Introduction
The somatic nervous system is very sensitive to weightlessness conditions in space as well as in simulated hypogravity on the Earth. Muscle weakness, atonia and atrophy of skeletal muscle fibres constitute the hypogravity motor syndrome that develops in response to microgravity (Grigoriev et al. Reference Grigoriev, Koslovakaya and Shenkman2004). Moreover, neuromorphological and histochemical studies of lumbar motoneurons of the spinal cord (SC) revealed a reduced functional activity of neurons after space flight (Krasnov Reference Krasnov1994). After a space flight, a low activity of succinate dehydrogenase and significantly reduced protein content were recorded in the cytoplasm of motoneurons in the ventral horns of the rat SC (Gorbunova & Portugalov Reference Gorbunova and Portugalov1976; Ishihara et al. Reference Ishihara, Yamashiro, Matsumoto, Higashibata, Ishioka, Shimazu and Ohira2006). However, in experiments with 35-day antiorthostatic hindlimb suspension the changes in spinal lumbar motoneurons neurons had a mostly functional character (Islamov et al. Reference Islamov, Mishagina, Tyapkina, Shajmardanova, Eremeev, Kozlovskaya, Nikolskij and Grigorjev2011).
Neurons of the SC are topographically organized into layers (Rexed's lamina). Lamina I and II neurons respond to noxious or thermal stimulation. Laminae IV and V include the neurons that give rise to the spinothalamic tract. Cells in laminae III and IV receive a convergent input from mechanoreceptors. Lamina V and VI neurons receive direct input from low-threshold Aδ and Aβ afferents and respond to touch and pressure. Lamina VI forms the base of the dorsal horn. It consists of a narrow band of darkly stained compactly arranged neurons and is present mainly in the enlargements. The borders with the neighbouring laminae V and VII are ambiguous. Lamina VII is often included in the ‘intermediate grey matter’ of the spinal cord. Lamina VII contains several well-defined nuclei, including the nucleus dorsalis (Clarke's column) and preganglionic neurons located in the intermediolateral nucleus (sympathetic neurons), and the sacral autonomic nucleus (parasympathetic neurons). Lamina VII neurons receive information from laminae II to V as well as visceral afferent fibres, and they serve as an intermediary relay in transmission of visceral motor neurons impulses. These interneurons often interconnect with one another and receive processed signals from sensory systems and eventually (often through some chain of interneurons) connect with motor neurons. Laminae VIII and IX are located in the ventral horn. Area X is the area surrounding the central canal (Willis & Coggeshall Reference Willis and Coggeshall1991).
Excitability of SC neurons increased under weightlessness conditions in space. It was supported by augmentation of H-reflex (Sato et al. Reference Sato, Miyoshi, Nakazawa, Yano and Takeoka2001). An amplitude of spinal reflexes is determined by functional condition of motoneurons and by level of presynaptic inhibition from peripheral and intraspinal structures. α-motoneurons make axonal collaterals projecting to Renshaw cells participating in recurrent inhibition of spinal motoneurons with inhibitory glycinergic synapses (Renshaw Reference Renshaw1941; Alvarez et al. Reference Alvarez, Benito-Gonzalez and Siembab2013). Renshaw cells and Ia inhibitory neurons of the intermediate zone of the SC also contain calcium-binding protein calbindin (CB) (Carr et al. Reference Carr, Alvarez, Leman and Fyffe1998).
CB is a calcium-binding protein involved in numerous functions, including cell signalling, calcium uptake and transport, cell motility and intracellular calcium buffering (Schwaller Reference Schwaller2012). CB-containing Renshaw cells located in the ventral horn receive glutamatergic inputs from muscle spindle afferent terminals and motoneuronal cholinergic terminals (Thirumalai et al. Reference Thirumalai, Behrend, Birineni, Liu, Blivis and O'Donovan2013). CB immunoreactivity disappeared from these cells following disconnection of motoneurons from their muscles either by axotomy or by intramuscular administration of botulinum toxin, only to reappear following functional reconnection of motoneurons and muscles (Sanna et al. Reference Sanna, Celio, Bloom and Rende1993).
In studies of the SC under microgravity condition, the most attention was focused on lumbar motoneurons. Interneurons as well as thoracic motoneurons were not studied. However, thoracic inspiratory skeletal muscles are also gravity-dependent (Venturoli et al. Reference Venturoli, Semino, Negrini and Miserocchi1998; Segizbaeva et al. Reference Segizbaeva, Pogodin, Lavrov, Balykin and Alexandrov2011). It is also known that the stimulation of muscle afferents or even passive movement of extremity may elevate the central inspiratory activity (Concu Reference Concu1989; Sonetti et al. Reference Sonetti, Wetter, Pegelow and Dempsey2001). Thus, hypokinesia may lead to decrease of respiratory activity and also influence to morphological and functional characteristics of not only lumbar, but also thoracic spinal neurons. The most works analysed the functional state of inspiratory muscles at space flight but not the morphology and physiology of the thoracic SC neurons.
Thoracic segments of SC have also preganglionic sympathetic neurons. There are some data that baseline sympathetic outflow increases during space flight (Eckberg & Neurolab Autonomic Nervous System Team, Reference Eckberg2003). Unfortunately, there are no data about changes in the morphology and neurochemistry of that group of neurons in the microgravity condition.
Thus, the aim of the current study was to determine the location and morphology of CB-immunoreactive (IR) spinal thoracic neurons in control mice and after a space flight.
Materials and methods
Animal groups
Pathogenic free male C57BL/6N 4–5-month-old mice were obtained from the Animal Breeding Facility – Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Pushchino, Moscow Region, Russia. There were three animals (n = 3) in this main study group. During the space flight, the animals were provided with paste-like feed with an energy value of 361.4 kcal/100 g of dried feed.
The following control groups were formed. There were three animals in control group 1 (n = 3), which were housed in the animal-breeding facility (vivarium) during the space flight of the BION-M1 biosatellite.
Control group 2 consisted of three animals (n = 3), which served as synchronous control animals and were left under 3 months surveillance (since the date of the biosatellite launch). These animals were housed in a model of a space flight vehicle and underwent the entire cycle of prelaunch preparation tests (including the 72 h prelaunch housing of the animals in the spacecraft with all conditions of a space flight artificially reproduced, including the dietary pattern and gas composition of the air inflated). The age of the animals at the start of the synchronous experiment was similar to the age of the animals of the main study group. They were provided with the same feed as the main study group.
The hardware for mice housing in-flight consisted of five individual habitats. Paste ‘space’ food was pumped from the container and distributed between individual habitats of the assembly six times daily (every 4 h at 00:00, 04:00, 08:00, 12:00, 16:00 and 20:00), a total of 54 g day−1 per three mice. During the initial stage of training, mice were provided with standard pelleted food (Assortiment Agro, Russia) and deionized water given ad libitum. Two weeks prior to launch, and the start of the ground control (control group 2) experiment, the animals were adapted to the flight paste food diet. Both the paste and standard diets (pelleted food and water) were first presented together; after 2–3 days the water bottle was removed, followed in another 2–3 days by the removal of the pelleted food. Vivarium control group (control group 1) was fed standard chow and water throughout the experiments. The differences in bodyweight between spaceflight, vivarium control and ground control groups were not significant (Andreev-Andrievskii et al. Reference Andreev-Andrievskii, Shenkman, Popova, Dolgov, Anokhin, Soldatov, Vinogradova, Ilyin and Sychev2014).
On April 19th, 2013, the Bion-M1 biosatellite was launched into orbit via a Soyuz 2-1a rocket from the Baikonur Cosmodrome. The Bion-M1 capsule flew a 30 day mission and landed in the Orenburg region of Russia. Recovery personnel retrieved the animal module habitats from the biosatellite and initial health inspections were performed onsite in a field laboratory. The space flight mice were then flown to Moscow, Russia and transported to the Institute for Biomedical Problems.
All of the animal experimental procedures were approved by the Commission on Biomedical Ethics of the State Scientific Center of the Russian Federation, Institute for Biomedical Problems, the Russian Academy of Sciences.
Tissue preparation
The animals were sacrificed 13–15 h after landing by cervical dislocation under the supervision of a NASA veterinarian. Mice in the control groups were sacrificed 2 days following experiments with the space flight mice. Methods of euthanasia and SC dissection were performed identically to that of the space flight group.
SC was rapidly removed and fixed overnight with 4% paraformaldehyde (PF) in 0.1 M phosphate buffer. Following fixation, SC was washed in three 30 min changes of phosphate-buffered saline (PBS; 0.01 M; pH 7.4), cryoprotected by overnight immersion in 30% buffered (pH 7.4) sucrose solution at 4°C, embedded in Tris-buffered saline tissue-freezing medium (Triangle Biomedical Science, Dirham, NC). Experiments were performed on the Th3–Th6 segments of the SC. Fourteen-μm-thick cross-sections were cut with a cryostat, mounted on poly-L-lysine-coated slides and air-dried for 1 h.
Immunohistochemistry
Transversal sections of the SC were processed for CB immunohistochemistry as described previously (Masliukov et al. Reference Masliukov, Korobkin, Nozdrachev and Timmermans2012). The sections were pre-incubated for 30 min at room temperature with the blocking buffer containing 5% normal donkey serum (NDS, Jackson ImmunoResearch Laboratories, USA) and 0.3% Triton X-100 (Sigma, USA) in PBS to prevent non-specific binding of secondary antibodies. The sections were then incubated overnight at 4°C in rabbit anti-CB serum (ab 11426, Abcam, UK) diluted 1 : 500 in blocking buffer. After five rinses in PBS, sections were incubated in FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, USA) diluted 1 : 200 in blocking buffer for 1.5–2 h at room temperature. After three rinses in PBS, sections were counterstained for 20 min at room temperature with NeuroTrace red (Molecular Probes, N-21482, USA) diluted 1 : 200 in blocking buffer. The sections were then rinsed three final times in PBS, mounted on glass slides, allowed them to dry overnight and coverslipped using VectaShield (Vector Bioproducts, USA). The control experiments were carried out with the primary antibody replaced with NDS.
Image processing and statistics
Different parts of the SC were identified according to Sidman et al. (Reference Sidman, Angevine and Pierce1971); the laminae of Rexed were identified according to the description by Molander & Grant (Reference Molander, Grant and Paxinos1995) for the rat, which equally applies to that of the mouse (Li & Clark Reference Li and Clark2001).
The specimens were examined using an Olympus BX43 fluorescence microscope (Tokyo, Japan) fitted with filter sets that allowed separate visualization of FITC or NeuroTrace red. Images from the fluorescence microscope were recorded using a TCH 5.0 cooled charge-coupled device digital camera and ISCapture for Windows imaging software (Tucsen, China). Each image was processed using a sharpen filter and contrast and brightness adjustment only. All photomicrographic plates were made using Adobe Photoshop 7.0 software (Adobe Systems, USA).
To calculate the number of CB-IR neurons in the SC, sections were taken at a distance of 70 μm (every fifth section, 15 sections per animal). To avoid duplicate counts of neurons in the serial sections of the SC, only those nerve cell bodies containing a clearly identified nucleus were counted in any given section. The number of CB-IR neurons and the cross-sectional areas of CB-IR neurons were determined at 200-fold magnification using ImageJ software (http://imagej.nih.gov/ij/index.html).
Statistical methods include calculation of the mean and standard error of the mean. Differences in means were subjected to one-way ANOVA, followed by Tukey's post-test of multiple comparisons. Differences were considered statistically significant if P < 0.05.
Results
The scientific programme of the Bion-M1 project was aimed at obtaining data on mechanisms of adaptation of muscle, bone, cardiovascular, sensorimotor and nervous systems to prolonged exposure in microgravity and during post-flight recovery. To this aim, functional measurements in vivo were combined with complementary morphological, biochemical, cellular and molecular studies performed in vitro. The principal animal species for physiological studies in this mission was the mouse. The programme had to be performed with a limited number of animals (Andreev-Andrievskii et al. Reference Andreev-Andrievskii, Shenkman, Popova, Dolgov, Anokhin, Soldatov, Vinogradova, Ilyin and Sychev2014). In our part of this large study, we obtained the data about the location and morphology of CB-IR spinal thoracic neurons in control mice and after a space flight.
CB-IR neurons in control mice
The results showed that in control mice (weight 26.9 ± 1.16 g), CB-IR interneurons were found in all sections of the SC. No statistically significant differences in size, number or location of the neurons were found between the control groups 1 and 2. Thus, the data from control groups were meaned.
CB-IR interneurons were found in the dorsal horn in laminae I–V, in intermediate zone – in lamina VII, in ventral horn – laminae VIII and IX and were not detected only in the lamina X (Fig. 1(a) and (b)). The intensity of fluorescence was similar for all groups of CB-IR neurons. CB immunoreactivity was determined in the neuronal somata and processes. CB in the nerve cell bodies filled the cytoplasm and proximal processes but appeared to be absent from the distal processes. The nucleus was IR, sometimes more so than the cytoplasm.

Fig. 1. Fluorescence micrographs of CB-IR interneurons (green) and CB-negative neurons (red) in the grey matter of the SC of control mice. Roman numerals – Rexed laminae. Lines – the boundaries of lamina X (area of the centre channel) of the laminae V (dorsal horn) and VII (intermediate region). (a) Dorsal horn and the intermediate zone; (b) Intermediate zone and the ventral horn. Bar, 200 μm.
CB-IR neurons of laminae I and II had oval or fusiform shape, CB was detected only in the somata (Fig. 2(a)). Interneurons of laminae I and II were located in the superficial region of the dorsal horn, where thermo- and nociceptive small myelinated Aδ and non-myelinated C fibres terminate (Craig et al. Reference Craig, Zhang and Blomqvist2002; Li et al. Reference Li, Sakamoto, Kawate, Cheng, Li, Shimada and Atsumi2005).
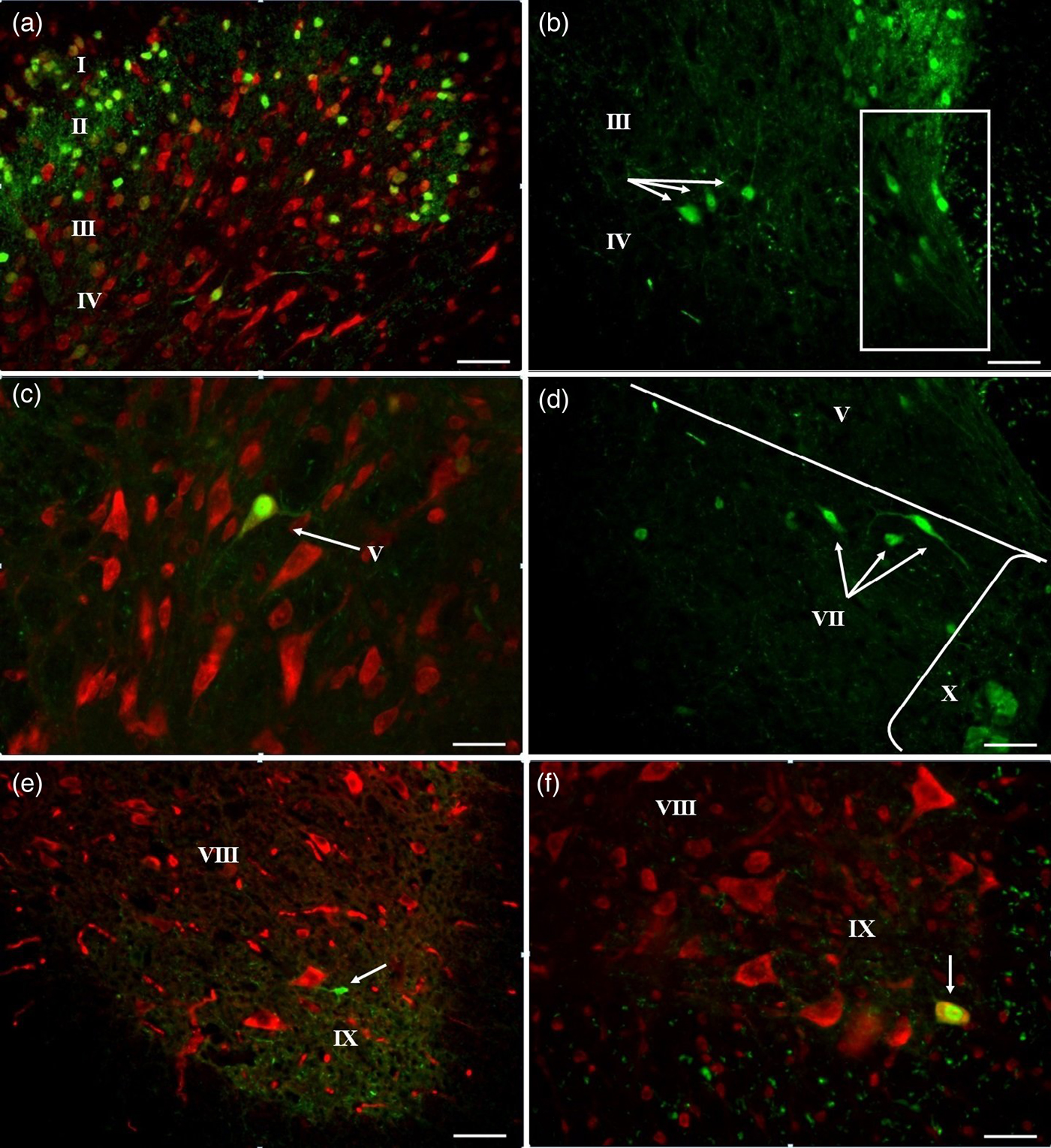
Fig. 2. Fluorescence micrographs of CB-IR neurons (green) in the thoracic segments of the SC in control mice. Legend: Roman Numerals – Rexed laminae, arrows – CB-IR cell, lines – the area of the grey matter. (a) Dorsal horn, (b) laminae III–IV and MB (in frame), (c) lamina V, (d) lamina VII, (e, f) laminae VIII and IX. Bar, 200 μm (a, e), 100 μm (b, d, f), 50 μm (c).
CB-IR neurons of laminae III and IV had only fusiform shape, CB immunofluorescence was found in the cell bodies and in the processes. Processes of CB-IR neurons projected in the dorsal (to lamina II) and ventral (to lamina X) directions, their length was 10 μm in both directions (Fig. 2(a) and (b)). Interneurons of laminae III and IV we combined into one group in accordance to their morphological similarity to antenna-like neurons (Schoenen Reference Schoenen1982) and functional unity of laminae III and IV where mechanosensory Aδ fibres end.
In lamina V, CB-IR interneuron profiles had triangle and fusiform shape and were oriented dorsoventrally (Fig. 2(c)). Their processes spread to 30 μm in dorsoventromedial direction. In the area of medial dorsal horn or medial border (MB) of the SC, CB-IR neurons had oval and fusiform shape with longer processes (length to 35 μm) oriented dorsomedially (Fig. 2(b), the region is marked by frame).
In the lamina VII, two subpopulations of CB-IR neurons were found. The first was presented by cells, located in the dorsal area and having fusiform and triangle shape of somata oriented mediolaterally. Cell bodies gave rise to 2–3 processes projected mediolaterally and intermixed with dendrites of neighbour cells forming cellular ‘chain’ (Fig. 2(d)). These cells were located in the area of the intermediolateral autonomic nucleus (Clarke et al. Reference Clarke, Dekaban and Weaver1998; Deuchars et al. Reference Deuchars, Milligan, Stornetta and Deuchars2005). Neurons from the second group were distributed diffusely and were presented by single cells or groups of 2–3 neurons. Neurons of this group had oval body with 3–4 radial processes or fusiform somata with 2–3 processes extended to the ventral horn (Fig. 1(a)). These neurons are similar with ‘partition cells’ making ipsilateral connections with motoneurons of the ventral horn, interneurons of the dorsal horn and with primary afferents (Barber et al. Reference Barber, Phelps, Houser, Crawford, Salvaterra and Vaughn1984; Stepien et al. Reference Stepien, Tripodi and Arber2010). Some authors referred them to commissural interneurons with radial processes making synapses with contralateral located neurons (Bertrand & Cazalets Reference Bertrand and Cazalets2011).
In the lamina VIII, CB-IR neurons were round-shaped; CB immunoreactivity in processes was not found (Fig. 1(b)).
In the lamina IX, also two groups of CB-IR neurons were found. The first one was located at the apex of the ventral horn. Neurons of this group had oval shape and 3–4 thin processes with length to 40 μm. Interneurons of lamina IX were often located at the origin of ventral horn and had smaller size (72.7 ± 14.14 μm2) (Fig. 2(e)) in comparison with CB-IR motoneurons of the ventromedial nucleus (216.2 ± 2.94 μm 2) (Fig. 2(f)) located in the ventral part of the lamina. On the basis of the presence of CB, location of cell bodies and distribution of neuronal processes at the area of the apex of the ventral horn, we suggested that these interneurons are Renshaw cells participating in recurrent inhibition of spinal motoneurons (Renshaw Reference Renshaw1941; Carr et al. Reference Carr, Alvarez, Leman and Fyffe1998; Alvarez et al. Reference Alvarez, Benito-Gonzalez and Siembab2013).
The maximal number of CB-IR interneurons was found in the lamina II (42 ± 4), the number of CB-IR neurons in lamina I was smaller (10 ± 1, Table 1). 3–4 cells per section were found in laminae III–IV–V–VII. Only single neurons on every third section were found in lamina VIII, and only one Renshaw cell and two motoneurons per section were revealed in lamina IX.
Table 1. Number of CB-IR neurons in the grey matter of the SC (per section, n = 45)

a Statistically significant differences in comparison with control mice (P < 0.05).
CB-IR motoneuron profiles were the largest in comparison with interneurons (Table 2). The cross-section area of neurons of laminae III–V and VII–VIII was smaller. Interneuron profiles of laminae I and II had the smallest size (P < 0.05).
Table 2. Cross-sectional area (μm2) of CB-IR neurons in the grey matter of the SC (n = 100 for laminae I and II, n = 20–40 for all other laminae)

a Statistically significant differences in comparison with control mice (P < 0.05).
CB-IR neurons in mice after space flight
In mice after a space flight (weight 28.9 ± 1.73 g), CB-IR interneurons were not found in every lamina. They were absent in the MB area, laminae VIII and X (Fig. 3(a)).

Fig. 3. CB-IR interneurons (green) in the thoracic segments of the SC after a space flight. Legend: Roman Numerals – Rexed laminae, arrows – IR cell, circle – CB-IR motoneuron in lamina IX, lines – the area of the grey matter. (a) Laminae VII–IX, (b) laminae I–IV, (c) lamina IV, (d) laminae V and VII, (e) lamina IX. Bar, 200 μm (a, d), 100 μm (b, e), 50 μm (c).
In laminae I–III CB-IR interneurons had oval (primarily in lamina II) and fusiform (primarily in laminae I and III) shape. In these neurons, CB immunofluorescence was observed in nuclei, and CB immunoreactivity was not found in the cytoplasm or processes (Fig. 3(b)).
In neurons of lamina IV, CB immunoreactivity was found in all cell structures, including nucleus (the brightest fluorescence) and cytoplasm with processes. The form of neuronal bodies was fusiform or triangle. But also neurons with another form and size were found on every section. They were divided into two groups. The first one was represented by very large neurons (Fig. 3(b) and (c)). Their cross-sectional areas (343.4 ± 57.37 μm2) were larger even in comparison with motoneurons (P < 0.05). Neurons from this group had round profile of cell body with large centrally located nucleus, immunonegative nucleolus and 5–6 long thin radial processes with the length to 100 μm.
In lamina V, fusiform and triangle CB-IR neurons were found. CB immunofluorescence was identified only in nuclei. In the MB, CB-IR interneurons were not detected.
In the intermediate region (lamina VII), two groups of the CB-IR neurons were found: sympathetic preganglionic neurons and ‘partition cells’ (Fig. 3(d)).
In lamina IX, CB-IR Renshaw cells were not observed but two groups of CB-IR motoneurons were found. The first one was located at the apex of the ventral horn (Fig. 3(a)). The cross-sectional area of profiles of these neurons was 210.7 ± 7.59 μm2. Neurons of the second group were found in the dorsal part of the lamina (Fig. 3(e)). The cross-sectional area of their profiles was 281.8 ± 6.59 μm2. In neurons of lamina IX, only nucleuses were CB-IR.
The maximal number of CB-IR neuron profiles was found in lamina II (67 ± 1), and the quantity of CB-positive cell profiles in lamina II was significantly smaller (17 ± 1) (P < 0.001). In laminae III–V, VII and IX, only 2–3 neuron profiles per section were observed (Table 1). Thus, the number of CB-IR in SC was not very large, except laminae I and II. In comparison with the control groups, the number of CB-IR neurons increased in laminae I, II and IX (P < 0.01) and decreased in laminae III–IV and VII ('partition сеlls’, P < 0.05).
Discussion
The present study reveals a number of morphological changes of CB-IR neurons of different laminae in the thoracic segments of the SC after space flight. The number of CB-IR neurons increased in laminae I, II and IX, but CB disappeared in neurons of MB (lamina V) and lamina VIII. Weightlessness did not affect the number of CB-IR neurons in laminae III–V (except MB) and VII, including preganglionic sympathetic neurons.
Thus, the maintenance of CB expression in different neuronal populations might depend on the activity and integrity of neurons. We suggest that the increasing in the number of CB-IR interneurons in laminae I and II is explained by the appearance of expression of CB in neurons that previously were CB-negative. Loss of afferent information under microgravity condition may lead to imbalance in the complexity of fine-tuned synaptic communication between excitatory and inhibitory synapses to hypo- and hyperexcitability of different groups of interneurons.
We suggest that in the MB, there is a separate group of CB-IR interneurons different on their morphology from dorsal horn interneurons. Our data are in agreement with earlier morphological studies of distribution of interneurons in lamina IV (Rexed Reference Rexed1952). The same groups of CB-IR lumbar interneurons were found after retrograde labelling of spinothalamic pathways (Craig et al. Reference Craig, Zhang and Blomqvist2002; Sojka et al. Reference Sojka, Zacharova, Spicarova and Palecek2010).
Motoneurons of the ventral horn (lamina IX) also contain choline acetyltransferase (ChAT), the enzyme that synthesizes acetylcholine. The percentage of ChAT-IR neurons colocolazing CB varies in different species from 2% in turtle to 56% in humans (Fahandejsaadi et al. Reference Fahandejsaadi, Leung, Rahaii, Bu and Geula2004; Morona et al. Reference Morona, Lopez, Dominguez and Gonzalez2007). In our previous work, we found that lumbar motoneurons have IR to neurofilament 200 (NF200) and some of them also colocalize CB. NF200 is a 200 kDa protein that is expressed in neurofilament-rich neurons and is often used as a marker for myelination. NF200 is usually located in large neurons (Porseva et al. Reference Porseva, Shilkin, Korzina, Smirnova and Maslyukov2013). NF200-IR neurons have larger size in comparison with Renshaw cells and thick processes (possibly myelinated axon) with bright fluorescence.
The absence of CB-IR neurons in the ventral horn (interneurons of lamina VIII and Renshaw cells) and MB can be explained by selective damage of this group interneurons resulted from their vulnerability to decreasing of afferent input. In avian embryos, deafferentation leads to loss of some interneurons. Loss of neurons was restricted to certain subpopulation of interneurons between the dorsal and ventral horn (McKay & Oppenheim Reference McKay and Oppenheim1991; Yin et al. Reference Yin, Johnson, Prevette and Oppenheim1994). Possibly, decrease of afferent information may loss of excitatory glutamatergic synaptic inputs to interneurons, and reduced excitability of neurons may enhance their vulnerability to degeneration and death (Tarabal et al. Reference Tarabal, Caraballo-Miralles, Cardona-Rossinyol, Correa, Olmos, Lladó, Esquerda and Calderó2014).
There are some new data indicating that although most primary nociceptive fibres terminate in the superficial dorsal horn laminae I and II, these neural signals are then relayed to the deep dorsal horn. Thus, medial deep dorsal horn neurons are likely involved in pain-withdrawal pathways and have direct monosynaptic input to motoneurons (Levine et al. Reference Levine, Hinckley, Hilde, Driscoll, Poon, Montgomery and Pfaff2014). Authors consider them as premotor neurons and they locate in the MB zone. Thus, CB immunoreactivity disappeared in interneurons with synaptic output to motoneurons. The reason of such vulnerability still remains incomplete.
Nevertheless, we keep in mind some limitation of these data. The first is that the results were obtained in a small amount of animals due to restrictions of the scientific programme of the Bion-M1 project. Also, the percentage of CB-IR interneurons was rather small, except laminae I and II.
The cross-sectional area of lamina IV interneuron and motoneuron profiles was the largest, and the same parameter of lamina II (43.5 ± 1.06 μm2) and I (57.9 ± 2.09 μm2) interneuron profiles was the smallest (Table 2, P < 0.05). In comparison with the control group, the mean of cross-sectional areas of neurons in laminae II and VII decreased and the same parameter increased in laminae III, IV and IX (P < 0.01).
The decreasing of cross-sectional areas of interneurons located in laminae II and VII may be explained by neurodegenerative processes due to reduction of afferent information under microgravity condition. In contrast, the increasing of cross-sectional areas of neurons in laminae III, IV and IX may appear due to the swelling of somata. The swelling may be linked to accumulation of Na+ and Ca+ in cells with hyperexcitability.
CB act as Ca2+ buffer by binding Ca2+, also regulate the intracellular Ca2+ homeostasis and may play an important role in controlling cell activity and plasticity (Baimbridge et al. Reference Baimbridge, Celio and Rogers1992; Schwaller Reference Schwaller2012). There are also some changes in CB expression after neuronal infections. For example, loss of CB immunoreactivity was observed in cervical SC of rabies-infected mouse (Monroy-Gómez & Torres-Fernández Reference Monroy-Gómez and Torres-Fernández2013). CB, by buffering Ca2+, can regulate intracellular responses to physiological stimuli and protect neurons against Ca2+-mediated neurotoxicity (Iacopino et al. Reference Iacopino, Christakos, German, Sonsalla and Altar1992; Airaksinen et al. Reference Airaksinen, Thoenen and Meyer1997). In the presence of CB, the Ca2+ channels enhance sensitivity to Ca2+ dependent inactivation. CB may also modulate Ca2+ influx through L-type voltage dependent Ca2+ channels in neurons and help to keep neuronal intracellular Ca2+ homeostasis (Xu et al. Reference Xu, Yang, Wang and Tang2014).
Weightlessness affects the electrophysiological properties of sensory neurons and makes these cells more excitable (Ren et al. Reference Ren, Fan, Song, Zhao, Chen and Shi2012). Increased activity in interneurons of the dorsal horn in particular may have the effect in subsets of interneurons of either causing excitotoxic cell death or inducing expression of CB as a neuroprotective response. In interneurons of laminae I and II, CB may participate in the regulation of nociceptive transmission process through interacting with the Cav1.3 subunit of the L-type voltage-dependent Ca2+ channel (Xu et al. Reference Xu, Yang, Wang and Tang2014). Increased calcium influx as a result of additional Cav1.2 or Cav1.3 channels could evoke changes in excitation–transcriptional signalling that may be responsible for the phosphorylation of transcription factors such as cJun and the activity-dependent expression of genes such as Tlx3 and even induce activity-dependent changes in neurotransmitter phenotype (Rosenberg & Spitzer Reference Rosenberg and Spitzer2011).
From the other hand, parameters of respiration (respiratory frequency, tidal volume, pulmonary ventilation and metabolic rate) were found to be reduced during space flight. It is possible that reduction of respiration could be a physiological response to reduced metabolic demand (Baevsky et al. Reference Baevsky, Baranov, Funtova, Diedrich, Pashenko, Chernikova, Drescher, Jordan and Tank2007). Reduced activity pattern of thoracic α-motoneurons during weightlessness, and the reduction of their input on Renshaw cells would be expected to lead to reduced activity of these neurons. As a result, these neurons should have a reduced need for Ca2+-buffering power, leading to reduced expression of CB.
Weightlessness does not affect the number and morphology of CB-IR preganglionic sympathetic neurons. However, only small population of preganglionic neurons exhibits CB immunoreactivity in mice. Additional studies are required to reveal changes in sympathetic neurons containing other neurochemical markers.
Conclusions
Space flight induces plasticity in the expression of CB in the thoracic part of the spinal cord. The number and morphological parameters of CB-IR neurons change multidirectionally. Nevertheless, the quantity and morphology of CB-IR preganglionic sympathetic neurons did not change after the space flight. We propose that the effect of weightlessness can be superimposed upon different patterns of neural activity in SC circuitry in either dorsal or ventral horns of the spinal cord, in inter- or motoneurons, causing an up- or downregulation of CB expression. CB, by buffering Ca2+, can regulate intracellular responses to physiological stimuli and protect neurons against Ca2+-mediated neurotoxicity due to presumable hyperexcitability of separate groups of interneurons. Multidirectional changes in sizes of neurons may be explained by dystrophy in some interneurons and swelling of somata in another group of neurons. The information provided here will also serve as a basis for future studies investigating mechanisms of the action of microgravity on the nervous system.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grants NN 12-04-00621 and 13-04-00059).







