Introduction
Cyanobacteria are important oxygenic photosynthetic organisms that play a major role in the production of organic matter and oxygen. They are ubiquitous on Earth and have adapted to live in some of the most extreme environments, which vary from the dry, high UV radiation conditions of the Artic and Antarctic to the hot dry conditions of the Atacama Desert (Friedmann Reference Friedmann1980; Wierzchos et al. Reference Wierzchos, Ascaso and McKay2006; Omelon Reference Omelon2008). Many cyanobacteria subsist and even thrive in these environments since they have adaptive strategies to cope with environmental stresses such as UV radiation and desiccation (Ehling-Schulz & Scherer Reference Ehling-Schulz and Scherer1999; Potts Reference Potts1999; Fleming & Castenholz Reference Fleming and Castenholz2007).
Exposure experiments have shown that cyanobacteria are also able to survive the adverse conditions of low Earth orbit (LEO) (Mancinelli et al. Reference Mancinelli, White and Rothschild1998; De la Torre et al. Reference de la Torre2010; Olsson-Francis et al. Reference Olsson-Francis, de la Torre and Cockell2010; Cockell et al. Reference Cockell, Rettberg, Rabbow and Olsson-Francis2011). Short-term experiments have demonstrated that endoevaporitic cyanobacteria from the Atacama Desert and the intertidal zone in Baja California, Mexico, are able to survive more than 10 days of exposure to LEO (Mancinelli et al. Reference Mancinelli, White and Rothschild1998; De la Torre et al. Reference de la Torre2010). More recently, an epilithic cyanobacterium (Gloeocapsa strain OU_20) from the rocky high-intertidal zone in Beer, United Kingdom, survived 548 days of exposure aboard the ESA EXPOSE-E facility outside the International Space Station (Cockell et al. Reference Cockell, Rettberg, Rabbow and Olsson-Francis2011). In this experiment, the natural rock-dwelling community was augmented with a biofilm of akinetes of Anabaena cylindrica and vegetative cells of Chroococcidiopsis and Nostoc commune, and only Gloeocapsa strain OU_20, Anabaena cylindrica and Chroococcidiopsis survived (Cockell et al. Reference Cockell, Rettberg, Rabbow and Olsson-Francis2011).
The aim of this study was to understand how cyanobacteria survive in LEO by characterizing their survival strategies in the natural environment. In this work, we focused on the epilithic cyanobacterium, Gloeocapsa strain OU_20, which we isolated from the rocky high-intertidal zone at Beer. We included Phormidium strain OU_10 and Leptolyngbya strain OU_13 in this study as we isolated them from the same community. Although they did not survive in LEO, previous ground-based experiments showed that they could survive periods of desiccation, vacuum (0.7×10−3 kPa) and low temperature (−80 °C), which are all prerequisites for survival in LEO (Olsson-Francis et al. Reference Olsson-Francis, de la Torre and Cockell2010).
In the high-intertidal zone, cyanobacteria are exposed to daily fluctuations in temperature, UV radiation, desiccation, salinity and intermittent water availability (Garbary Reference Garbary and Seckbachs2007). The physiological challenges that the high-intertidal zone creates result in a loss of microbial diversity (Rothrock & Garcia-Pichel Reference Rothrock and Garcia-Pichel2005; Abed et al. Reference Abed, Kohls, Schoon, Scherf, Schacht, Palinska, Al-Hassani, Hamza, Rullkotter and Golubic2008; Bauersachs et al. Reference Bauersachs, Compaore, Severin, Hopmans, Schouten, Stal and Damste2011). Yet, cyanobacteria can survive in this environment due to physiological adaptation. For example, analysis of an intertidal flat, on the Arabian Gulf, demonstrated that mats located at high tide have an increased level of the trans/cis ratio (0.6–1.6%) of the cyanobacterial fatty acid n-18 : 1ω9 (salinity of the higher tidal zone is 20‰ compared with 6‰ in the lower zone) (Abed et al. Reference Abed, Kohls, Schoon, Scherf, Schacht, Palinska, Al-Hassani, Hamza, Rullkotter and Golubic2008).
In this paper, we demonstrate that the survival mechanisms required for life in the high-intertidal zone are important for surviving the environmental conditions associated with LEO.
Material and methods
Sample site
The sample site for this work was a cliff face in Beer, Devon, United Kingdom, 50°41.50′N, 3°08.19′W, that is made of Cretaceous nodular chalk limestone (Griffiths Reference Griffiths2000). The cyanobacteria inhabit the surface of the rocks and form a homogenous epilithic covering. We collected samples from the upper greensand layer, which is in the high-intertidal zone. Data loggers demonstrated that the cyanobacteria were exposed to an air temperature range of 6.28–27.76 °C and a light intensity of 0–73.3 μmol m−2 s−1 (Olsson-Francis et al. Reference Olsson-Francis, de la Torre and Cockell2010).
Micro-organisms and growth conditions
Phormidium strain OU_10, Gloeocapsa strain OU_20 and Leptolyngbya strain OU_13 were previously isolated from the high-intertidal zone at Beer (Olsson-Francis et al. Reference Olsson-Francis, de la Torre and Cockell2010). The control cultures were grown in a modified BG-11 medium (salinity of 6.5‰) and the stressed cells were grown in a BG-11 medium with 500 mM NaCl (salinity of 35.7‰, which is comparable to the salinity of seawater (35‰) (Kester et al. Reference Kester, Duedall, Connors and Pytkowic1967)). For the Transmission Electron Microscope (TEM) analysis, the cyanobacteria were grown on BG-11 agar plates (1.5% agar) with a salinity of 6.5‰ and 35.7‰. All of the cultures were grown under a day cool-white fluorescent light (40.5 μmol m−2 s−1) with a night cycle of 8 hours (LEEC, PL2), at 25 °C (Olsson-Francis et al. Reference Olsson-Francis, de la Torre and Cockell2010).
To monitor cyanobacterial growth, cell counts were conducted and the specific growth rate constant (k) was determined, as previously described (Pirt Reference Pirt1978). For accurate enumeration, cells were dissociated from the cyanobacterial mats by sonication, at 3 hHz (Morris et al. Reference Morris, Monier and Jacques1998). The biochemical and morphological analyses were carried out on exponentially grown cells (this is where the number of cells doubles within a fixed time), which were aseptically harvested.
TEM analysis
TEM analysis was used to characterize the morphology of the cyanobacteria. Sections of agar (1×32 mm) containing exponentially grown cyanobacteria were placed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.4, containing 0.1% ruthenium red (cacod/RR). By aspiration at each step, the samples were post-fixed in 2% osmium tetroxide in cacod/RR, dehydrated through an ascending series of acetone infiltrated in Epon resin (Shell Chemical Company, San Francisco, CA, USA), at 60 °C with six changes. The samples were finally embedded in complete Epon and polymerized at 60 °C for 48 hours. Ultrathin sections of approximately 70 nm were cut using a diamond knife and collected on copper slot grids with a formvar film. The sections were counter-stained with uranyl acetate and Reynolds lead citrate before examination in a JEM 1400 transmission electron microscope (JEOL, Tokyo, Japan) operating at 80 kV. Digital images were acquired using an AMT XR60 digital camera (AMT Imaging, Danvers, MA, USA).
Determination of gas chromatography-mass spectrometry (GC-MS)
The lipids were extracted from exponentially grown cells using a modified Bligh and Dyer extraction protocol (Bligh & Dyer Reference Bligh and Dyer1959). A total of 10–20 mg of freeze-dried material was extracted using a monophasic solvent mixture of H2O, methanol and chloroform (4 : 10: 5). Three ml of solvent was added to the cells, sonicated for 5 minutes and then centrifuged for 3 minutes at 12 000 g. The supernatant was removed from the tubes and replaced with 3 ml of solvent mixture; this process was repeated three times. An equal volume of chloroform and water (3 ml each) was added to the supernatant and shaken for 5 minutes. To separate the organic and aqueous phases the samples were centrifuged. The chloroform phase was removed and the aqueous phase was extracted twice more with chloroform, and then combined with the extract.
GC-MS analysis of the fatty acids (as methyl esters) was carried out using an Agilent Technology 6890 gas chromatogram coupled to a 5973 mass spectrometer. Separation was performed on a SGE BPX90 column (30 m length, 0.25 mm internal diameter, 0.25 mm film thickness). Helium at a column flow rate of 1.1 ml min−1 (constant flow rate) was used as the carrier gas. Injection was splitless with an injector temperature of 250 °C. The GC oven temperature was held for 2 minutes at 50 °C and then programmed at 5 °C min−1 to 250 °C. The final temperature was held for 8 minutes.
Sunscreen pigment analyses
For community analysis, the photosynthetic community, which consisted of a mixture of cyanobacterial species, was removed from the rock using a scalpel and homogenized in ddH2O with a glass rod. For analysis of the isolates, 20 ml of cells were harvested by centrifugation (10 000 g, 10 minutes) and dried overnight at 80 °C. Each of the samples was divided into three equal volumes for further analyses.
To extract the mycosporine-like amino acids (MAAs), the cell suspension was resuspended in 1 ml of 20% methanol for 2 hours at 45 °C. The MAAs content was calculated by the method of Garcia-Pichel & Castenholz (Reference Garcia-Pichel and Castenholz1993) using the average extinction coefficient of 120 L g−1 cm−1 determined by Oren & Gunde-Cimerman (Reference Oren and Gunde-Cimerman2007). The scytonemin was extracted by resuspending the cell suspension in 1 ml of 100% acetone and incubating overnight, at 4 °C, in the dark. The content of scytonemin was calculated using the method of Garcia-Pichel & Castenholz (Reference Garcia-Pichel and Castenholz1991).
To determine protein concentration the cells were lysed by heating the cells at 90 °C, for 10 minutes, with 1 N NaOH and the concentration was measured using the Markwell method (Markwell Reference Markwell, Haas, Bieber and Tolbert1978).
Exposure experiments
The cyanobacteria were exposed independently to vacuum, desiccation, UV radiation (254 nm) and a range of temperatures (−20 to 70 °C). The cyanobacteria were washed three times in ddH2O (to remove excess salt) and dried overnight, under ambient conditions, prior to desiccation and vacuum exposure. Exponential grown cells were used for the UV radiation experiment.
For the controls, replicas for each experiment were stored under ambient conditions, in the dark. Survivability was measured as a percentage of the control cells, for each time interval (Olsson-Francis et al. Reference Olsson-Francis, de la Torre, Towner and Cockell2009).
The effect of desiccation was measured at room temperature in a desiccator containing silica gel beads, which were heated overnight at 80 °C to eliminate moisture. We also measured the effect of temperature on desiccated cells, over a temperature range of −20 to 70 °C. The cells were independently exposed to vacuum (0.7×10−3±0.01 kPa), which was conducted in a simulation chamber, as previously described (Olsson-Francis et al. Reference Olsson-Francis, de la Torre, Towner and Cockell2009). For the above experiments, cells were exposed for 1, 3, 7, 14, 21 and 28 days.
UV exposure was examined using a MRL-58 multiple-ray germicidal lamp, which emits at 254 nm, with an irradiation intensity of 10 W m−2. Exponentially grown cells were washed and resuspended in ddH2O (to ensure that the salt was removed from the stressed cells), using a pipette, to give a final cell concentration of approximately 1×107 ml−1. Preliminary work demonstrated that there was no significant difference in survivability between cells resuspended in a BG-11 medium or ddH2O, which suggests that osmotic stress did not affect survivability. Five ml of cell suspension was placed in the lid of a Petri dish (9 cm diameter) and 500 μl aliquots were removed from the irradiated samples after a total UV dosage of 0–1000 J m−2. The cells were either incubated directly in the BG-11 medium or diluted appropriately prior to incubation. The cultures were analysed once the cells reached the late exponential phase and the survival percentage was determined, as previously described (Knudson Reference Knudson1986; Billi et al. Reference Billi, Friedmann, Hofer, Caiola and Ocampo-Friedmann2000). The results were plotted and the UV fluence lethal to 90% of the population (LD90) was calculated (Tauscher et al. Reference Tauscher, Schuerger and Nicholson2006).
After exposure, the samples were incubated in 15 ml sterile glass bottles containing 5 ml of BG-11 medium and incubated under a day cool-white fluorescent light (40.5 μmol m−2 s−1) with a night cycle of 8 hours (LEEC, PL2), at 25 °C. Microbial growth was routinely monitored using a Leica DMRP microscope (1000×), equipped with epifluorescence. Cell counts were conducted, when the cells reached the late exponential phase (after 10 weeks), as previously described (Olsson-Francis et al. Reference Olsson-Francis, de la Torre and Cockell2010).
Results
Microbial growth
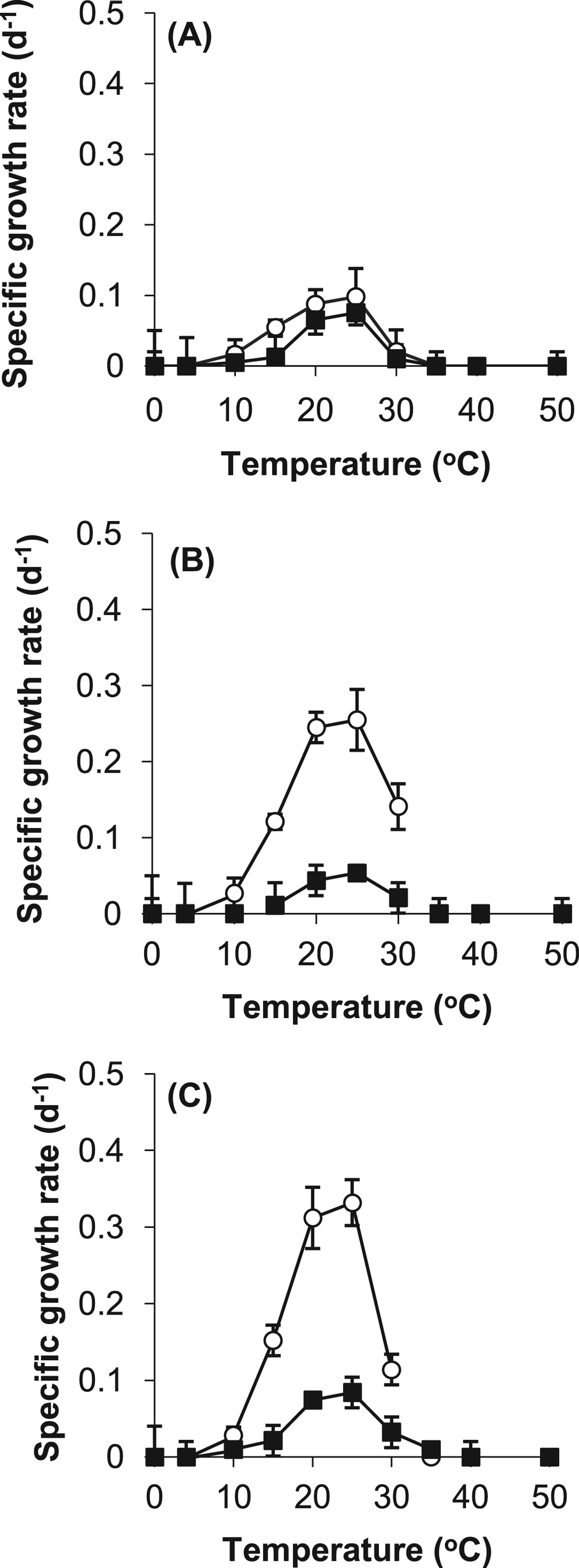
Both the control and the stressed cells grew optimally between 20 °C and 25 °C. None of the cyanobacteria were able to grow below 5 °C, or above 35 °C, as shown in Fig. 1. The optimal specific growth rate for Leptolyngbya strain OU_13 was 0.255 d−1, for Phormidium strain OU_10 was 0.332 d−1and for Gloeocapsa strain OU_20 was 0.098 d−1. The addition of 500 mM NaCl to the growth medium had a detrimental effect on the specific growth rates. For example, at 25 °C the specific growth rate of Leptolyngbya strain OU_13 was 0.054 d−1 compared with 0.255 d−1, as shown in Fig. 1. None of the cyanobacteria strains were hypersaline strains, as they were unable to grow above 130‰ salinity (data not shown).

Fig. 1. Specific growth rates of control (○) and stressed (■) cells of (A) Gloeocapsa strain OU_20, (B) Leptolyngbya strain OU_13 and (C) Phormidium strain OU_10. The values reported are the means of three independent experiments, and the standard error associated with these determinations is shown.
Cellular morphology
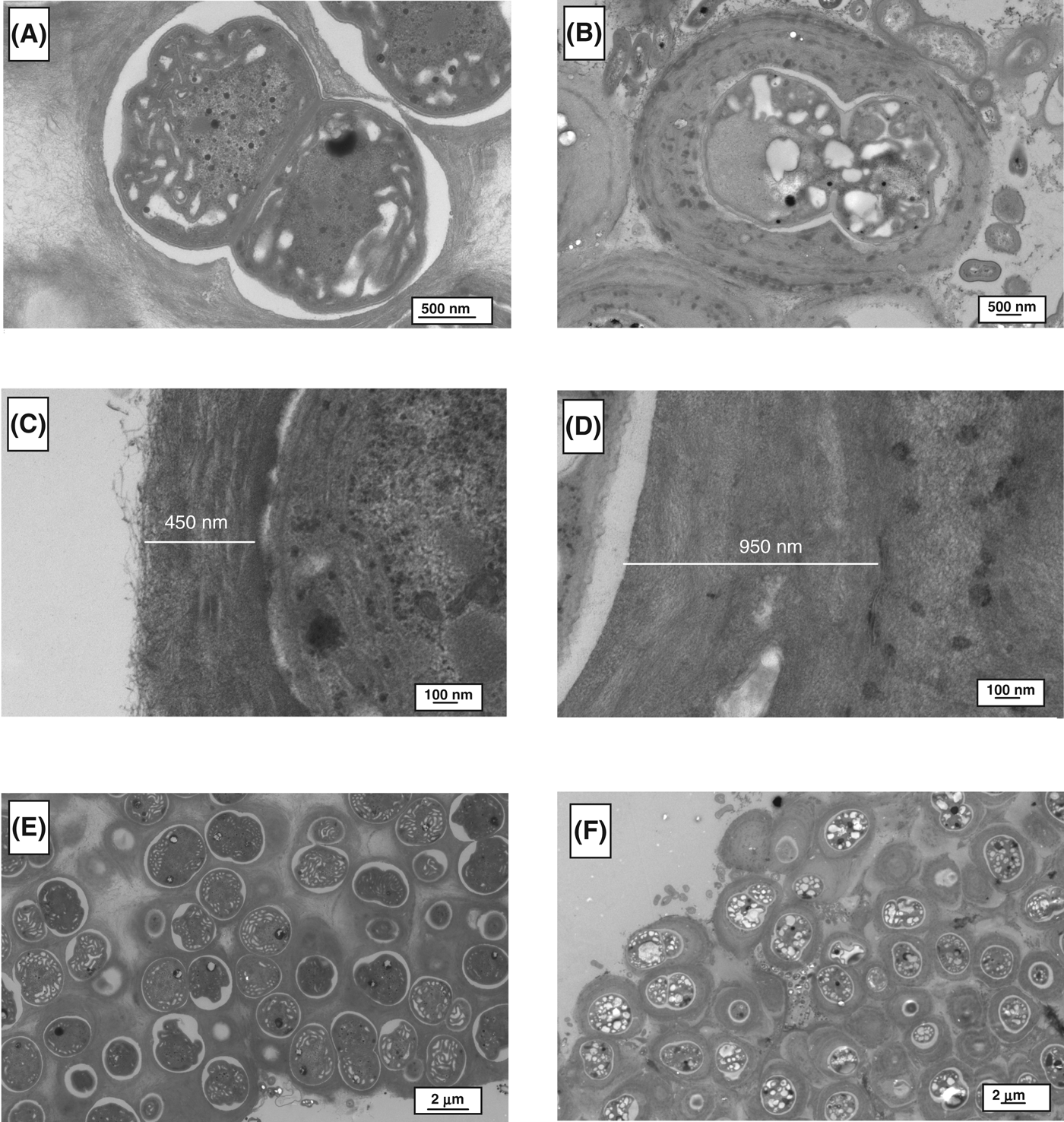
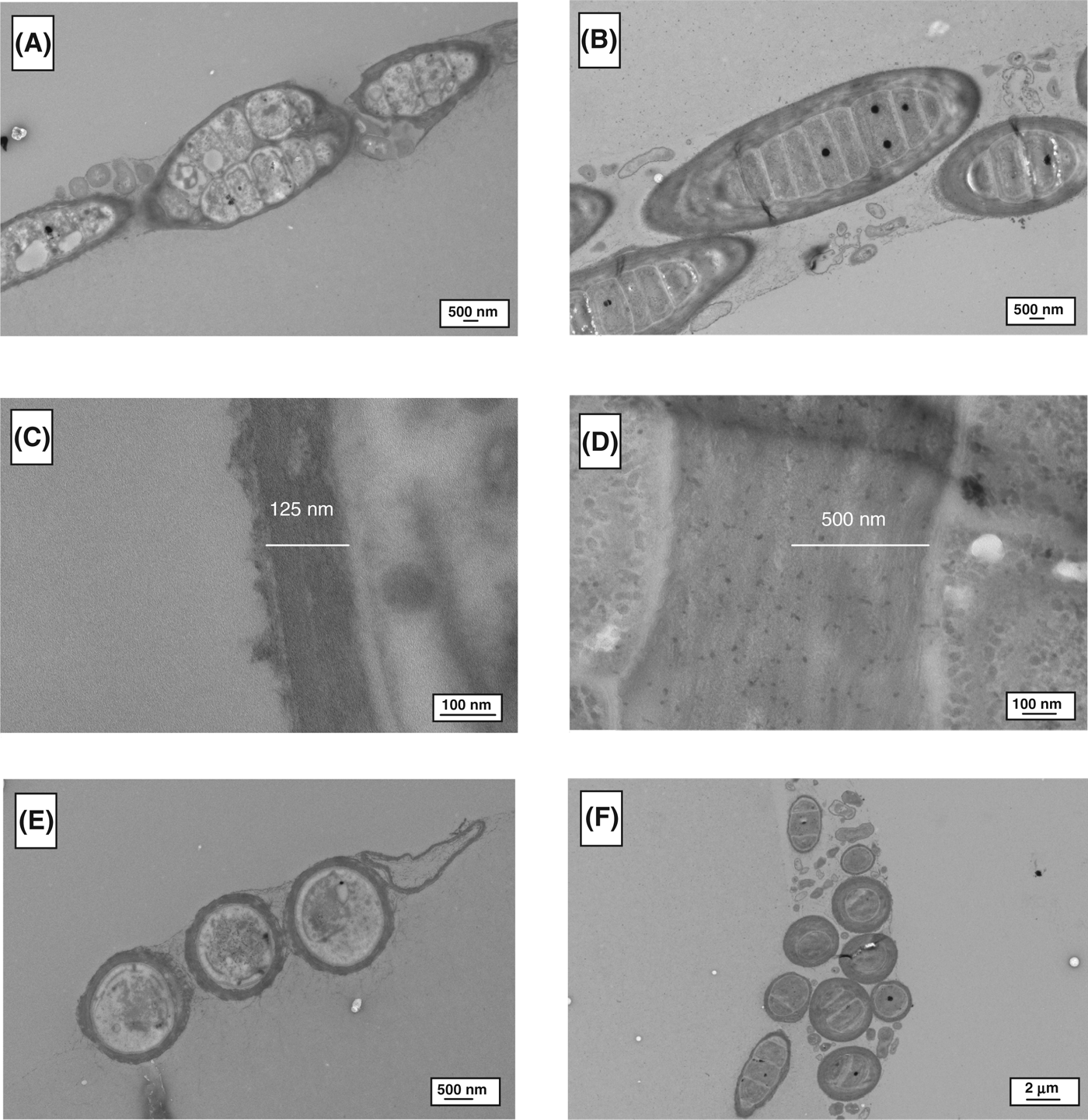
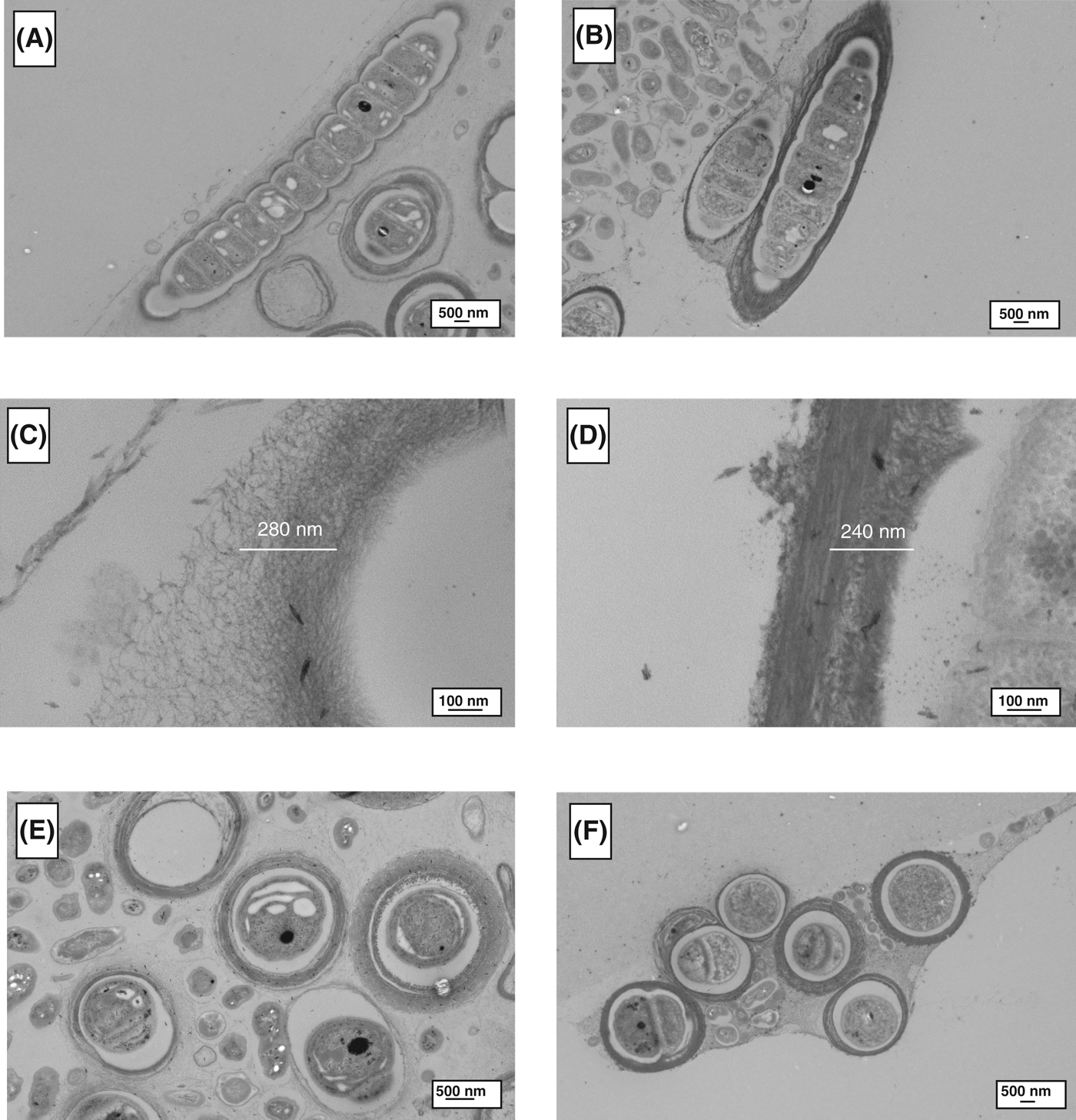
TEM analysis was used to determine morphological differences between the control and stressed cells, as shown in Figs. 2–4. For all of the cyanobacteria, the major difference was related to the extracellular sheath. The micrographs demonstrated that the fibrillar material was more compact in the stressed cells, this is particularly evident in Gloeocapsa strain OU_20 (Fig. 2). The extracellular sheath was also thicker in the stressed cells. For example, the thickness of the sheath was 4.0-fold (Leptolyngbya strain OU_13), 2.1-fold (Gloeocapsa strain OU_20) and 1.2-fold (Phormidium strain OU_10) greater in the stressed cell, as calculated from Figs 2C, D, 3C, D, 4C and D. The filamentous cyanobacteria, Phormidium strain OU_10 and Leptolyngbya strain OU_13 formed thin mats, whereas Gloeocapsa strain OU_20 formed aggregates.

Fig. 2. TEM images of Gloeocapsa strain OU_20. (A), (C) and (E) are micrographs of control cells; while (B), (D) and (F) are of stressed cells.

Fig. 3. TEM images of Leptolyngbya strain OU_13. (A), (C) and (E) are micrographs of control cells; while (B), (D) and (F) are of stressed cells.

Fig. 4. TEM images of Phormidium strain OU_10. (A), (C) and (E) are micrographs of control cells; while (B), (D) and (F) are of stressed cells.
Lipid analysis
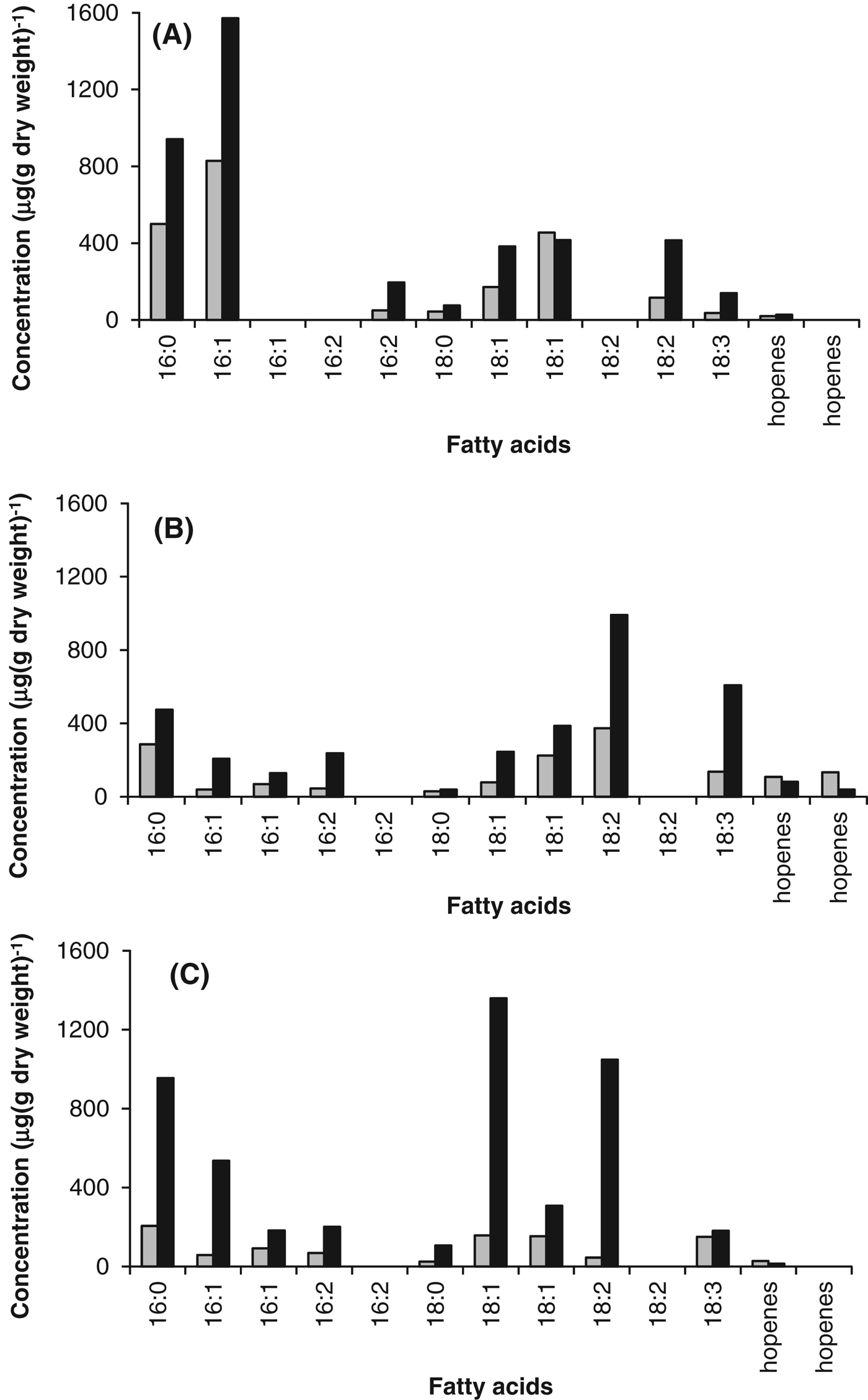
GC-MS analysis demonstrated that the lipid composition varied between the three cyanobacteria; however, the saturated fatty acids, 16 : 0 and 18 : 0, and the unsaturated fatty acids, 16 : 1, 16 : 2, 18.1, 18 : 2 and 18 : 3, dominated each of the cyanobacteria. It is evident from Fig. 5 that the abundance of fatty acids was greater in the stressed cells. The total amount of fatty acids in the control cells was 0.99 mg (g dry weight)−1 (Phormidium strain OU_10), 1.52 mg (g dry weight)−1 (Leptolyngbya strain OU_13) and 2.22 mg (g dry weight)−1 (Gloeocapsa strain OU_20), whereas the total amount in the stressed cells was 4.83 mg (g dry weight)−1 (Phormidium strain OU_10), 3.44 mg (g dry weight)−1 (Leptolyngbya strain OU_13) and 4.16 mg (g dry weight)−1 (Gloeocapsa strain OU_20). Although the different isomers of the unsaturated fatty acids were separated on the column, the specific isomers were not identified.

Fig. 5. Lipid content of control (grey) and stressed (black) cells of (A) Gloeocapsa strain OU_20, (B) Leptolyngbya strain OU_13 and (C) Phormidium strain OU_10. GC-MS analysis was used to determine the fatty acid content of the cells.
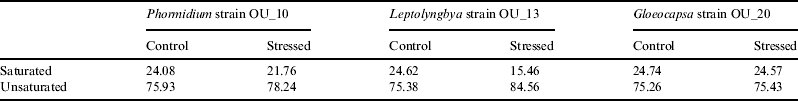
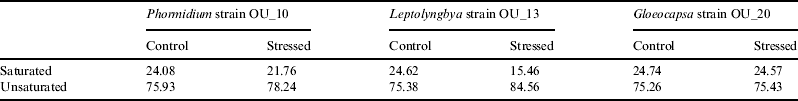
For Phormidium strain OU_10 and Leptolyngbya strain OU_13, the percentage of unsaturated fatty acids was 3.04% (Phormidium strain OU_10) and 12.17% (Leptolyngbya strain OU_13) greater than in the stressed cells, as shown in Table 1. However, the difference for Gloeocapsa strain OU_20 was insignificant (0.17%).
Table 1. Fatty acid composition (%) of Phormidium strain OU_10, Leptolyngbya strain OU_13 and Gloeocapsa strain OU_20
Sunscreen pigmentation analysis
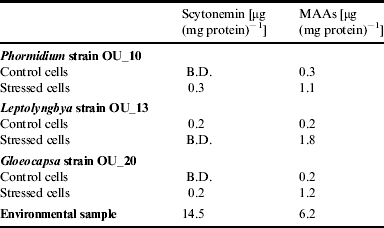
The scytonemin content that we measured using the method of Garcia-Pichel et al. (Reference Garcia-Pichel, Wingard and Castenholz1993) was low. We were unable to detect any scytonemim in the control cells of Phormidium strain OU_10 and Gloeocapsa strain OU_20, and in the stressed cells of Leptolyngbya strain OU_13. The effect of salinity on the sycotonemin content was ambiguous. However, the MAAs content was affected by salinity and the content was higher in the stressed cells. For example, the MAAs content of Leptolyngbya strain OU_13 was 0.2 μg (mg protein)−1 (control cells) compared with 1.8 μg (mg protein)−1 in salt stressed cells.
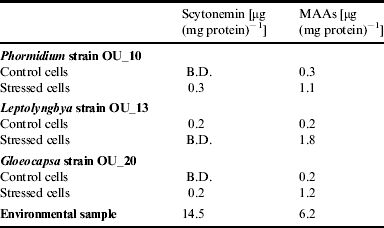
When we compared the values obtained for the cyanobacteria in the batch culture experiment with those obtained for the natural community, we noted that the scytonemin and MAAs contents were much lower, as shown in Table 2. For example, the scytonemin content was 14.5 μg (mg protein)−1 compared with 0.2 μg (mg protein)−1 (Gloeocapsa strain OU_20).
Table 2. Scytonemin and MAA content measured in cells from the batch culture experiment and from the epilithic community
B.D.=below detection limits.
Exposure experiments
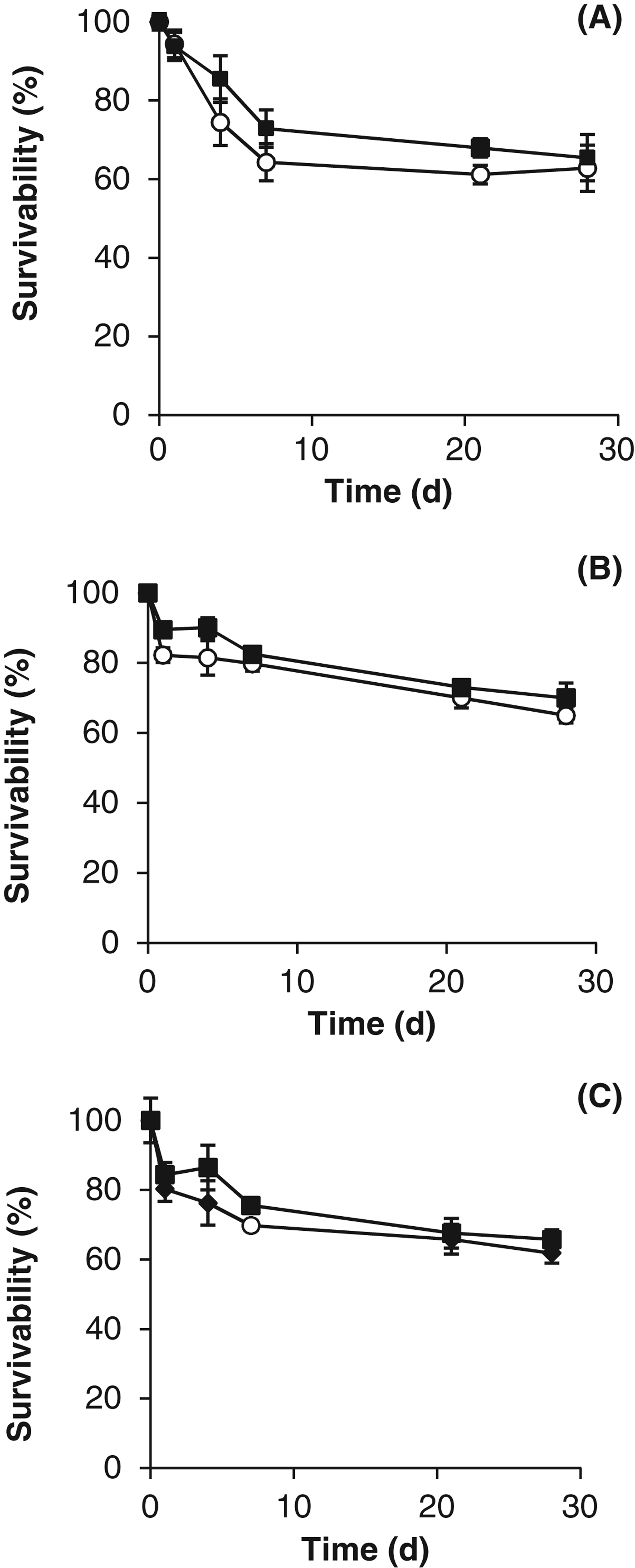
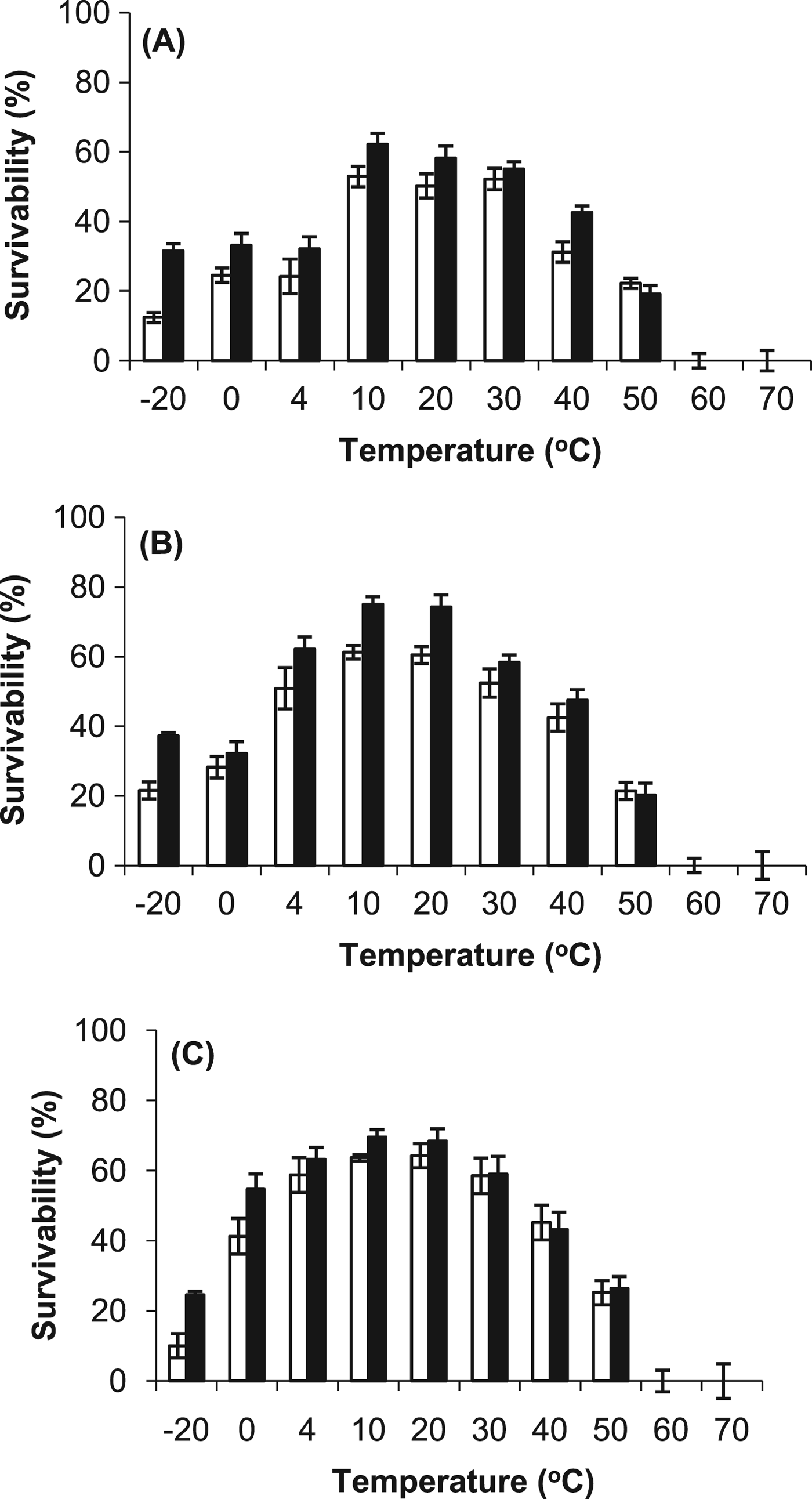
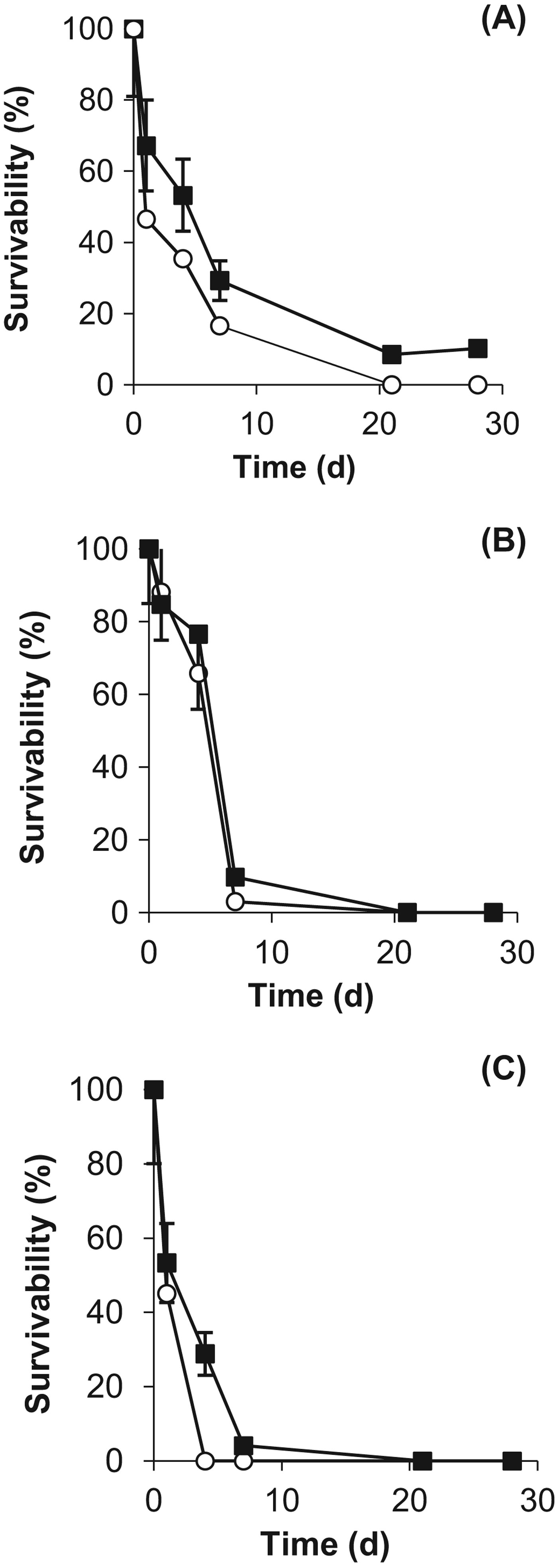
Exposure experiments were conducted to compare the resistance of the cyanobacteria with desiccation, temperature, vacuum and UV radiation. The results demonstrated that all of the cyanobacteria were resistant to desiccation (>60% survival after 28 days) and there was no major difference caused by the growth conditions (Fig. 6). However, the desiccated cells were unable to survive temperatures above 50 °C (no difference was observed between the stressed and control cells), as demonstrated in Fig. 7. At the lower temperatures, for example at −20 °C, the survival rate was more than 15% higher in the stressed cells.

Fig. 6. Desiccation resistance of control (○) and stressed (■) cells of (A) Gloeocapsa strain OU_20, (B) Leptolyngbya strain OU_13 and (C) Phormidium strain OU_10. The values reported are the means of three independent experiments, and the standard error associated with these determinations is shown.
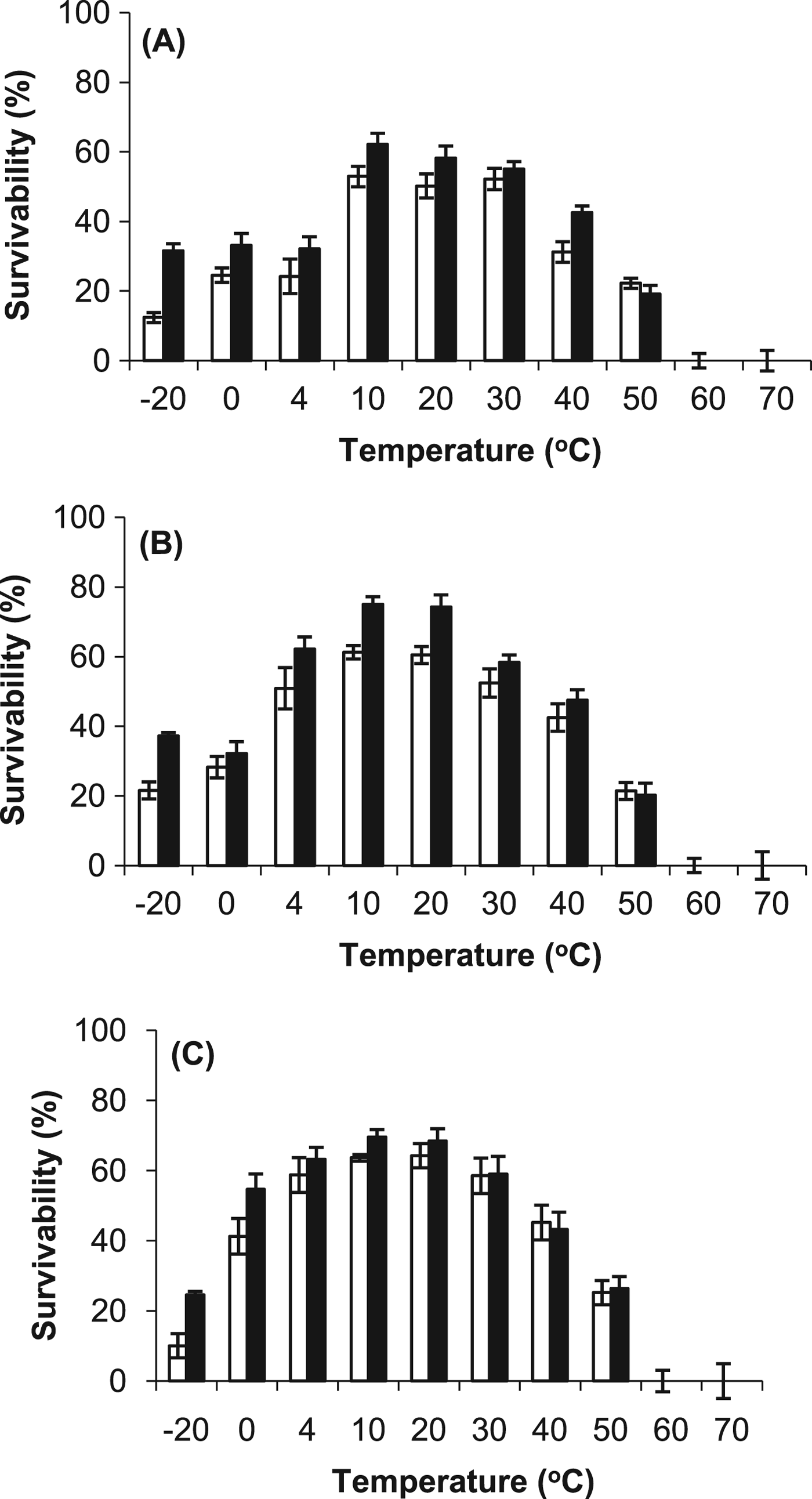
Fig. 7. Temperature resistance of control (○) and stressed (■) desiccated cells of (A) Gloeocapsa strain OU_20, (B) Leptolyngbya strain OU_13 and (C) Phormidium strain OU_10. The cells were exposed to a range of temperatures (−20 °C to 70 °C) for 28 days. The values reported are the means of three independent experiments, and the standard error associated with these determinations is shown.
When we exposed the cyanobacteria independently to vacuum the stressed cells of Gloeocapsa strain OU_20 were more resistant as shown in Fig. 8. For example, after 28 days, 10.5% of the stressed cells survived compared with only 1.5% of the control cells. However, there was little difference between stressed and control cells of Leptolyngbya strain OU_13 and Phormidium strain OU_10, and both strains survived over 20 days of exposure (Fig. 8).

Fig. 8. Vacuum (0.7×10−3 kPa) resistance of control (○) and stressed (■) cells of (A) Gloeocapsa strain OU_20, (B) Leptolyngbya strain OU_13 and (C) Phormidium strain OU_10. The values reported are the means of three independent experiments, and the standard error associated with these determinations is shown.

Fig. 9. UV resistance (254 nm) of control (○) and stressed (■) cells of (A) Gloeocapsa strain OU_20, (B) Leptolyngbya strain OU_13 and (C) Phormidium strain OU_10.
Exponentially grown cells were exposed independently to a total UV dosage of 0–1000 J m−2 (Fig. 9). The stressed cells were more resistant to UV exposure. This was clearly demonstrated when we calculated the LD90 (the dosage that reduces the population to 10%). For example, 130 J m−2 compared with 95 J m−2 (Phormidium strain OU_10); 195 J m−2 compared with 150 J m−2 (Gloeocapsa strain OU_20), and 120 J m−2 compared with 100 J m−2 (Leptolyngbya strain OU_13). When we compare the cyanobacteria, Gloeocapsa strain OU_20 was the most UV resistant.
Discussion
To understand how cyanobacteria survive in LEO we focused our work on the cyanobacteria Gloeocapsa strain OU_20, Phormidium strain OU_10 and Leptolyngbya strain OU_13. In their natural environment, they are able to survive fluctuations in environmental parameters, such as UV radiation, desiccation and changes in temperature, which are ideal prerequisites for survival in LEO. We studied the physiology of the cyanobacteria in batch culture, using a medium that had a similar salinity to seawater. Although salinity is not associated with LEO it is one of the most conspicuous environmental changes in the intertidal zone. The lower-intertidal zone is only out of seawater for short periods; while the high-intertidal zone is frequently exposed to high salinity water (evaporated seawater) as well as low salinity water (rainwater) (Garbary Reference Garbary and Seckbachs2007). The salinity of the medium that we used is lower than expected in the high-intertidal zone; however, it is sufficient to cause a physiological response, as demonstrated by TEM (Figs 2, 3 and 5) and lipid analysis (Fig. 5).
Physiological studies have demonstrated that salinity promotes a stress response in cyanobacteria similar to that found for desiccation, cold temperature and UV resistance (Potts Reference Potts1999; Dillon et al. Reference Dillon, Tatsumi, Tandingan and Castenholz2002; Tamaru et al. Reference Tamaru, Takani, Yoshida and Sakamoto2005; Oren & Gunde-Cimerman Reference Oren and Gunde-Cimerman2007; Ozturk et al. Reference Ozturk, Aslim and Suludere2009). For example, the formation of an extracellular sheath is associated with salinity as well as desiccation and cold temperature resistance (Tamaru et al. Reference Tamaru, Takani, Yoshida and Sakamoto2005); furthermore, UV absorbing pigments, which are required for UV resistance, have also been shown to be induced by salinity (Dillon et al. Reference Dillon, Tatsumi, Tandingan and Castenholz2002).
The stressed cells were more resistant to cold temperatures and UV radiation, while stressed cells of Gloeocapsa strain OU_20 were also more resistant to vacuum. Although Gloeocapsa strain OU_20 did not survive the full adverse effect of extraterrestrial UV fluxes in LEO, the LD90 values were 120 J m−2 (control) and 195 J m−2 (stressed), which were higher than the other cyanobacteria. Previous work has demonstrated that UVC (280–100 nm) resistance is linked to the production of the pigment scytonemin (Sagan Reference Sagan1973; Pierson et al. Reference Pierson, Mitchell and Ruff-Roberts1993; Proteau et al. Reference Proteau, Gerwick, Garcia-Pichel and Castenholz1993; Dillon & Castenholz Reference Dillon and Castenholz1999). In the intertidal zone, there is a positive correlation between scytonemin production and exposure to solar irradiation (Abed et al. Reference Abed, Kohls, Schoon, Scherf, Schacht, Palinska, Al-Hassani, Hamza, Rullkotter and Golubic2008).
The effect of salinity on the scytonemim content was ambiguous (we were unable to detect any scytonemim in the control cells of Phormidium strain OU_10 and Gloeocapsa strain OU_20, and in the stressed cells of Leptolyngbya strain OU_13 with the Garcia-Pichel et al. (Reference Garcia-Pichel, Wingard and Castenholz1993) method). Although Dillon et al. (Reference Dillon, Tatsumi, Tandingan and Castenholz2002) demonstrated that salinity enhances scytonemin production at low light in Chroococcidiopsis, this was not observed in this study and future work is required to optimize the method for detecting scytonemin under the conditions used in this study. However, the MAAs content was affected by salinity and the content was higher in the stressed cells, although the difference was only small with Gloeocapsa strain OU_20. MAAs is a UV screening pigment typically accumulated in the cytoplasm, but derivatives can be excreted into the sheath (Böhm et al. Reference Böhm, Pfleiderer, Böger and Scherer1995). They present a unique absorption band that has a maximum between 309 and 362 nm, and have no absorption bands down to 210 nm (Gao & Garcia-Pichel Reference Gao and Garcia-Pichel2011). They are also thought to serve as osmotic solutes in high salinity and have been shown to be induced by osmotic stress without UVB (Oren). Analysis of the photosynthetic community showed that the levels of UV screening pigments, both MAAs and scytonemin, were much higher in the environmental samples than the laboratory grown cultures. This could be due to the diversity of cyanobacteria present in the environmental samples or the fact that they were exposed to natural UV (A and B) radiation exposure.
A higher concentration of lipids was measured in the stressed cells, for all of the cyanobacteria, compared with the controls. Although previous work has studied the effect of salinity on lipid composition, the total concentration of lipids has not been determined; instead, work has focused on the percentage of unsaturated and saturated fatty acids (Gombos et al. Reference Gombos, Wada and Murata1992; Tasaka et al. Reference Tasaka, Gombos, Nishiyama, Mohanty, Ohba, Okhi and Murata1996; Allakhverdiev et al. Reference Allakhverdiev, Kinoshita, Inaba, Suzuki and Murata2001). A high percentage of unsaturated fatty acids is associated with adaptation to low temperature, desiccation and salinity, while a decrease in unsaturated fatty acids is associated with UV radiation (Gombos et al. Reference Gombos, Wada and Murata1992; Gupta et al. Reference Gupta, Bhadauriya, Chauhan and Bisen2008). Although the percentage of unsaturated fatty acids was higher in Phormidium strain OU_10 and Leptolyngbya strain OU_13, there was no difference with Gloeocapsa strain OU_20.
Nevertheless, a thick mucilaginous sheath encapsulated cells of Gloeocapsa strain OU_20. Although the sheath was affected by the growth conditions, the thickness of the sheath in the control cells of Gloeocapsa strain OU_20 was greater than in the stressed cells of Phormidium strain OU_10 and Leptolyngbya strain OU_13. It is thought that the mucilaginous sheath forms a buffer zone between the cell and the physico-chemical environment, which plays a crucial role in stress tolerance, such as desiccation, freezing and thawing, UV, salinity and heavy metal contamination (Tamaru et al. Reference Tamaru, Takani, Yoshida and Sakamoto2005; Wright et al. Reference Wright, Smith, Joardar, Scherer, Jervis, Warren, Helm and Potts2005; Ozturk et al. Reference Ozturk, Aslim and Suludere2009; Ozturk & Aslim Reference Ozturk and Aslim2010). Gloeocapsa strain OU_20 also formed aggregates, which consisted of a large number of cells. It is thought that the formation of aggregates increase the effective cellular path lengths for radiation and the overall protection against UV radiation (Garcia-Pichel et al. Reference Garcia-Pichel, Wingard and Castenholz1993). The cells located in the centre of the aggregate are protected from the external environment.
Conclusion
In conclusion, from studying the response of Gloeocapsa strain OU_20 to intertidal conditions we postulate that the dense extracellular mucilaginous sheath and the formation of aggregates both contribute to its ability to survive in LEO. Other factors, such as the concentration of fatty acids and UV pigments, may also play a role and future work is required to conclusively determine their part in LEO survival.
Acknowledgement
We would like to thank Heather Davies for the TEM analysis.