (a) FTIR spectrum dominated by sulphate oxyanion, whereas FTIR spectrum (b) shows additional bands corresponding to carbonate oxyanion. Both spectra show bands suggestive of organic compounds (indicated by *).
Potential sources of bio/organic compounds
There are several potential sources that might account for the presence of bio/organic compounds in the secondary mineral deposits, including accidental emplacement of compounds and microbial activity. Emplacement of extraneous bio/organic compounds into the precipitates may be due to (1) plant detritus moving downward through the basalts via cracks and fissures with subsequent deposition with the secondary deposits, (2) organic aerosols carried through the cave openings and (3) post-depositional interaction with cave animals. Plant detritus is not likely because surface vegetation is extremely sparse in the Blue Dragon flow (Fig. 1(b)). Transportation of organic aerosols in the caves and their subsequent emplacement on the ceiling and floor deposits seems highly unlikely, especially considering the extent and complexity of some of the subsurface features (i.e. Arco Tunnel). Post-depositional interaction with cave animals is also unlikely to produce the detected organic compounds, especially for ceiling and wall deposits. To limit the possibility post-depositional interaction with cave animals, mineral samples were retrieved from the interior of the deposits rather than from the surfaces. Furthermore, no evidence of animal interaction was found near the secondary deposits. Microbial activity occurring in or around the basalt is another potential source of bio/organic compounds.
Proposed mechanisms for secondary deposits
A plausible explanation for the presence of secondary Na-sulphate deposits is the abiotic and biotic physiochemical weathering of the overlying basalt (Fig. 7). Downward migrating meteoric water interacts with primary alkali-rich plagioclase minerals (which constitutes a majority of the groundmass phase) leaching sodium ions. The Na-rich deposits are consistent with the elevated alkali concentrations reported in the Blue Dragon flow (Stout et al. Reference Stout, Nicholls and Kuntz1994; Richardson et al. Reference Richardson, Hinman, McHenry, Kotler, Knipe and Scott2012). Na-leaching has been observed as a source for Na-rich secondary minerals (halides) in other basaltic cave settings (Forti Reference Forti2005). Isolated regions of basaltic glass could be another source of sulphur, especially since the Blue Dragon flow has a high concentration (∼25 wt% by volume) of glass (Stout et al. Reference Stout, Nicholls and Kuntz1994). Varying oxidation states of sulphur (S6+ and S2−), along with Fe-sulphide globules, have been reported in basaltic glasses (Métrich et al. Reference Métrich, Berry, O'Neill and Susini2009). Thus, bio-oxidation of Fe-sulphides and S2− in basaltic glass could account for the sulphate oxyanion observed in the secondary deposits. Sulphur-oxidizing endolithic organisms, such as certain Thiobacillus species, have been reported in basaltic cracks and glass (Thorseth et al. Reference Thorseth, Furnes and Tumyr1995; McKinley et al. Reference McKinley, Stevens and Westall2000; Edwards et al. Reference Edwards, McCollom, Konishi and Buseck2003; Cousins et al. Reference Cousins, Smellie, Jones and Crawford2009; Izawa et al. Reference Izawa, Banerjee, Flemming, Bridge and Schultz2010). Abiotic and biotic-mediated oxidation of these reduced sulphur species could produce and subsequently introduce the sulphate oxyanion into migrating water. Bio/organic compounds (such as lipids, sugars, proteins, amino acids and their degradation products) could be incorporated into migrating water along with sodium and sulphate ions. Upon evaporation in the ceiling cracks and cave floors, a fraction of the bio/organic compounds would be incorporated in the secondary deposits (Fig. 7).

Fig. 7. Schematic illustrating biotic and abiotic weathering of the overlying basalts at COM and subsequent mineralization of secondary Na-sulphate minerals on the cave floors.
The need for sulphur oxidation, in conjunction with FTICR-MS and FTIR spectra, demonstrate that bio/organic compounds are associated with the secondary deposits, and thus, some degree of biogenic activity is probably involved in the formation of secondary sulphate deposits. While plausible, evidence for the proposed scheme is not definitive. To the authors’ knowledge, no investigation of biogenic activity related to Na-sulphate mineralization in volcanic settings has been conducted. Therefore, in the future, an exhaustive microbial study would need to be conducted to identify and confirm microbial species associated with secondary mineralization.
Conclusions
The secondary sulphate deposits in the subsurface of COM are products of biotic and abiotic weathering and subsequent precipitation. Peaks suggestive of bio/organic compounds observed in the FTICR-MS spectra imply that the secondary sulphate deposits are directly or indirectly associated with biological activity, which is further supported by field observations, infrared spectroscopy and sulphur fractionation. The effectiveness of natural Na-sulphate minerals to assist in the ionization/desorption of associated bio/organic compounds further establishes the importance of Na-sulphate minerals for future laser desorption mass spectrometry instruments proposed for planetary investigations. The occurrence of biological activity associated with the formation of secondary minerals in the lava tubes and caves of COM, in addition to the chemical and morphological similarities between the COM and Martian basalts, offers further evidence as to the importance of the subsurface as an auspicious environment in the search for life on Mars.
Acknowledgements
The authors acknowledge support by the National Aeronautics and Space Administration (NASA) Exobiology Programme (grant no. NNX08AP59G) and permission to conduct this study from the National Park Service at Craters of the Moon National Monument. CDR would also like to thank Dawn Knipe, J. Michelle Kotler and Doug Owen for their valuable insight and assistance. Research performed at the Idaho National Laboratory under United States Department of Energy (DOE) Idaho Operations Office Contract DE-AC07-05ID14517.