Introduction
Endoliths
Endoliths, referring to microorganisms that inhabit rocks, are divided into several categories. Most familiar are epiliths, such as lichens that colonize the surface of a rock. Chasmoendoliths typically inhabit cracks, fissures and vesicles in rocks (McLoughlin et al. Reference McLoughlin, Brasier, Wacey, Green and Perry2007), often due to their more favourable microclimate compared to the harsher outside extremes, such as desiccation. Cryptoendoliths are most typically characterized by the colonization of pore spaces within sandstones and other sedimentary rocks, such as the well-documented sandstones in the Antarctic and the Canadian High Arctic (e.g. Friedmann Reference Friedmann1982; Omelon et al. Reference Omelon, Pollard and Ferris2007). The endoliths of relevance for this study are the so-called euendoliths (Golubic et al. Reference Golubic, Friedmann and Schneider1981). This term is assigned to those microorganisms that actively burrow into a rock substrate, a common example being that of basaltic volcanic glass. Microbial endoliths have been the focus of much research over the past few decades. Their ability to survive a multitude of environmental extremes (notably oligotrophy, desiccation and elevated ultraviolet (UV) radiation) has made them prime candidates for the search for life on Mars. Their ability to be detected is based on a wide range of biosignatures (identifiable, e.g., by Raman spectroscopy, morphology and geochemistry) and has further demonstrated their valuable role within astrobiology (e.g. Edwards et al. Reference Edwards, Russell and Wynn-Williams1997; Villar et al. Reference Villar, Edwards, Wynn-Williams and Worland2003).
Abiotic and biotic textures in oceanic basalts
Basaltic lavas that abound on the sea floor are readily colonized by microbes, often to depths of 500 m (Furnes & Staudigel Reference Furnes and Staudigel1999). The colonization produces identifiable trace fossils in the form of tunnels, etching and pitting along alteration fronts in volcanic glass. The first description of this alteration was by Ross & Fisher (Reference Ross and Fisher1986), and since then, similar characteristic textures have been found in oceanic basalts at numerous locations, including the East Pacific Rise, Mid Atlantic Ridge, Juan de Fuca ridge and Ontong Java plateau (Thorseth et al. Reference Thorseth, Furnes and Heldal1992, Reference Thorseth, Furnes and Tumyr1995, Reference Thorseth, Torsvik, Torsvik, Daae and Pederson2001, Reference Thorseth, Pederson and Christie2003; Furnes et al. Reference Furnes, Thorseth, Tumyr, Torsvik, Fisk, Alt, Kinoshita, Stokking and Michael1996, Reference Furnes, Staudigel, Thorseth, Torsvik, Muehlenbachs and Tumyr2001b, Reference Furnes, Thorseth, Torsvik, Muehlenbachs, Staudigel, Tumyr, Smellie and Chapman2002b; Fisk et al. Reference Fisk, Giovannoni and Thorseth1998, Reference Fisk, Storrie-Lombardi, Douglas, Popa, McDonald and Di Meo-Savoie2003; Torsvik et al. Reference Torsvik, Furnes, Muehlenbachs, Thorseth and Tumyr1998). In these studies, biotic and abiotic alteration fronts were shown to be distinguishable. Abiotic alteration consists of palagonitization induced by hydrous alteration, and forms smooth alteration fronts of yellow palagonite that are continuous along, and parallel to, glass boundaries. By contrast, biologically mediated dissolution of glass is characterized by complex, irregularly distributed features (Fig. 1). In previous studies, the biotic textures have been divided into ‘granular’ and ‘tubular’ types (Furnes et al. Reference Furnes, Banerjee, Staudigel, Muehlenbachs, McLoughlin, de Wit and Van Kranendonk2007). Granular textures consist of micrometre-sized coalesced spherical bodies that form irregularly distributed alteration fronts along glass boundaries and fractures. They are generally the most abundant type of bioalteration seen in oceanic basaltic lavas (Furnes & Staudigel Reference Furnes and Staudigel1999). Tubular textures are less common, and form long hair-like or tufted tubules protruding from fractures or other boundaries (e.g. vesicles) in the glass. Like the granular texture, the tubules are irregularly distributed, but consist of long narrow tubes with constant width. The tubes often display branching patterns, segmentation and irregular swellings (Furnes et al. Reference Furnes, Banerjee, Staudigel, Muehlenbachs, McLoughlin, de Wit and Van Kranendonk2007). Similar textures have also been identified in ancient oceanic crust sequences spanning the Phanerozoic, Proterozoic and Archean (Furnes et al. Reference Furnes, Muehlenbachs, Tumyr, Torsvik and Xenophontos2001a, Reference Furnes, Muehlenbachs, Torsvik, Tumyr and Shi2002a, Reference Furnes, Banerjee, Muehlenbachs, Staudigel and de Wit2004, Reference Furnes, Banerjee, Muehlenbachs and Kontinen2005, Reference Furnes, Banerjee, Staudigel, Muehlenbachs, McLoughlin, de Wit and Van Kranendonk2007). The fact that these biosignatures can survive the dynamic tectonic environment on Earth for periods of billions of years holds promise for their survivability on Mars.

Fig. 1. Difference in morphology between tubular bioalteration (a) (Fisk et al. Reference Fisk, Storrie-Lombardi, Douglas, Popa, McDonald and Di Meo-Savoie2003) and abiotic alteration (b).
Controls on bioalteration
The environmental controls on bioalteration formation are poorly understood. Changes in fluid flux, nutrient supply and local temperature are believed to be important controls on bioalteration in sea floor lavas (Furnes et al. Reference Furnes, Banerjee, Staudigel, Muehlenbachs, McLoughlin, de Wit and Van Kranendonk2007), as are the salinity and nutrient content of the circulating fluids (McLoughlin et al. Reference McLoughlin, Brasier, Wacey, Green and Perry2007). The next step in the study of biomediated processes is to understand better the environmental controls that affect the distribution of endolithic microborings. The freshwater environment in particular has been overlooked, as found in subglacial volcanic settings. Alteration fluids formed as a result of subglacial eruptions will differ significantly from those in an oceanic setting, whilst the basaltic host lithologies remain essentially the same (i.e., hyaloclastites and pillow basalts).
Bioalteration as a biosignature for Mars
Bioalteration is a remarkably good morphological biosignature, particularly for exploration for exobiology on Mars. Firstly, despite the ubiquity of aqueous alteration in basalt, there is no known abiotic mechanism that can reproduce these textures (Fisk et al. Reference Fisk, Popa, Mason, Storrie-Lombardi and Vicenzi2006; Walton Reference Walton2008). Secondly, the textures are distinctive in themselves (e.g. segmentation, invariant tubule widths, and tubule bifurcation) and indicative of biological activity (Staudigel et al. Reference Staudigel, Furnes, Banerjee, Dilek and Muehlenbachs2006; McLoughlin et al. Reference McLoughlin, Brasier, Wacey, Green and Perry2007). Thirdly, the identification of DNA lining the inner surfaces of microborings in fresh lavas provides additional support for a biological origin (Giovannoni et al. Reference Giovannoni, Fisk, Mullins, Furnes, Alt, Kinoshita, Stokking and Michael1996; Torsvik et al. Reference Torsvik, Furnes, Muehlenbachs, Thorseth and Tumyr1998; Furnes et al. Reference Furnes, Staudigel, Thorseth, Torsvik, Muehlenbachs and Tumyr2001b).
The presence of basaltic volcanism on Mars, coupled with the evidence for aqueous alteration, is permissive for such biosignatures to form there. Phyllosilicates – the major constituent of palagonite – have also been identified on Mars by the OMEGA instrument (Poulet et al. Reference Poulet, Bibring, Mustard, Gendrin, Mangold, Langevin, Arvidson, Gondet and Gomez2005), and are indicative of aqueous alteration of basalt and there was probably a high potential for endolithic microorganisms to alter subaqueous basalt in the early wet Martian environment (Fisk & Giovannoni Reference Fisk and Giovannoni1999a,Reference Fisk and Giovannonib; Banerjee et al. Reference Banerjee, Furnes, Muehlenbachs and Staudigel2004a,Reference Banerjee, Muehlenbachs, Furnes, Staudigel and de Witb, Reference Banerjee, Furnes, Simonetti, Muehlenbachs, Staudigel, deWit and Van Kranendonk2006). Recent studies have hinted that this may be the case with the discovery of alteration textures in Nakhla meteorites that bear a striking similarity to microborings in seafloor lavas (Fisk et al. Reference Fisk, Popa, Mason, Storrie-Lombardi and Vicenzi2006).
Lastly, the colonization of subglacial basalts on Earth is potentially important for bioalteration on Mars where basaltic glaciovolcanism is believed to have occurred in the past (e.g. Chapman Reference Chapman1994; Chapman & Tanaka Reference Chapman and Tanaka2001; Ghatan & Head Reference Ghatan and Head2002; Head & Wilson Reference Head, Wilson, Smellie and Chapman2002; Chapman & Smellie Reference Chapman, Smellie and Chapman2007). Indeed, some of the first work on volcanic endolithic microborings was on Icelandic subglacial hyaloclastites (Thorseth et al. Reference Thorseth, Furnes and Heldal1992), but there has been little subsequent work on lavas from that environmental setting.
Aims of this study
This work contains the first petrographic description of euendolithic microborings within Antarctic hyaloclastites. Additionally, this work aims to provide insights into how the local environment affects the occurrence of endolithic microborings within basaltic sequences that have experienced both freshwater (subglacial) and marine conditions. Basaltic subglacial eruptions result in similar lithologies (i.e., pillow basalts and hyaloclastites) and volcanic edifices to those erupted on the sea-floor at mid-ocean ridges due to analogous processes of rapid lava quenching upon contact with cold water or ice and the formation of abundant glass (cf. Jones Reference Jones1969; Staudigel & Schmincke Reference Staudigel and Schmincke1984). As a result, subglacial and marine volcanic sequences have the potential to host comparable microbial activity. In this study, a sequence of Antarctic lava-fed deltas erupted under well-constrained environmental conditions (Smellie Reference Smellie2006; Smellie et al. Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008) was studied to identify the major environmental controls on any endolithic microborings. The Antarctic sequences were initially erupted subglacially, but most were subsequently affected by percolating marine water (Johnson & Smellie Reference Johnson and Smellie2007). By studying the bioalteration in a series of lavas from the same volcanic province under well-known but variable environmental conditions, we should be able to identify more confidently which are the major controls that determine the distribution and abundance of endolithic microbial activity in basaltic glass.
Sample location
James Ross Island, Antarctica
The James Ross Island Volcanic Group (JRIVG) is a large Neogene alkaline basaltic volcanic province that crops out extensively in the northern Antarctic Peninsula region (Nelson Reference Nelson1975; Smellie Reference Smellie1999; Fig. 2). The island is a polygenetic stratovolcano 40–60 km in basal diameter rising to about 1600 m. It contains a uniquely valuable record of the characteristics and configuration of the Antarctic Peninsula Ice Sheet during glacial and interglacial periods (Smellie et al. Reference Smellie, McArthur, McIntosh and Esser2006, Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008, and submitted). The island was probably surrounded by at least seasonally ice-free conditions during multiple interglacial periods, when seawater was apparently able to percolate into the lava-fed deltas. The sequences are also well dated by the 40Ar/39Ar method (Smellie et al. Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008). Most of the more than 50 eruptions documented were subglacial, producing multiple, voluminous and geographically extensive lava-fed delta sequences, each composed of subaqueous hyaloclastite foreset units and subaerial capping lavas (Skilling Reference Skilling, Smellie and Chapman2002; Smellie Reference Smellie2006). The hyaloclastites are composed of coarse angular fragments of sideromelane (basaltic glass) with minor olivine phenocrysts and often abundant small plagioclase crystals. The glass fragments are characteristically partially altered around their margins to palagonite, whilst the smaller fragments are usually completely replaced. Secondary minerals, predominantly zeolites (chabazite and phillipsite), are present in the pore spaces, and are a direct result of aqueous alteration throughout the lava sequence (Nelson Reference Nelson1975; Johnson & Smellie Reference Johnson and Smellie2007).

Fig. 2. Map of James Ross Island, northern Antarctic Peninsula, showing the location of the type localities for the five hyaloclastite-bearing lava-fed deltas selected for study.
Following initial alteration of the hyaloclastite under freshwater (meltwater) conditions, the lower parts of many of the deltas were affected by influxes of seawater, which affected the type of alteration, through crystallization or possibly recrystallization of zeolites and clays (Johnson & Smellie Reference Johnson and Smellie2007). Whilst reducing the porosity of the rock, the later influx of marine fluid was also apparently able to percolate through the delta hyaloclastites via grain boundaries, cracks and fissures. Elevation data are also available for the individual hyaloclastite samples and were used in our new study to determine which samples had experienced freshwater or seawater conditions, or both.
Methods
Morphology
54 thin sections of hyaloclastite from five lava-fed deltas selected from the JRIVG were analysed petrographically and by scanning electron microscopy (SEM) to identify bioalteration textures that matched those previously described from oceanic basalts. For SEM study, thin sections were carbon coated and analysed using a Jeol Scanning Electron Microscope (JSM-6480LV) at University College London. Using the morphological data obtained, the biogenicity of these microborings was reviewed based on the biogenicity criteria proposed by McLoughlin et al. (Reference McLoughlin, Brasier, Wacey, Green and Perry2007).
Quantitative analysis
To estimate the modal percentage of microborings, a method was devised whereby the fraction of microborings that have affected the available alteration boundaries (grain boundaries, cracks and vesicles) was visually estimated for suitable basaltic glass clasts (sideromelane clasts) in each thin section. Results are recorded as numbers varying from 0 (i.e., absent) to 10 (all boundaries affected), which we term ‘bioalteration values’. Example thin section images of bioalteration values 0, 5 and 10 are shown in Table 1. A typical thin section contains between 35 and 50 suitable clasts. Any clasts that are completely palagonitized were omitted from the assessment, as it has been widely reported that such microborings are not observed within palagonite itself, possibly because they have been destroyed by the alteration process (Furnes & Staudigel Reference Furnes and Staudigel1999). 20 of the samples are from marine-altered hyaloclastite; the remainder are thought to have only experienced freshwater (i.e., meltwater) conditions. In total, over 2500 individual measurements were made. For each thin section, the mean bioalteration value was calculated from all sideromelane clasts analysed within the thin section. To avoid bias in the results, thin sections were analysed blind and in a random order. Additionally, a random selection of thin sections were later analysed a second time, and results compared to verify the initial estimation. Results of these mean bioalteration values for each thin section are presented in Table 2.
Table 1. Example bioalteration values used to determine the extent of bioalteration within the JRIVG hyaloclastites. A bioalteration value represents the visually estimated fraction of all alteration boundaries within a sideromelane clast that show textural evidence of bioalteration. Bioalteration values range from 0 to 10, and examples of values 0, 5, and 10 are shown

Table 2. Mean bioalteration values for hyaloclastites from James Ross Island. Random re-test mean values are also shown

a From Smellie et al. (Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008).
b From Johnson & Smellie (Reference Johnson and Smellie2007) ; samples listed as marine have also previously experienced freshwater alteration.
c See text for details of method.
Mean bioalteration values for all the thin sections were processed using a K-means clustering algorithm in Matlab using three clusters to divide the data into absent, present, and abundant bioalteration values. A K-means algorithm aims to cluster data into groups (of a pre-defined number) whilst minimizing intra-cluster variance. The percentage of these individual clusters that consisted of marine and glacially-altered lavas was used to identify any correlation between bioalteration and alteration environment (see Fig 5). The value of using statistical cluster analysis of large geobiological data sets of this nature is discussed in Storrie-Lombardi & Fisk (Reference Storrie-Lombardi and Fisk2004).
One delta (Dobson Dome; delta names after Smellie et al. Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008) experienced only freshwater conditions whilst a second (Lachman Crags main) was probably wholly marine emplaced. Another three deltas (Forster Cliffs, Patalamon Mesa main and Tumbledown Cliffs) were initially emplaced in a glacial setting (i.e., freshwater alteration), but subsequently were affected by marine incursions (Johnson & Smellie Reference Johnson and Smellie2007). The Dobson and Lachman deltas were selected to act as possible controls (end-members) to help interpret the deltas that experienced both freshwater and marine conditions. The deltas range in age from less than 80 ka to 5.15 Ma, thus giving the potential to identify any age-related effects on bioalteration. Much of the alteration probably took place shortly after initial emplacement, while the permeable hyaloclastite pile was cooling and the pore fluids were still relatively warm, but the marine alteration may have taken place at somewhat cooler temperatures. A typical petrological description of the hyaloclastite can be found in Johnson & Smellie (Reference Johnson and Smellie2007).
Results
Endolithic microboring morphology
The bioalteration textures in our samples are predominantly tubular, consistent with a relatively low temperature alteration environment (Furnes et al. Reference Furnes, Banerjee, Staudigel, Muehlenbachs, McLoughlin, de Wit and Van Kranendonk2007). Granular alteration is consistently uncommon (but present), although it is the commonest form of bioalteration in oceanic lavas, even at low temperatures. Textural identification of bioalteration was based on comparisons with published examples of characteristic features of biologically mediated dissolution of glass. We note in particular the restriction of bioalteration in our samples to glass boundaries, cracks and vesicles (i.e., places where liquid water can circulate); the association with palagonite formation (and therefore water); and most importantly, the characteristic ‘tubular’ appearance and other distinctive morphological features (segmentation, bifurcation, irregular pathways, constant microtubule width (typically 2–20 μm)) of the endolithic microborings, which closely resemble previously published examples from sea-floor lavas and are thought to be indicative of biological behaviour (Staudigel et al. Reference Staudigel, Furnes, Banerjee, Dilek and Muehlenbachs2006). Examples are shown in Fig. 3. Under SEM, the edges of glass clasts and fractures also display irregular pitting and etching patterns (see Fig. 4).

Fig. 3. Examples of microborings (a, c, d, e and f) and abiotic alteration (b) seen in the JRIVG hyaloclastites (M=microborings, F=fresh glass, P=palagonite, Z=zeolite). Arrows in photograph (c) indicate bifurcation.

Fig. 4. Bioalteration ‘pitting’ and ‘etching’ into glass boundaries under SEM. These features clearly extend from the fracture into the adjacent glass.
Biogenicity
The microborings observed here fulfil two biogenicity criteria described by McLoughlin et al. (Reference McLoughlin, Brasier, Wacey, Green and Perry2007):
1. ‘ a geological context that demonstrates the syngenicity and antiquity of the putative biological remains'. The JRIVG microborings are restricted to areas previously exposed to external water. They are associated entirely with vesicles, fractures and sideromelane clast boundaries;
2. ‘evidence of biogenic morphology and behaviour'. The JRIVG microborings display evidence of branching, segmentation, spiral pathways and a consistent tubule width.
Additionally, evidence of a biological origin for such microborings, based on morphology, is evaluated by Walton (Reference Walton2008). Here, Walton discusses and refutes the possible abiotic alternatives for these bioalteration textures, such as skeletal crystals, healed fractures and ambient inclusion trails.
Bioalteration values and alteration environment
Fig. 5 shows the clustering of the data as revealed by K-means analysis. A clear division between the three groups can be seen, and is taken to be representative of differing levels of bioalteration: ‘absent/low’, ‘present’ and ‘abundant’. The percentage of the two different environments (marine and glacial) for each group clearly shows a strong trend of increasing bioalteration with a marine environment, and decreasing bioalteration with a glacial environment. The overall trend is that bioalteration is typically higher in hyaloclastite samples that experienced marine conditions than those that simply experienced freshwater (meltwater). Additionally, a t-test value (a test of whether the means of two groups are statistically different from each other) of p<0.0001 was calculated for the data, strongly suggesting this observed pattern is not simply a result of chance.

Fig. 5. Plot of K-means clustering of the mean bioalteration values obtained on samples from James Ross Island hyaloclastite. The algorithm shows three distinct clusters representative of increasing bioalteration values. Grey stars represent the centroid for each cluster. Each group represents the different clusters assigned by the algorithm. The dashed field line has been added to clearly show the parameters of these clusters. The ‘abundant bioalteration’ group is dominated by marine altered hyaloclastites, whereas the ‘absent/low bioalteration’ group is dominated by hyaloclastites altered by freshwater (glacial meltwater). The middle group ‘bioalteration present’ is slightly dominated by hyaloclastites altered by marine water.
Elevation and age
Parameters such as elevation and age may be directly related to the alteration environment, since the lava-fed deltas on James Ross Island are known to have undergone a history of initial freshwater alteration followed by marine alteration (Johnson & Smellie Reference Johnson and Smellie2007) and they vary in age by up to 6 Myr (Smellie et al. Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008), thus giving the deltas time to be altered by percolating fluids. We normalized our samples relative to the elevation of the surfaces that separate marine from freshwater alteration (identified by Johnson & Smellie (Reference Johnson and Smellie2007) for each delta). In addition, one delta (Dobson) only experienced freshwater conditions, whilst another (Lachman main) was probably marine emplaced; these two deltas were used as controls for distinguishing marine from freshwater bioalteration effects. There is a relatively strong correlation between those samples with low bioalteration values (<2) that were affected only by freshwater, and marine-affected samples showing significantly higher values (Fig. 6), with only c. 15% of samples contradicting this observation. Note that the marine–freshwater transition may be a zone several metres thick rather than a precise elevation. The elevations used here (from Johnson & Smellie Reference Johnson and Smellie2007) may only be accurate to within ±5–10 m, and the presence of ‘marine-affected’ samples with apparently anomalous values close to the transition surface may simply be an artefact of the artificially ‘precise’ elevation used in our calculations (e.g. three supposedly marine-affected samples from the Forster Cliffs delta showing low (i.e., ‘freshwater’) values; Fig. 6).
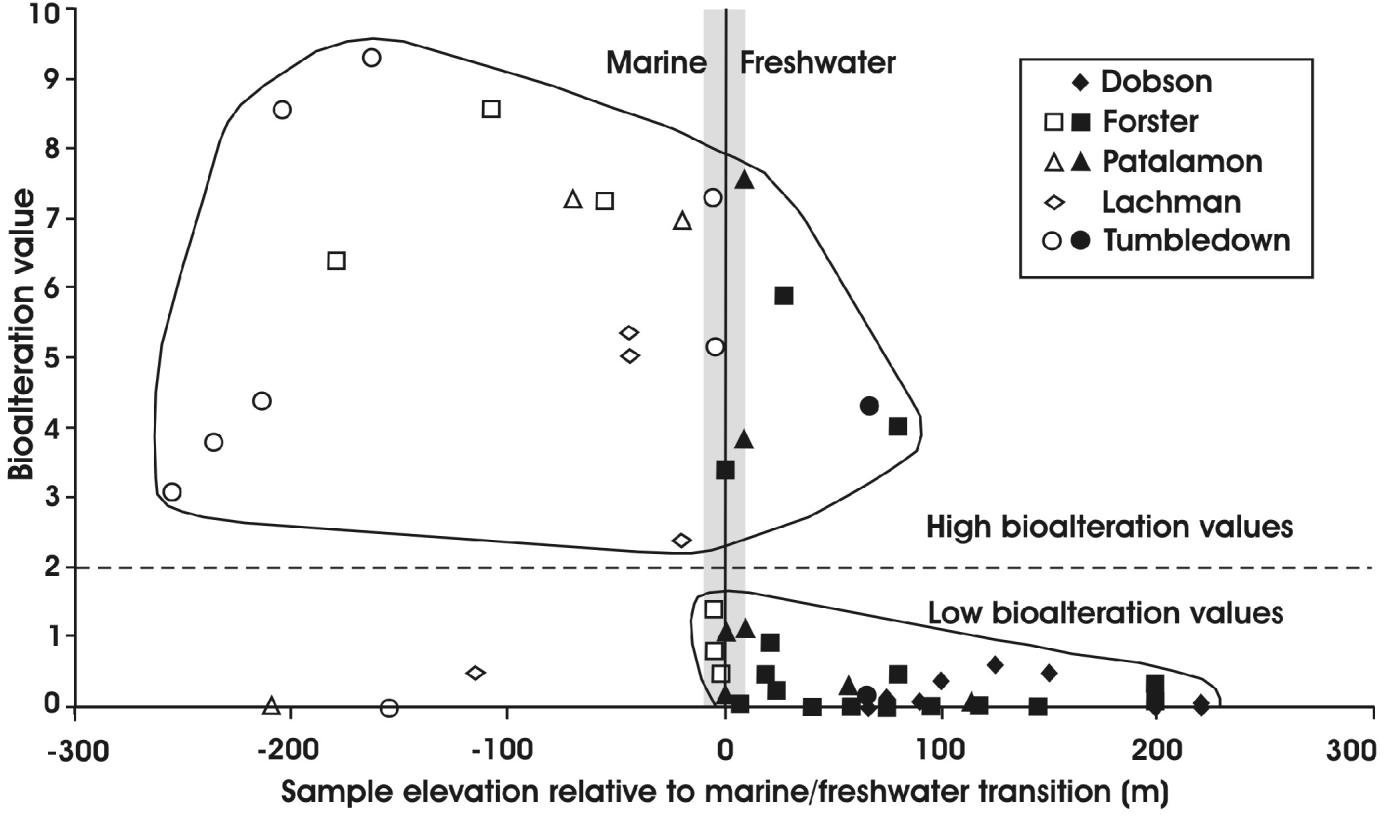
Fig. 6. Plot showing sample elevation and bioalteration values for James Ross Island hyaloclastites. Elevation is sample height (in metres) relative to the upper surface reached by seawater during later marine influxes (shown as vertical line at 0 m; surface identified by Johnson & Smellie Reference Johnson and Smellie2007). Samples below that surface experienced marine conditions after an initial freshwater environment, whilst higher samples experienced only freshwater conditions. The elevation of the marine surface is not precisely determined in all deltas, an uncertainty indicated here schematically by the grey band. Outside of the grey band only six samples out of 53 (11%) plot ‘anomalously’, and the dataset shows clearly that marine samples typically have significantly higher bioalteration values compared with those at higher elevations that experienced only freshwater conditions.
Fig. 7 shows bioalteration values plotted against delta age. Only the youngest delta (Dobson; <80 ka) appears to be almost free of bioalteration, whilst the other deltas show similar, widely varying levels of bioalteration values.

Fig. 7. Plot of bioalteration values against hyaloclastite age (i.e., emplacement age of the host lava-fed delta; ages from Smellie et al. Reference Smellie, Johnson, McIntosh, Esser, Gudmundsson, Hambrey and van Wyk de Vries2008). Although the youngest delta (Dobson Dome) shows very limited signs of bioalteration, there is overall no obvious relationship between bioalteration intensity and sample age.
Discussion
Our results for James Ross Island hyaloclastites are the first evidence for microbial (endolithic) alteration in basaltic glass in Antarctica, and they are also the largest dataset obtained for a glaciovolcanic sequence anywhere. They also demonstrate a relationship between bioalteration extent and alteration fluid source: seawater-affected samples generally show a much higher level of bioalteration compared with samples affected only by freshwater (glacial meltwater). The latter are characterized by extremely low bioalteration values (c. 83% of freshwater-affected samples have ‘absent/low’ bioalteration values; Figs 5 and 6). Thus, glacially-derived meltwater does not appear to strongly promote bioalteration, implying a very low bacterial biomass. Conversely, there appears to be no difference between the types of microbial textures found in either freshwater or marine altered lavas, pointing towards a common method by which the microbes dissolve or etch the glass, regardless of alteration fluid.
There are three main factors that could possibly explain the relationship between alteration fluid composition and the abundance of microborings. Firstly, the microbial biomass of seawater is significantly higher than that of glacial meltwater. During a subglacial eruption, the primary source of microbes is the overlying glacial ice. As a lava-fed delta eruption progresses, microbes might enter via meltwater pathways, fractures in the ice and from subaerial exposure of the delta top. Other (non-delta-forming) glaciovolcanic eruptions may remain entirely subglacial (e.g. eruptions that cease at the pillow volcano or tindar-forming stages; Smellie (Reference Smellie and Elias2007) ). Secondly, seawater is more nutrient rich than glacial meltwater, and combined with the basaltic glass substrate, may provide ideal conditions for microboring formation.
Intuitively, hyaloclastites that are older have had a longer residence time and thus might have had more time or opportunities to be altered by endolithic microorganisms. However, there appears to be no obvious relationship between delta age and intensity of bioalteration (Fig. 7). The youngest delta (Dobson; <80 ka) shows the least effects of bioalteration so we cannot discount the possibility that insufficient time has elapsed for bioalteration to occur in this case.
Fig. 8 shows a schematic sequence of events that might explain our observations of freshwater versus seawater bioalteration intensity and sample elevation. Delta hyaloclastite is initially altered by glacial meltwater, probably soon after emplacement when the volcanic pile is still warm. The hyaloclastite is a coarse-grained lithofacies relatively free of material finer than coarse sand, which facilitates the entry and circulation of freshwater (glacial meltwater). Our results suggest that only minor bioalteration takes place at this stage. During interglacial periods, the ice retreats and seawater penetrates the hyaloclastite, probably along fractures, grain boundaries and any unfilled pore spaces (cf. Johnson & Smellie Reference Johnson and Smellie2007). This is probably when the bulk of the microbial community was introduced, with a consequent increase in the intensity of microboring activity.

Fig. 8. Diagram of a simplified James Ross Island subglacial lava sequence showing different stages of fluid alteration and microboring production.
It is possible that it is simply the intensity of aqueous (abiotic) alteration experienced by the glass that promotes the development of endolithic microborings. If the thickness of palagonite rims on glass fragments and amount of zeolite formation within hyaloclastite are interpreted as a measure of the intensity of aqueous alteration, then comparison of our datasets for the two different environments suggests that there is no correlation with intensity of bioalteration. Our marine altered samples do not contain more secondary minerals than the glacially (freshwater) altered lavas. This observation is consistent with that of Johnson & Smellie (Reference Johnson and Smellie2007), who suggested that the majority of the alteration (of original glass and filling pore spaces) took place relatively soon after delta emplacement under freshwater conditions, with subsequent marine conditions mainly simply modifying the zeolite (and palagonite) compositions and filling any (few?) remaining pore spaces and fractures. This further implies that the formation of endolithic microborings in this volcanic province is controlled largely by the aqueous chemistry and origin of the alteration fluids.
Depth is thought to be one of the most important controls of bioalteration in oceanic lavas, whereby bioalteration decreases with depth due to elevated temperatures and lack of fluids (Furnes & Staudigel Reference Furnes and Staudigel1999; Furnes et al. Reference Furnes, Staudigel, Thorseth, Torsvik, Muehlenbachs and Tumyr2001b; Fisk et al. Reference Fisk, Storrie-Lombardi, Douglas, Popa, McDonald and Di Meo-Savoie2003). The greater intensity of bioalteration in our hyaloclastites occurs in the marine-affected samples, which probably took place at lower temperatures. However, our results suggest that the frequency of microborings in our samples is predominantly controlled by the type of alteration fluid.
Finally, our study seems to demonstrate that glacial meltwater is largely free of microbes, with consequently little bioalteration produced in glassy host rock such as delta hyaloclastites. If this is generally true, the consequences for Mars are important and we suggest that the chances of successfully detecting any exobiology in putative subglacially erupted volcanic constructs there (e.g. Chapman Reference Chapman1994; Chapman & Tanaka Reference Chapman and Tanaka2001; Ghatan & Head Reference Ghatan and Head2002; Head & Wilson Reference Head, Wilson, Smellie and Chapman2002; Chapman & Smellie Reference Chapman, Smellie and Chapman2007) are much reduced.
Conclusions
The study of the bioalteration of volcanic glass has almost exclusively focused on lavas in an oceanic setting, either associated with spreading ridges or oceanic plateaux. In contrast, our study contributes to the knowledge of bioalteration in a terrestrial subaqueous setting. The principal conclusions are as follows.
1. The JRIVG hyaloclastites show numerous examples of bioalteration, particularly formation of microtubules, which are characteristic of endolithic microboring biogenicity (cf. McLoughlin et al. Reference McLoughlin, Brasier, Wacey, Green and Perry2007).
2. Quantitative analysis of the intensity of bioalteration shows that there is much greater bioalteration intensity in samples that experienced marine conditions. Indeed, bioalteration is minor and is even absent in samples that were only affected by freshwater (in this case, glacially-derived meltwater). This shows that, additionally to eruptive setting, the composition of the alteration fluids exerts an important control on the intensity of endolithic microborings.
3. Variables that affect alteration fluids derived from different sources include microbial biomass, nutrient supply and aqueous chemistry. One or a combination of these could be the cause of the observed bias of microboring formation towards marine-affected samples.
4. Finally, this study shows that it is important to characterize bioalteration textures in environments other than seafloor lavas to gain a fuller understanding of their distribution and formation, particularly if such morphological features are to be used as Martian biosignatures. For example, if glacial meltwater is generally very poor in microbes, as suggested by our results, the chances of detecting exobiology in subglacially erupted Mars glassy rocks are significantly reduced.
Acknowledgements
This paper is dedicated to the memory of Dr David Wynn-Williams, formerly of BAS, an endolithic bacteria expert and pioneer astrobiologist who tragically died during an accident in 2002. It is published as a spin-off from the British Antarctic Survey's GEACEP programme (ISODYN Project) that seeks to investigate climate change over geological time scales. Claire Cousins acknowledges receipt of an EPSRC studentship award obtained through a UCL strategic, interdisciplinary research initiative. We also thank Dr Michael Storrie-Lombardi for reviewing the manuscript and his helpful comments, which have greatly improved the manuscript, and Bryan Prosser for advice on statistical analysis.












