Introduction
In the most arid deserts of Earth photosynthetic activity is restricted to microbial communities under or in translucent rocks in hypolithic or endolithic communities: Highly specialized communities dominated by cyanobacteria that support diverse heterotrophic assemblages (Antony et al. Reference Antony, Cockell and Shouche2012; Chan et al. Reference Chan, Lacap, Lau, Ha, Warren-Rhodes, Cockell, Cowan, McKay and Pointing2012). In those areas where water is too scarce the dry limit of life is reached because adaptation to desiccation is no longer possible and soil can lack detectable DNA (Navarro-González et al. Reference Navarro-González2003; Wierzchos et al. Reference Wierzchos, de los Ríos and Ascaso2013). Understanding the dry limit of life is critical when considering the possibility of life elsewhere; field investigations have shown that in extremely dry deserts life is rare occurring in isolated islands in depauperated bare soils; thus suggesting that if life ever existed or exists on Mars, microhabitats would be widely dispersed among virtually lifeless surroundings (Warren-Rhodes et al. Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gómez-Silva, Amundson, Friedmann and McKay2006).
The study of hypoliths across a moisture gradient in the Atacama Desert revealed that the community diversity decreased as rain levels decreased. As the mean annual precipitation fell from 10 cm year−1 to about 1 cm year−1 the fraction of quartz stones on the desert floor that were colonized dropped from almost 30% to <0.1% (Warren-Rhodes et al. Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gómez-Silva, Amundson, Friedmann and McKay2006). In the hyper-arid core of the Atacama Desert endolithic life forms thrive inside halites (Wierzchos et al. Reference Wierzchos, Ascaso and McKay2006; Stivaletta et al. Reference Stivaletta, Barbieri and Billi2012), where absorption of atmospheric water vapour by deliquescence provides an alternative source of water for life other than rainfall, fog or dew (Davila et al. Reference Davila, Hawes, Ascaso and Wierzchos2013). In the Namib Desert, the hypolithic community structure and the colonization rate were reported to be influenced by the moisture source changing from fog-dominated to rain-dominated (Stomeo et al. Reference Stomeo, Valverde, Pointing, McKay, Warren-Rhodes, Tuffin, Seely and Cowan2013; Warren-Rhodes et al. Reference Warren-Rhodes2013). An additional example of successful microbial exploitation in an otherwise harsh environment is given by hypoliths occurring under quartz pebbles in the Mojave Desert (Schlesinger et al. Reference Schlesinger, Pippen, Wallenstein, Hofmockel, Klepeis and Mahall2003, Bishop et al. Reference Bishop, Schelble, McKay, Brown and Perry2011).
A key aspect of the hypolithic and endolithic colonizations is given by the intensity of light reaching the organisms in the photosynthetically active radiation (PAR, 400–700 nm). Recently, a Monte Carlo model of light propagation in translucent stones was developed to describe the light levels on the bottom and subsurface edges of desert quartz (Jolitz & McKay Reference Jolitz and McKay2013) and showed the variation in light levels; for instance available light resulted 13–24 times higher at the sunward subsurface edge than at the basal surface.
In deserts, lithic communities are often dominated by cyanobacteria of the genus Chroococcidiopsis (Billi et al. Reference Billi, Baqué, Smith and McKay2013). The study of these cyanobacteria is of growing interest, because they thrive in the closest terrestrial analogues of Mars, and also because, by tolerating desiccation, high doses of UVC and ionizing radiations (Billi et al. Reference Billi, Friedmann, Hofer, Caiola and Ocampo-Friedmann2000; Billi Reference Billi2009; Baqué et al. Reference Baqué, Viaggiu, Scalzi and Billi2013c), they are suitable astrobiology model systems (for a review see Billi et al. Reference Billi, Baqué, Smith and McKay2013) and are currently part of two astrobiological experiments for the next EXPOSE-R2 mission on the International Space Station (de Vera et al. Reference De Vera2012; Baqué et al. Reference Baqué, Scalzi, Rabbow, Rettberg and Billi2013a, Reference Baqué, de Vera, Rettberg and Billib)
The Mojave Desert has long been studied for its characteristics similar to the terrain observed on Mars (e.g. Abbey et al. Reference Abbey, Salas, Bhartia and Beegle2013) and was listed as a Mars analogue by the Terrestrial Analogs Panel of the National Science Foundation-sponsored Decadal Survey (Farr Reference Farr2004). One of the primary rock types we consider here, carbonates, have been detected on Mars (see e.g. Brown et al. Reference Brown, Hook, Baldridge, Crowley, Bridges, Thomson, Marion, de Souza Filho and Bishop2010). In addition, images from the Mars Science Laboratory Curiosity rover have shown rocks with Mg-rich surface coatings which may be similar to desert varnish common in the Mojave (Lanza et al. Reference Lanza, Ollila, Cousin, Hardgrove, Wiens, Mangold and Vaniman2014). The red coating on the rock type labelled in our study as ‘red carbonate’ may be a type of desert varnish. Bishop et al. (Reference Bishop, Schelble, McKay, Brown and Perry2011) considered whether remote spectral observations could identify such carbonates on Mars. They showed that the red coatings greatly suppress the spectral features of the underlying carbonate.
In this paper, we investigate if translucent rocks with lithologies and slightly different optical properties influence hypolithic colonization when environmental conditions are virtually identical. Samples of talc, marble, quartz, carbonate and red-coated carbonate rocks inhabited by photosynthetic organisms were collected within a distance of a few kilometres in the Silver Lake region of the eastern part of the Mojave Desert. Rocks were characterized for the transmission of natural sunlight in the UV to the near infra-red (IR) (300–800 nm), while the cyanobacterial component of the hypoliths was investigated by in vivo detection of the emission spectra of the photosynthetic pigments and by assessing the genetic diversity by sequencing data inferred from clone libraries of the 16S rRNA gene.
Materials and methods
Rock samples
Marble, quartz, talc, white carbonate and red-coated carbonate rocks with visible hypolithic growth were collected within a few kilometres distance in five sites of the Silver Dry Lake bed in the arid region of the Mojave Desert (Fig. 1).

Fig. 1. Sites in the vicinity of Silver Lake, CA from which the colonized stones were collected. Numbered markers indicate locations for (1) marble, (2) quartz, (3) talc, (4) white carbonate and (5) red-coated carbonate. Centre of this image is at N35°22′18″, W116°12′00″ GPS coordinates. The distance between the two furthest sites is ∼10 km.
Light transmittance
Each rock was cleaned and the organisms were removed from the rock by removing the colonies with a small brush wetted with 70% isopropanol, the rock was then allowed to air dry. Once dried the rock was placed on top of the optical probe for transmittance measurements. The lip of the optical probe was flush with the rock to exclude ambient light.
All measurements in this study were conducted between 2 and 3 p.m. local time on a sunny, cloudless July day, under blue skies and low relative humidity (<10%). An Ocean Optics 4000× spectrometer, an Ocean Optics fibre optic cable (HR2B622) and the Ocean Optics SpectraSuite© software were used to obtain the light transmittance measurements. Measurements were taken at the beginning and at the end of the sampling period with the optical probe completely covered (with a brass hose cap) to determine darkness (zero light) and in full sunlight to assess complete transmittance (100%). To ensure that only light that had passed through the rock would be measured by the Ocean Optics fibre optics cable a seal was formed between the probe lip and the rock. The ‘transmittance’ feature in SpectraSuite was employed to determine the light transmittance from 200 to 900 nm for each rock. Three measurements were taken for each rock and averaged. Three rocks of each type were measured and averaged and normalized to 600 nm as a representative value in the visible. The results presented in Fig. 3 are from nine measurements of each rock type (3 rocks/type×3 measurements/rock=9). Our estimated error is ∼10% at 600 nm and increases in the near IR.
Confocal laser scanning microscopy
Small rock fragments with visible green growth were spotted onto 1.5% agarose in phosphate buffer and observed with a 60× (1.35 NA) Plan Apochromat oil immersion objective with a 3× zoom. Autofluorescence from the photosynthetic pigments was acquired by using a confocal laser scanning microscopy (CLSM; Olympus Fluoview 1000) by excitation with a 488, 543 and 635-nm laser, and collecting the emitted fluorescence in three channels simultaneously: blue (503–524 nm), green (555–609 nm) and red channel (655–755 nm emission range).
Three-dimensional images were captured every 0.5 μm and images were subsequently processed with Imaris v. 6.1.0 software (Bitplane AG Zürich, Switzerland) to obtain maximum intensity projections. The emission spectra of the photosynthetic pigments at the single cell were obtained by using a CLSM coupled to a spectrofluorometric detector (CLSM-λ scan); after excitation with a 543 nm laser fluorescence spectra were recorded from 553 to 800 nm and each image sequence (wavelength scan) was obtained by scanning the same x–y optical section using a bandwidth of 5 nm and steps of 5 nm. The region of interest (ROI) function of the software was used to determine the spectral signature of a selected area from the scanned image. The mean and standard errors for all the ROIs were calculated (n=20). Curve plotting and fitting were performed using the GraphPad Prism program (GraphPad Software, San Diego, CA). The sum of two Gaussians fitting was used to illustrate the presence of two main peaks in the fluorescence emission of the main pigments (phycobiliproteins and chlorophyll a). The goodness of fit was illustrated by the calculation of the R 2: control=0.982; white carbonate=0.986; marble=0.973; red-coated carbonate=0.965; quartz=0.972 and talc=0.931. The maximum of the two peaks was calculated by the software for hypolithic phototrophs under each rock type: CCMEE029 651 and 681 nm; white carbonate 660 and 686 nm; marble 615 and 683; red-coated carbonate 650 and 680; quartz 659 and 686 talc 654 and 682 nm.
DNA extraction from hypoliths
Genomic DNA was obtained from hypolithic growth visible under each rock by using a method previously used with environmental rock samples with phototrophic communities (Stivaletta et al. Reference Stivaletta, Barbieri and Billi2012). Briefly, after washing with sterile water, pellets were resuspended in 300 μl of phenol saturated with 0.1 M Tris hydrochloride (pH 7.4) and glass beads (20%, vol/vol) and subjected to four 2-min cycles of heating at 65 °C and vortexing for 30 s. After centrifugation the aqueous phase was extracted once with tris-phenol/chloroform/isoamyl alcohol (25 : 24 : 1); then 1/5 volume of TE buffer (1 mM EDTA [pH 8.0] and 10 mM Tris-hydrochloride [pH 7.4]) was added and the pellet extracted again with phenol. Finally the aqueous phases were extracted with chloroform/isoamyl alcohol (24 : 1) and nucleic acids precipitated overnight at −20 °C with cold ethanol and 0.3 M sodium acetate.
Amplification of 16S ribosomal RNA genes and clone library construction
The 16S rRNA genes were amplified by using as forward primer the cyanobacteria-specific primer CYA359F (5′-GGGGAATTTTCCGCAATGG-3′; Rudi et al. Reference Rudi, Skulberg and Jakobsen1998) and as reverse the universal primer C (5′-ACGGGCGGTGTGTAC-3′) corresponding to Escherichia coli numbering 1406–1392; the amplified fragments were used to construct clone libraries into pGEM-T Easy Vector (Promega) as described previously (Stivaletta et al. Reference Stivaletta, Barbieri and Billi2012). The PCR products were cloned using the PGEM-T Cloning kit (Promega, USA). After the screening of the positive clones (from 30 up to 50 clones for each environmental sample) the plasmid inserts were amplified using the M13 forward and reverse primer (Promega) and after purification digested with the restriction enzyme AluI (BioLabs) as described previously (Stivaletta et al. Reference Stivaletta, Barbieri and Billi2012). Clones with different digestion patterns were chosen for sequencing (BMR-Genomics, Padova, Italy). Sequence data have been submitted to NCBI GenBank database with accession numbers KF932305 to KF9323017.
Results
Different rock types, namely marble, quartz, talc, white carbonate and red-coated carbonate rocks from the Silver Dry Lake bed in the arid region of the Mojave, showed visible green hypolithic colonization (Fig. 2). Although the underside of the investigated rock was partially covered by phototrophic communities; phototrophic colonization did not seem to be influenced by specific rock features. No variation in thickness (minimum, maximum and most populated) was observed between colonized and non-colonized rock sections.

Fig. 2. Rock types colonized in the arid region of the Mojave Desert near Silver Dry Lake bed. Scale: each square is 0.5 cm on a side.
The spectral transmission measurements, shown in Fig. 3, are normalized to 600 nm. For the rocks measured, the absolute transmission at 600 nm was about 10% of incident solar and reflects a choice of locations on the rock that would give a strong signal to the spectrometer. In this study, we did not determine the lower limits of light that allow for hypolith growth under small translucent stones. Warren-Rhodes et al. (Reference Warren-Rhodes2013) using a sensitive PAR photometer did report such a study and found 0.1% of incident sunlight (∼2 μmol m−2 s−1) as the lower limit for hypolithic growth on small quartz rocks such as those studied here. As seen in Fig. 3, all rock types blocked UVB (the solar flux was recorded to 300 nm by the instrument but for all the rock types the normalized transmission fell to <1% before 350 nm). The rock types differed in transmitting violet-blue (400–495 nm) and UVA (320–400 nm) light (Fig. 3). The main differences in light transmission were in the wavelength at which the normalized transmission fell to 1%. This was 475 nm for white carbonate and quartz, 425 nm for red-coated carbonate and talc and 380 nm for marble.

Fig. 3. Light transmittance through diverse hypolithic colonized rocks found in the Mojave Desert. The light transmittance presented is for the rock only, after the biofilm of cyanobacteria were removed. All curves have been normalized to have the same value at 600 nm. Solar flux was recorded to 300 nm by the instrument but for all the rock types the normalized transmission fell to <1% before 300 nm.
Chroococcidiopsis-dominated hypoliths under different rocks
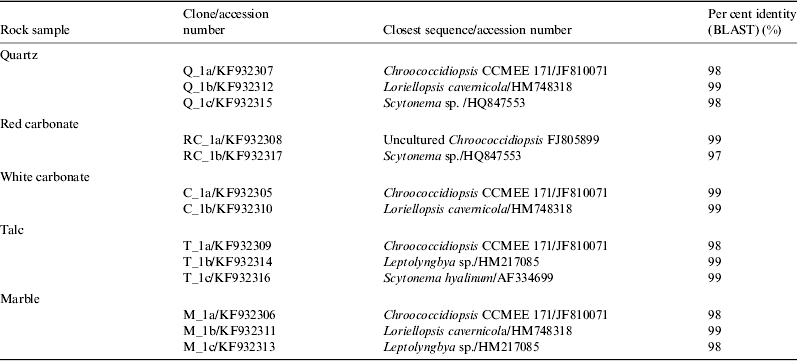
The comparative analysis of the cyanobacterial composition in hypoliths under five rock types was carried out by screening and sequencing five 16S rRNA gene clone libraries, of about 50–100 clones. A minimum of 20 clones per library were screened by restriction fragment length polymorphism (RFLP) analysis and the screening of a given library was considered exhaustive when no new RFLP-defined profiles with unique banding patterns were identified. BLAST analysis of the sequences of the selected clones showed that Chroococcidiopsis was the most commonly recovered phylotype from each rock (70–80%), although less abundant phylotypes were assigned to filamentous cyanobacteria of the genera Loriellopsis, Leptolyngbya and Scytonema (Table 1). An exhaustive CLSM analysis was performed to confirm the occurrence of rare filamentous cyanobacteria in addition to representatives of the genus Chroococcidiopsis: however not all the phylotypes identified by 16S rRNA gene sequencing were visualized (Fig. 4).

Fig. 4. CLSM 3D images of cyanobacterial communities in rocks from the Mojave Desert showing sporadic filamentous cyanobacteria in talc (a) and in marble (b), whereas aggregates of coccalean cyanobacteria were abundant in all rock types, including red-coated carbonates (c). Images were acquired by using 488, 543 and 635-nm laser and collecting the emitted fluorescence in the blue channel (503–524 nm) for calcium carbonate precipitation (grey), in the green channel (555–609 nm) for phycobiliproteins and in the red channel (655–755 nm) for chlorophyll a autofluorescence. Bar=5 μm.
Table 1. Phylotypes identified in different rocks from the Mojave Desert based on the phylogenetic analysis of 16S rRNA gene sequences

Different spectral fingerprints of Chroococcidiopsis under different rocks
The emission spectra of the photosynthetic pigments in Chroococcidiopsis occurring in different rocks were obtained with CLSM-λ scan analysis after excitation with a 543 nm laser. The spectra were confidently ascribed to Chroococcidiopsis on the basis of the cell morphology and the absence in the samples of 16S rRNA gene sequences of other chroococcalean cells (Table 1). The comparison of the mean fluorescence spectra of Chroococcidiopsis from marble, white carbonate and red-coated carbonate were not significantly different from that of Chroococcidiopsis sp. CCMEE 029 (Negev desert, Israel) grown under white light under laboratory-controlled conditions (Fig. 5). In particular, these spectra showed a broad emission peak at 650–660 nm from the overlap of chlorophyll a and phycobiliproteins (phycocyanin and allophycocyanin) and lacked the emission peak at 580 nm due to the absence of phycoerythrin (Roldán et al. Reference Roldán, Thomas, Castel, Quesada and Hernández-Mariné2004). When compared to the emission spectrum of the laboratory reference strain, the spectral fingerprints of Chroococcidiopsis- in quartz and carbonate showed a 10 nm red shifted and that of cells from talc a 5 nm red shift (Fig. 5).

Fig. 5. CLSM-λ scan of Chroococcidiopsis in different rocks from the Mojave Desert and Chroococcidiopsis sp. CCMEE 029. (a) Spectral emissions of the photosynthetic pigments with a 543-nm excitation laser, representing the mean fluorescence intensity versus emission wavelength. Data are mean fluorescence intensity±standard deviation. (b) CLSM images (optical sections) corresponding to the maximum emission peaks of the photosynthetic pigments of cells from talc, quartz and carbonate excited with the 543-nm laser. Bar=5 μm.
Discussion
The Mojave Desert in Southern California contains within a distance of a few kilometres, a variety of hypolithic rocks (talc, marble, quartz, carbonate and red-coated carbonate), as such this location provides the opportunity to determine if rocks with different lithologies and slightly different optical properties influence hypoliths when environmental conditions are virtually identical. Hence, the present work provides the first investigation of hypoliths developed in a given environment but under rocks with different optical properties.
The assessment of the transmission of the sunlight in the UV to the near IR (300–800 nm) revealed that all the rocks blocked UVB radiation but differed in the transmission of blue and UVA light; indeed light transmission fell to 1% of the transmission at 600 nm at 475 nm for white carbonate and quartz, at 425 nm for red-coated carbonate and talc and at 380 nm for marble.
Despite differences in light availability for photosynthesis, the comparative analysis of the cyanobacterial component in the different hypoliths, as revealed by 16S rRNA gene clone libraries, showed no apparent variation with rock type. No exclusive phylotypes were identified in a given rock type thus suggesting that despite differences in light availability, cyanobacteria phylotypes under the different rocks showed no apparent variation: hypoliths were dominated by phylotypes of the genus Chroococcidiopsis, although less abundant environmental sequences of the genera Loriellopsis, Leptolyngbya and Scytonema, were identified; furthermore, each stone supported a number of unique 16S rRNA gene-defined genotypes. These results are in line with previous reports on Chroococcidiopsis-dominated hypoliths in hot deserts worldwide (reviewed by Chan et al. Reference Chan, Lacap, Lau, Ha, Warren-Rhodes, Cockell, Cowan, McKay and Pointing2012). The 16S rRNA gene libraries here generated were judged large enough to cover the genetic diversity of the environmental samples due to an even frequency distribution of phylotypes along with the preponderance of the same phylotypes in each library were observed (Kemp & Aller Reference Kemp and Aller2004). Furthermore, a 16S rDNA library of about 100 clones was reported to cover the diversity of hypoliths in the Atacama Desert due to the relatively low diversity occurring in this extreme environment (Lacap et al. Reference Lacap, Warren-Rhodes, McKay and Pointing2011).
Variations in the spectral emissions of Chroococcidiopsis cells living in different rocks were evaluated by means of CLSM-λ scan; a method that allows in vivo determinations of fluorescence spectra at single-cell levels (Roldán et al. Reference Roldán, Thomas, Castel, Quesada and Hernández-Mariné2004). The spectral fingerprints of cells in red-coated carbonate and marble did not differ from that of a laboratory-grown strain (Chroococcidiopsis sp. CCMEE 029); whereas spectral fingerprints with a red shift of 10 nm occurred in Chroococcidiopsis in quartz and carbonate and a 5 nm red shift in cells from talc. Since cyanobacteria in the emission peak at 650–660 nm is considered due to the overlap of chlorophyll a and phycobiliproteins, phycocyanin and allophycocyanin (Roldán et al. Reference Roldán, Thomas, Castel, Quesada and Hernández-Mariné2004), the observed red shift might depend on modifications of the phycobilisome composition of Chroococcidiopsis under quartz and carbonate and talc. Since only Chroococcidiopsis phylotypes occurred under each rock, it was not possible to determine if different phylotypes under the same rock exhibited the same peaks. When possible, such as for talc sample the comparison of the spectrum of Leptolyngbya and Chroococcidiopsis indicated that they were similar in shape and different in the height of the emission peaks (not shown).
Cyanobacteria alter the phycobilisome composition to maximize light-harvesting efficiency in fluctuating light environments, a phenomenon known as chromatic adaptation (Gutu & Kehoe Reference Gutu and Kehoe2012). A few cyanobacteria were recently reported to have red-shifted chlorophyll d and f (Chen et al. Reference Chen, Schliep, Willows, Cai, Neilan and Scheer2010, Reference Chen, Li, Birch and Willows2012), being thus able to extend oxygenic photosynthesis from visible to IR. In the present paper, even though red-shifted chlorophylls were not revealed by CLSM-λ scan, the potential for exotic pigments cannot be ruled out, and therefore, it is worthwhile to further investigate this possibility by exposing cyanobacteria from red-coated carbonates to red-light, as it was reported with stromatolites when isolating cyanobacteria with red-shift chlorophyll (Chen et al. Reference Chen, Li, Birch and Willows2012). The observed red shift might reflect the versatility of Chroococcidiopsis in inhabiting dry niches with different light availability for photosynthesis, and supports the role of this cyanobacterium as pioneer phototroph in different extreme environments on Earth.
In the present work, the assessment of the light transmission of different rock types pointed out that the red-coated carbonate had the highest IR transmission and that in principle might support near IR chlorophylls; this is interesting because the possibility of IR oxygenic photosynthesis has implications for exotic photosynthesis (O'Malley-James et al. Reference O'Malley-James, Raven, Cockell and Greaves2012).
As mentioned above, rocks on Mars, imaged by Curiosity's MastCam at Yellownife Bay in Gale Crater, Mars, bear a superficial resemblance to Mojave rocks and in particular the red-coated carbonate type. Fig. 6 shows a comparison of the red-coated carbonate to the Sutton_Inlier rock imaged on the surface of Mars after the rover cracked the rock by rolling over it. It was suggested that the Mojave red-coated carbonates are a model for carbonates on Mars and that the coatings on carbonate rocks reduce the strength of the carbonate bands, causing changes in the shape of some bands – consistent with orbital data from Mars (Bishop et al. Reference Bishop, Schelble, McKay, Brown and Perry2011).

Fig. 6. Comparison between a red-coated carbonate hypolith from the Mojave (a) and a close up of a rock on Mars called ‘Sutton_Inlier’ (b). The Mars rock is about 12 cm wide at the end closest to the camera and was imaged on the surface of Mars after the rover cracked it by rolling over it. NASA Image PIA16804.
Acknowledgements
This research was funded by the Italian Ministry of Foreign Affairs, Direzione Generale per la Promozione del Sistema Paese and NASA's Astrobiology Science and Technology for Exploring Planets (ASTEP) and the NASA Graduate Student Research Programme. The authors thank Dr Elena Romano, Centre of Advanced Microscopy of ‘Tor Vergata’ University, for skilful assistance in using the facility.