Introduction
Candida lusitaniae and C. guilliermondii are rare pathogens that have gained interest since they easily acquire resistance to antifungal treatment (Hawkins and Baddour, Reference Hawkins and Baddour2003). They can undergo morphological switching and form pseudohyphae structures. Phenotypic switching is induced in the yeast population by the external signals such as pH, temperature and mating-associated factors. Pseudohyphae morphology is associated with various processes related to Candida virulence: colonization, rapid dissemination in the host tissues, adhesion to host surfaces, biofilm formation (Thompson et al., Reference Thompson, Carlisle and Kadosh2011). Pseudohyphae phenotype displays an increased antifungal drug resistance up to ten times as well (Miller et al., Reference Miller, Dick and Merz2006). Antifungal treatment of Candida-caused diseases is difficult since the metabolism and cell structure of the pathogen are similar to the host. The side effect of the antifungal treatment as well as growing resistance to the antifungal treatment and reported multidrug resistance cases (Khan et al., Reference Khan, Malik and Ahmad2012; Asner et al., Reference Asner, Giulieri, Diezi, Marchetti and Sanglard2015) promote the new studies of the alternative methods for the biocontrol of Candida yeasts. Particularly the problem has high actuality in space stations where the access to drugs and treatment options are limited.
Environment of the spaceships and international space stations is very specific and colonized by different types of bacteria, viruses and yeast (Castro et al., Reference Castro, Thrasher, Healy, Ott and Pierson2004; Cohrs et al., Reference Cohrs, Mehta, Schmid, Gilden and Pierson2008; Horneck et al., Reference Horneck, Klaus and Mancinelli2010). Microorganism can persist on the surfaces of the devices in the air, water and other biological objects including astronauts (Nickerson et al., Reference Nickerson, Ott, Wilson, Ramamurthy and Pierson2004; Ott et al., Reference Ott, Bruce and Pierson2004). Candida yeasts are commensal microorganisms that can colonize human body. Their growth is limited by the host immune system and competition with other microorganisms occupying the same locations. In the patient suffering skin diseases, when the immune system is reduced or after antibiotic treatment, Candida yeasts can easily spread in the human body. During long spaceflights, organisms of the astronauts are affected by ionizing radiation, UV radiation, stress, closed environment thus can lead to the decreased immunity, allergic diseases and increased risk to suffer microorganism-caused infections (Aviles et al., Reference Aviles, Belay, Vance and Sonnenfeld2005; Sonnenfeld, Reference Sonnenfeld2005; Crucian et al., Reference Crucian, Stowe, Pierson and Sams2008, Reference Crucian, Zwart, Mehta, Stowe, Uchakin, Quiriarte, Pierson, Smith and Sams2013). Disbalance in immune system leads to the increased rate of infections and allergies as well (Montoro et al., Reference Montoro, Mullol, Jáuregui, Dávila, Ferrer, Bartra, Del Cuvillo, Sastre and Valero2009). The pathogenicity of the microorganisms is altered in space and microgravity conditions and non-pathogenic yeast can change the gene expression programmes and cause diseases (Crabbé et al., Reference Crabbé, Pycke, Van Houdt, Monsieurs, Nickerson, Leys and Cornelis2010). The use of the antimicrobial and antifungal drugs during the long spaceflights under the weakened immune system conditions is strictly limited. Therefore, search for the alternative methods for the elimination of the microorganisms as well as analysis of the microorganism physiology under microgravity conditions is one of the main tasks of NASA (Castro et al., Reference Castro, Thrasher, Healy, Ott and Pierson2004; Horneck et al., Reference Horneck, Klaus and Mancinelli2010). For the simulation of the low shear microgravity conditions in earth, rotary cell cultivation system (RCCS) is used. Cell growth in RCCS affects the main cell processes such as DNA replication and repair, response to environmental conditions, mitochondria functions and changes cell viability and resistance to the various stress conditions (Nislow et al., Reference Nislow, Lee, Allen, Giaever, Smith, Gebbia, Stodieck, Hammond, Birdsall and Hammond2015). In this work, we study the effects of modelled microgravity conditions on C. lusitaniae and C. guilliermondii cells and evaluate the feasibility of electroporation for biocontrol of RCCS affected and non-affected Candida species.
Electroporation (electropermeabilization) is the disruption of the cell membrane potential resulting in the hydrophilic pore formation (Teissie et al., Reference Teissie, Golzio and Rols2005; Teissie, Reference Teissie2014), therefore permeability of the cell membrane can be increased when external electric field pulses are applied to the cells. Electroporation is successfully used in vitro and in vivo applications and the most attractive feature is that it is universal for all the cell types (Rems and Miklavčič, Reference Rems and Miklavčič2016). Electropermeabilization can be reversible when pores in the cell membrane are closed after a certain period, and irreversible, that leads to the cell death (Krassowska and Filev, Reference Krassowska and Filev2007). Electroporation is successfully applied in electrochemotherapy (Mir, Reference Mir2001; Sersa et al., Reference Sersa, Miklavcic, Cemazar, Rudolf, Pucihar and Snoj2008; Sersa et al., Reference Sersa, Cemazar and Snoj2009; Miklavčič et al., Reference Miklavčič, Mali, Kos, Heller and Serša2014), electrogenetransfer (Li, Reference Li2004; Andre and Mir, Reference Andre and Mir2010), food industry (Sack et al., Reference Sack, Sigler, Frenzel, Eing, Arnold, Michelberger, Frey, Attmann, Stukenbrock and Müller2010; Saulis, Reference Saulis2010) as well it has potential for wound treatment similarly to electrotherapy (Ferguson et al., Reference Ferguson, Byrnes, Sun, Marti, Bonde, Duncan and Harmon2005; Kambouris et al., Reference Kambouris, Zagoriti, Lagoumintzis and Poulas2014; Novickij et al., Reference Novickij, Grainys, Lastauskienė, Kananavičiūtė, Pamedytytė, Zinkevičienė, Kalėdienė, Novickij, Paškevičius and Švedienė2015).
Material and methods
Yeasts and cultivation conditions
Previously described C. lusitaniae C18 and C. guilliermondii C94 clinical isolates were used in our research (Zinkeviciene et al., Reference Zinkeviciene, Vaiciulioniene, Baranauskiene, Kvedariene, Emuzyte and Citavicius2011; Lastauskiene et al., Reference Lastauskiene, Zinkevičiene, Girkontaite, Kaunietis and Kvedariene2014).
For the electroporation assay, yeast cells were grown in 10 ml YPD medium (2% glucose, 2% peptone, 1% yeast extract) on a rotary shaker at 130 rpm, overnight at 30°C. Then, cells were harvested by centrifugation at 2000 rpm for 5 min, three times washed with 1 M sorbitol and resuspended in 1 ml 1 M sorbitol. The final concentration was 109 CFU ml−1.
Antifungal assay
For the pseudohyphae induction, C. lusitaniae and C. guilliermondii cell suspensions (1 × 103–5 × 103 CFU ml−1) were plated on YPD plates supplemented with 1 mM CuSO4 (Favel et al., Reference Favel, Michel-Nguyen, Peyron, Martin, Thomachot, Datry, Bouchara, Challier, Noël, Chastin and Regli2003; Miller et al., Reference Miller, Dick and Merz2006). Yeasts were incubated for 5 days at 35°C in the dark and the number of colonies exhibiting the original and variant phenotypes were counted. For the reversal studies, colonies showing different phenotypes were separately grown in the liquid YPD medium for 48 h. On the YPD-CuSO4 medium, 100 µl of 1 × 103–5 × 103 CFU ml−1 suspension was plated again and the ability to maintain parental phenotype was evaluated, fulfilling the criteria for phenotype switching and showing that pseudohyphae formation occurred as a morphology switching, but not as a random mutation event.
Determination of the minimal inhibitory concentration (MIC) of amphotericin B was performed by microdilution in 96-well plate and agar diffusion methods. For the microdilution method, 100 µl of 106–107 CFU ml−1 suspension was added to the 100 µl of the YPD medium supplemented with 0.005, 0.01, 0.1, 1 and 2 mg ml−1 of amphotericin B dissolved in dimethyl sulfoxide and incubated at 35°C for 48 h. As the sterility control, YPD medium alone was used. Cell suspension in YPD medium served as a positive control. For the agar diffusion method, 106 CFU ml−1 suspension was mixed with the YPD medium. One hundred microlitres of the tested substance of concentrations listed above was added to the well. MIC was determined as the concentration with the visible inhibition zone around the well. All tests were performed in triplicate.
RCCS conditions
Rotary cell culture system Synthecon RCCS-1(USA) was used. This system ensures bubble-free membrane-based oxygenation, which results in a gentle culturing and the vertical move rotation. C. lusitaniae and C. guilliermondii cell suspensions were grown overnight in the rotary shaker at 130 rpm, 30°C. One microlitre of the suspension was transferred to the 10 ml sterile YPD medium and grown for 16 h in the rotary cell culture system at 30°C, 22.5 rpm.
Electroporation setup and parameters
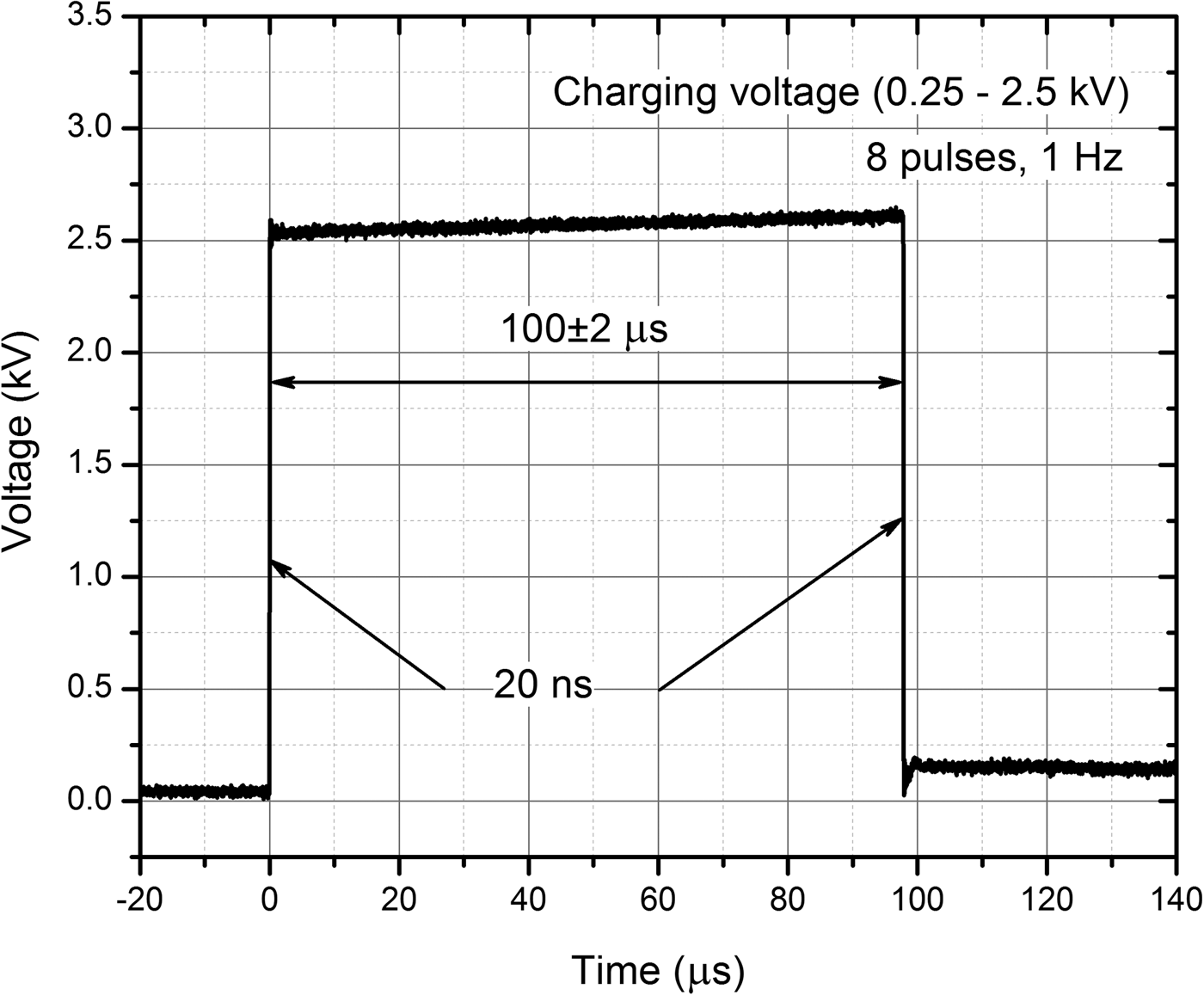
For pulse generation, the 0–3 kV, 60 A square wave pulse generator (100 ns–1 ms) that has been developed in the Institute of High Magnetic Fields (VGTU, Vilnius, Lithuania) was applied (Novickij et al., Reference Novickij, Grainys, Lastauskienė, Kananavičiūtė, Pamedytytė, Kalėdienė, Novickij and Miklavčič2016). The electric pulses were generated in electroporation cuvette with 1 mm gap between the electrodes (VWR International, Radnor, PA, USA). The square wave 100 µs × 8 pulse bursts of 2.5–25 kV cm−1 have been generated at the repetitive frequency of 1 Hz. The waveform of the pulse is presented in Fig. 1. In order to determine the threshold electric field when electroporation occurs, the amplitude of electric field was increased with a step of 2.5 kV cm−1.
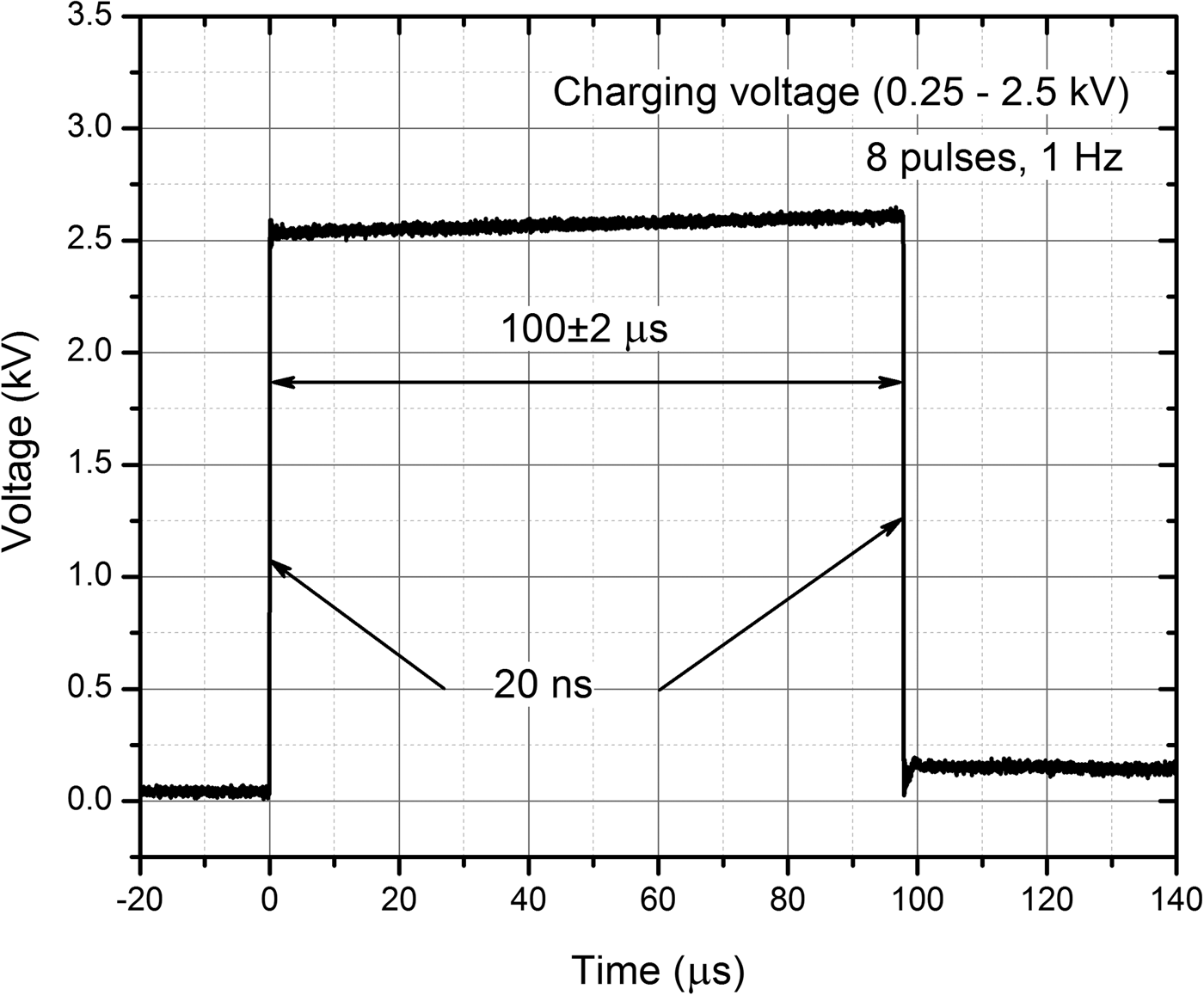
Fig. 1. The waveform of the applied pulses.
For the evaluation of cell permeabilization, 73 µl of 109 cell suspension was mixed with 7 µl, 50 µM propidium iodide (PI) and transferred to electroporation cuvette for further pulsed treatment. After electropermeabilization, yeast cells were incubated for 5 min at room temperature. Evaluation of the membrane permeabilization was carried out by flow cytometry at 550 nm wavelength, using Amnis Flow Sight system (Amnis, Seattle, WA, USA).
For the cell viability analysis, CFU count was performed. Seventy-three microlitres of 109 cell suspension was subjected to the various strength pulsed electric field (PEF). After the treatment, cells were plated onto YPD plates and incubated at 30°C in the dark. After 48 h of growth, appeared colonies were counted.
Results
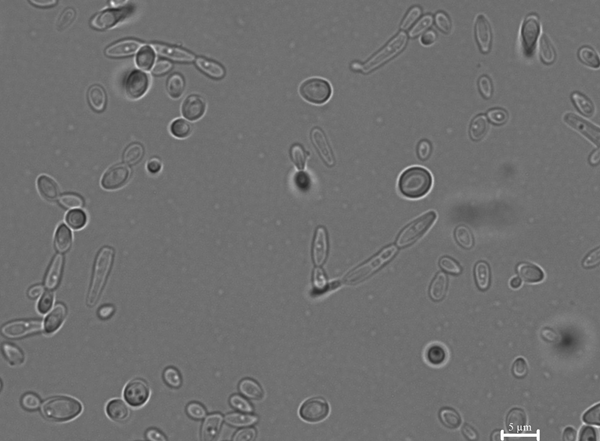
Analysis of pseudohyphae structures showed that altered phenotypes appeared in the C. lusitaniae and C. guilliermondii populations with the frequency of 10−2. They were reversible and parental phenotypes after the reversal studies were maintained, indicating the morphology switching – not a random mutation event (Fig. 2).
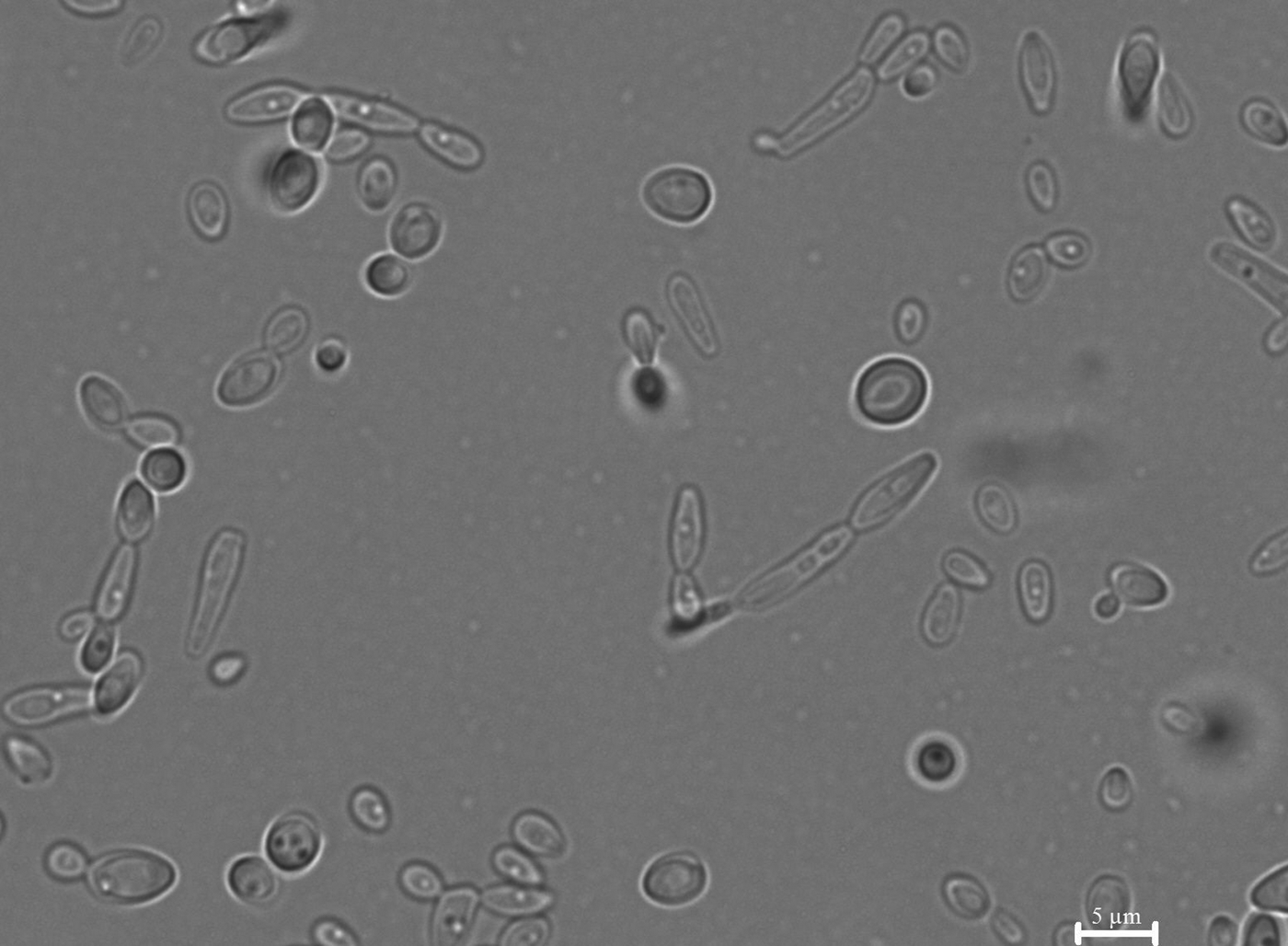
Fig. 2. Pseudohyphae formed by C. lusitaniae. Microscopy analysis showed pseudohyphae formation and cells with the altered phenotype that can undergo further phenotypic switching.
After successful pseudohyphae induction, evaluation of the antifungal drug resistance was performed. In the antifungal drug susceptibility assay, amphotericin B was used for identification of the increased drug resistance phenotypes. The MIC detected by both – microdilution and agar diffusion methods showed 0.01 mg ml−1 MIC of amphotericin B for the cellular phenotype of both Candida strains. After the pseudohyphae induction, the MIC of amphotericin B increased significantly – ten times for C. lusitaniae and 20 times for C. guilliermondii pseudohyphae morphology. Cell cultivation in the RCCS system led to the formation of even more resistant phenotype of both tested Candida species. MIC of amphotericin B grew up to 0.2 mg ml−1 for C. lusitaniae and to 0.32 mg ml−1 for C. guilliermondii cells grown in RCCS conditions (Table 1).
Table 1. Minimal inhibitory concentrations of amphotericin B in three types of C. lusitaniae and C. guilliermondii cells

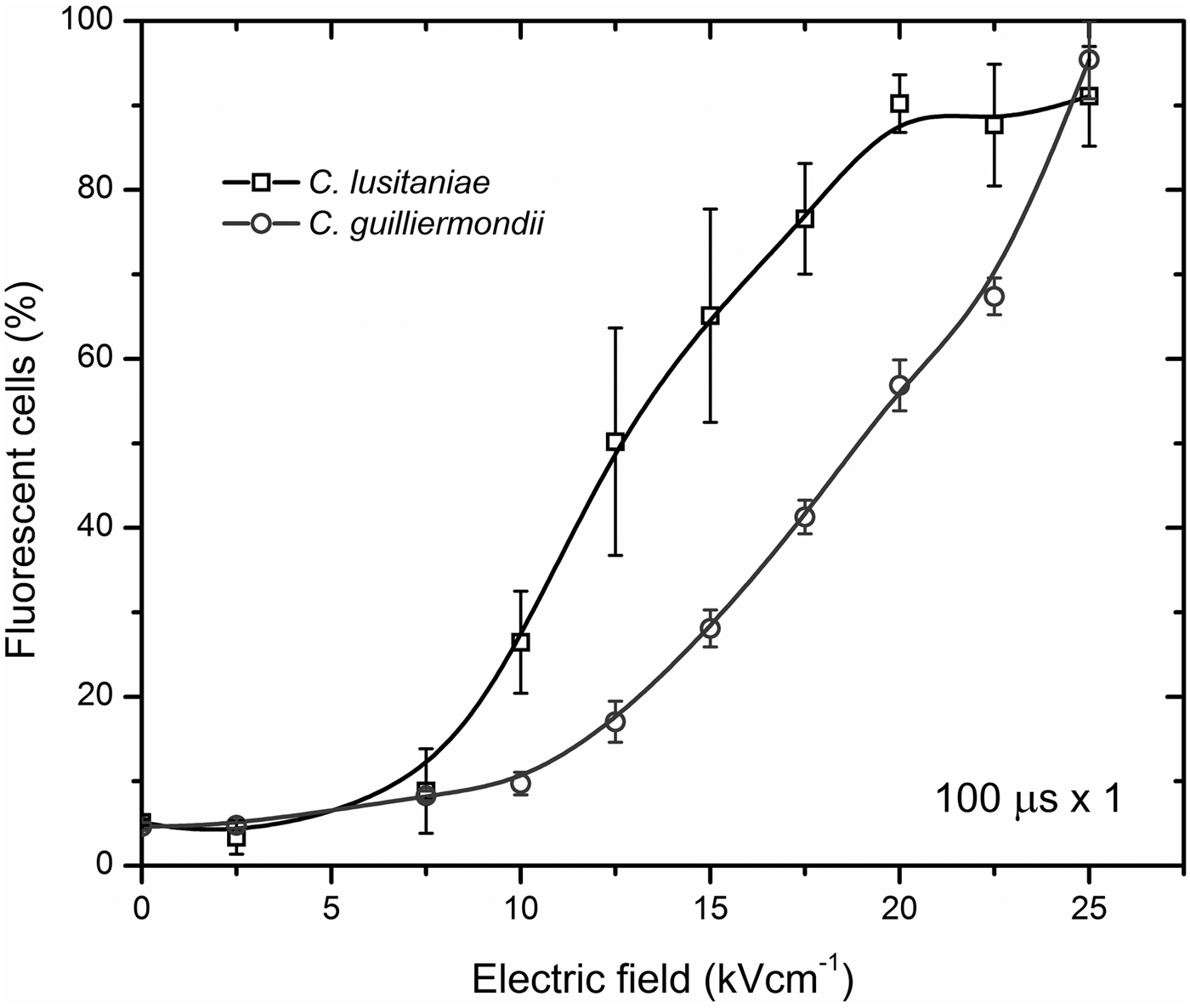
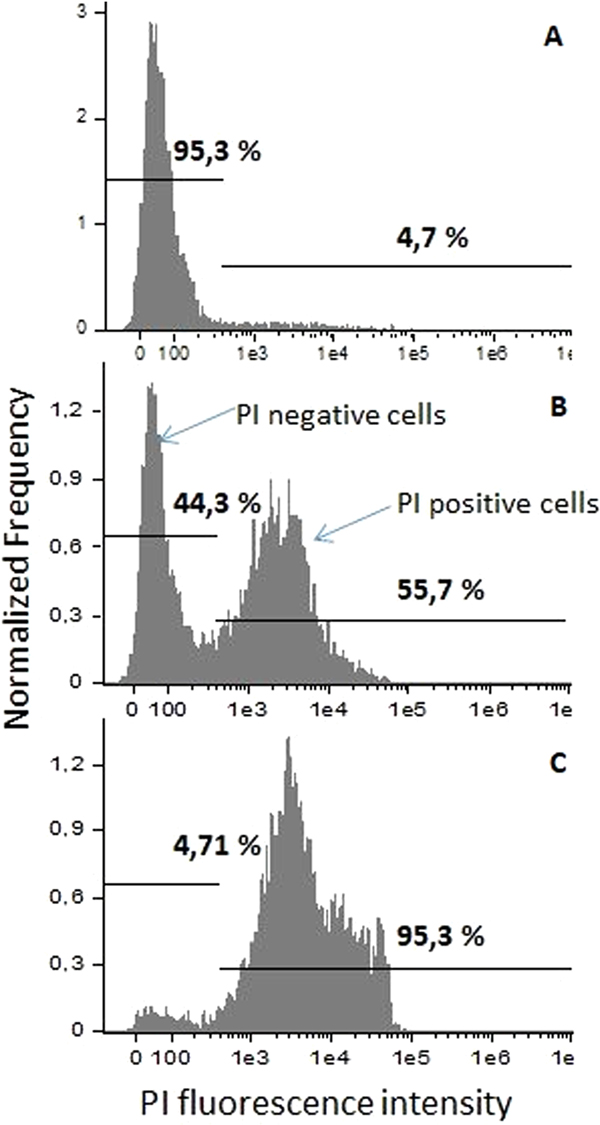
C. lusitaniae and C. guilliermondii cells were subjected to the 2.5–25 kV cm−1 PEF of 8 × 100 µs duration pulses. Results of the cell permeabilization analysis performed by flow cytometry are presented in Fig. 3. Statistically significant difference (P < 0.05) in fluorescent dye uptake can be observed already after applying 10 kV cm−1 PEF. Further increase of PEF amplitude results in an increased permeabilization rate until it reaches saturation at 20 kV cm−1. The percentage of PI-positive cell in the control samples did not exceed 7%. The gates were positioned in the PEF-untreated control sample according to the results obtained by staining Candida cells with PI and performing fluorescent microscopy analysis (Nicon Eclipse 80i, Japan). The shift of the fluorescence spectra can be observed after treatment or the cells with PEF showing increased number in PI-positive (permeabilized cells) (Fig. 4). All the experiments were repeated in triplicates and reliability of the results was confirmed by fluorescent microscopy as well.

Fig. 3. Flow cytometry analysis results of C. lusitaniae and C. guilliermondii permeabilization rate after applying 2.5–25 kV cm−1 PEF.
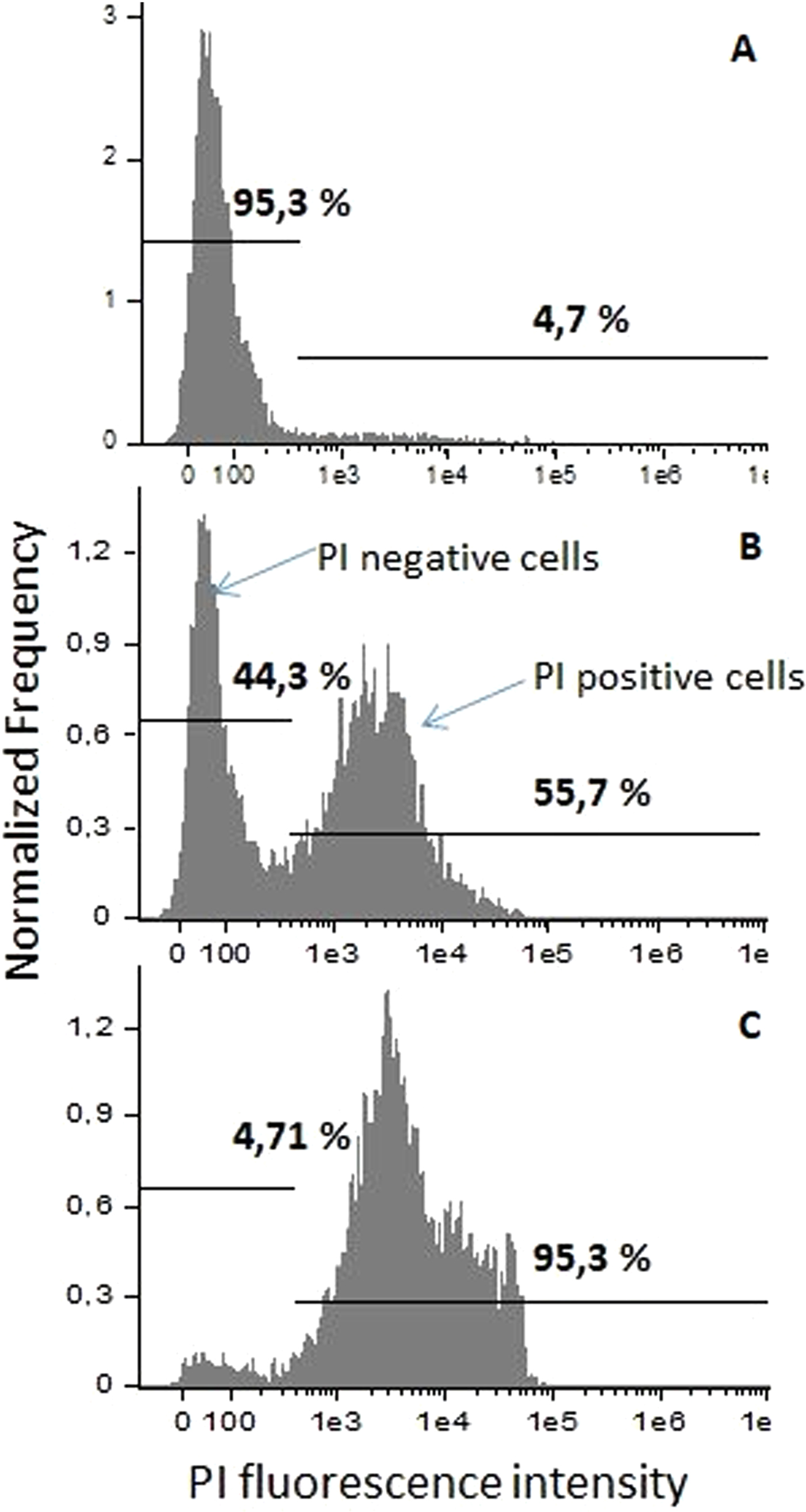
Fig. 4. Fluorescence spectra and determination of the analysis gates. (A) 0 kV cm−1, PEF untreated, control sample; (B) 15 kV cm−1 8 × 100 µs PEF; (C) 25 kV cm−1 8 × 100 µs PEF.
Since the electric field in the range of 2.5–7.5 kV cm−1 did not induce a statistically significant increase in reversible cell permeabilization, further in the study, the C. lusitaniae and C. guilliermondii cellular and pseudohyphae phenotypes were subjected to 7.525 kV cm−1 PEF.
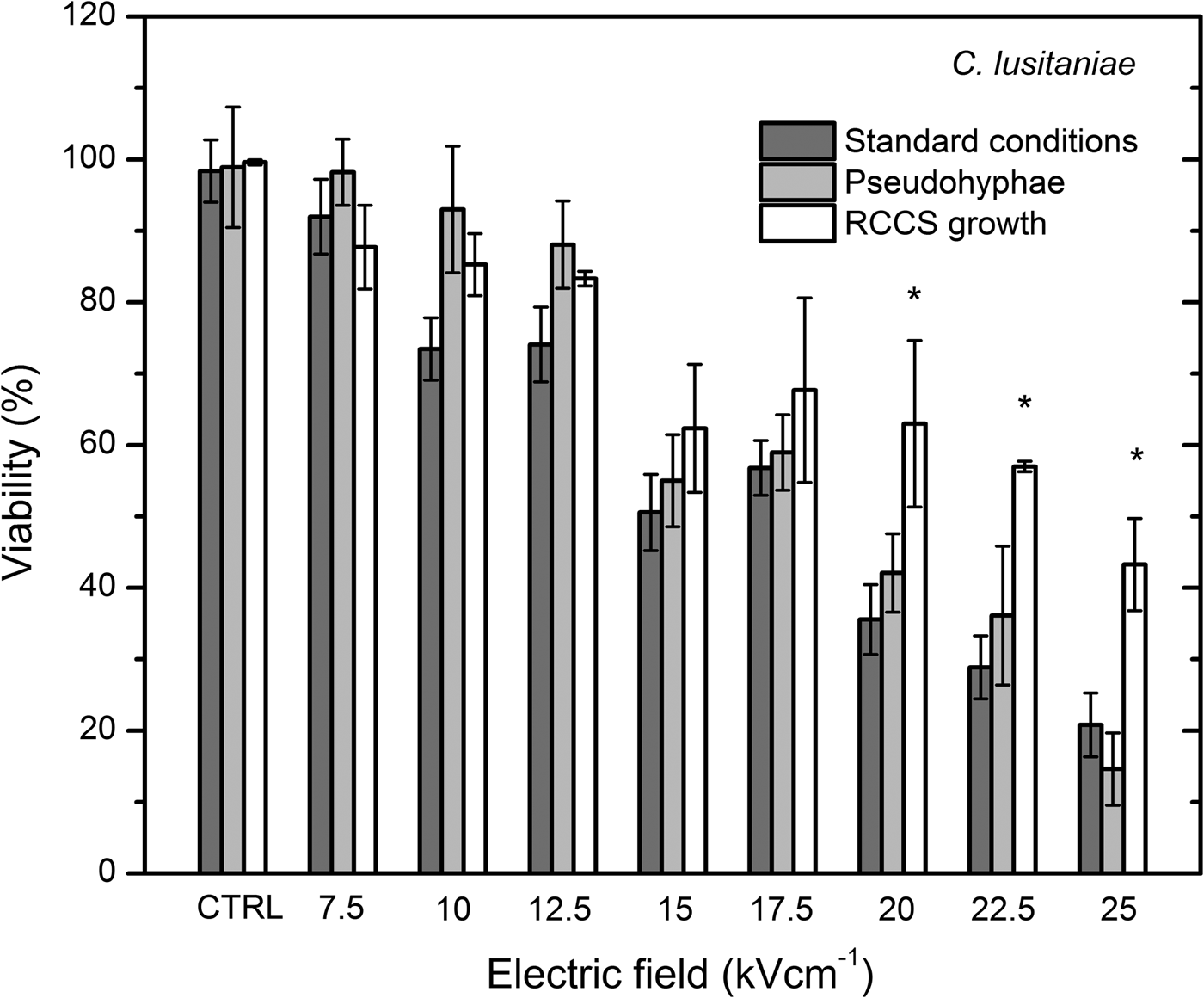
After applying 10 kV cm−1 PEF, cell viability decreased significantly if compared with the untreated control (Fig. 5). Percentage of the irreversibly permeabilized cells increased in a dose-dependent manner. After applying 20, 22.5 and 25 kV cm−1, 36 ± 5%, 29 ± 4% and 21 ± 4% of CFU were detected, respectively. Permeabilization rate of the pseudohyphae phenotype of C. lusitaniae showed a similar dose-dependent result. At 7.5 and 12.5 kV cm−1, no significant CFU loss was observed when compared with the untreated control. The significant loss in viability in pseudohyphae morphology was observed after applying 15 kV cm−1 PEF and it decreased in the dose-dependent manner. These results showed that applied PEF parameters are suitable for the inactivation of both morphological forms.
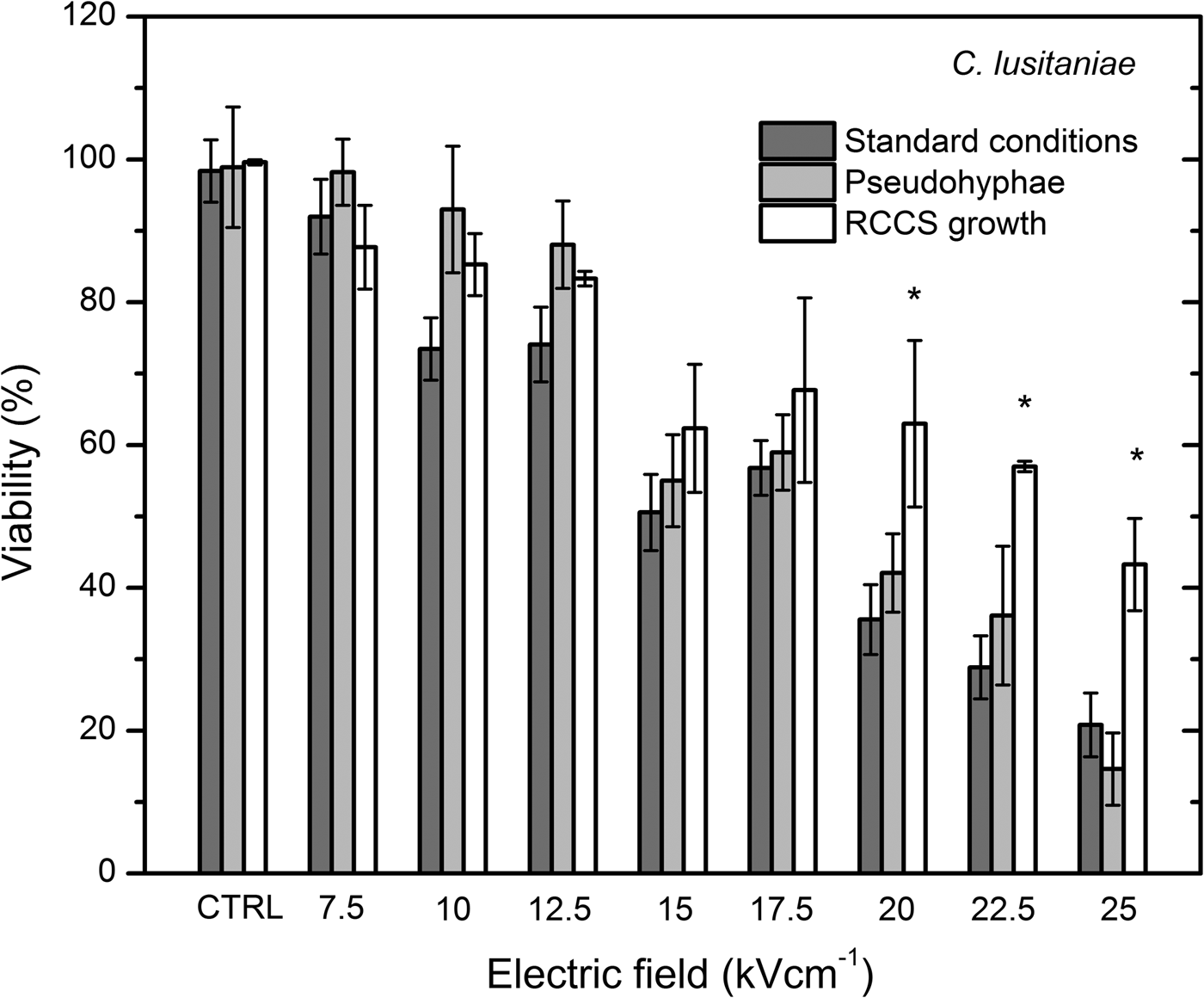
Fig. 5. CFU of the PEF-treated C. lusitaniae budding cells grown in standard conditions, pseudohyphae phenotype and under microgravity grown cells showed significantly increased resistance after cultivation under microgravity conditions.
The cellular and pseudohyphae morphology in RCCS grown cells exhibited significantly increased resistance (P < 0.05) to the applied 20, 22.5 and 25 kV cm−1 PEF.
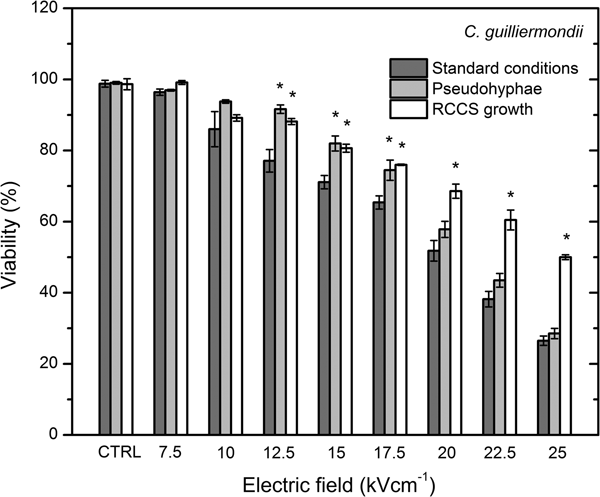
C. guilliermondii showed in generally around 10% higher cell viability in test conditions as compared with C. lusitaniae (Fig. 6).

Fig. 6. Susceptibility to the applied PEF of C. guilliermondii cells grown in standard conditions, pseudohyphae phenotype and under microgravity grown cells.
C. guilliermondii viability displayed dose-dependent inactivation rate. RCCS growth has increased the resistance to the applied PEF (P < 0.05) already after applying 12.5 kV cm−1 PEF indicating the new, highly resistant phenotype.
Discussion
Numerous studies have confirmed that growth, physiology, morphology and virulence are altered in microorganisms during the spaceflights (Nickerson et al., Reference Nickerson, Ott, Wilson, Ramamurthy and Pierson2004). Specific environment in the space ships and space stations is affecting both: the microorganism and the host. Microgravity, UV and ionizing radiation, stress and limited space conditions can weaken organism immunity (Crucian et al., Reference Crucian, Stowe, Pierson and Sams2008). Candida-caused infection are closely related with the weak immune system.
In this study, we applied electroporation for the biocontrol of amphotericin B-resistant phenotypes of Candida yeasts. We showed that phenotype switching due to the pseudohyphae formation and growth under microgravity conditions were associated with changes in amphotericin B MIC profiles. In this study, MICs of at least ten dilutions higher were apparent for pseudohyphae phenotype compared with the cellular morphology. Yeast cell grown under microgravity conditions exhibited 20 and 32 dilutions higher resistance to amphotericin B when compared with the normal cells and two and 1.6 times higher resistance when compared with the pseudohyphae morphology for C. lusitaniae and C. guilliermondii, respectively. The results indicate the new possible phenotypes that are associated with the extreme resistance to amphotericin B. Resistance breakpoints for polyenes have not been determined, but most clinicians use a MIC of 1.0 µg ml−1 to indicate resistance to amphotericin B (Messer et al., Reference Messer, Jones and Fritsche2006; Messer et al., Reference Messer, Moet, Kirby and Jones2009; Pfaller et al., Reference Pfaller, Castanheira, Messer, Moet and Jones2010).
To our knowledge, this research is the first study which uses electroporation for the inactivation of both morphological forms and microgravity-affected Candida yeasts. We have shown that pseudohyphae morphology displayed slightly higher viability rate if compared with the cellular phenotype after applying 7.5–12.5 kV cm−1 PEF (Fig. 4), meanwhile the inactivation rate for the microgravity-affected cells was the same (Fig. 5).
However, the 15–22.5 kV cm−1 treatment resulted in a similar cell inactivation efficacy for both morphological types, however the resistance to PEF increased in the cells grown under microgravity conditions. We believe that the result was influenced by the dependence of the induced transmembrane potential on the morphological structure of the cells. In case of a cellular morphology, the dependence is straight-forward, having a linear proportionality with increase of PEF amplitude (Krassowska and Filev, Reference Krassowska and Filev2007); however, for irregularly shaped cells, the distortion of the spatial distribution of the induced transmembrane potential occurs (Pucihar et al., Reference Pucihar, Kotnik, Valič and Miklavčič2006). As a result, the activation of the increased membrane permeability is completed by both the spatial position of the cells and angular distribution of the irregularly shaped cells in respect to the electric field vector, which both affect the density of induced pores, number of pores and the efficacy of permeabilization in overall (Valič et al., Reference Valič, Pavlin and Miklavčič2004; Pucihar et al., Reference Pucihar, Miklavčič and Kotnik2009; Kotnik et al., Reference Kotnik, Pucihar and Miklavčič2010).
At the same time, microgravity conditions seem to alter the gene expression pattern in C. lusitaniae causing increased resistance to both chemical compounds and physical treatment. Distribution of the nutrients and metabolites is changed around the microorganisms due to the lack of sedimentation and can alter the cell growth (Nickerson et al., Reference Nickerson, Ott, Wilson, Ramamurthy and Pierson2004); this leads to shortened lag phase, increased biomass and increased production of the secondary metabolites (Klaus, Reference Klaus2003). Changed fluid dynamics and extracellular transport efficiency might be the cause of changed cell growth in microgravity conditions (Kacena and Todd, Reference Kacena and Todd1997). The mechanism of the antifungal resistance is remaining unclear. The possible explanation could be related to the microbial growth kinetics and changes in cellular transport causing massive efflux of antifungal compounds from the cell (Tixador et al., Reference Tixador, Richoilley, Gasset, Templier, Bes, Moatti and Lapchine1985). It was shown that transcriptional profiling and gene expression analysis of bacteria show alterations in the metabolic pathways that can be related to the increased resistance to antibiotics in bacteria (Wilson et al., Reference Wilson, Ott, Höner zu Bentrup, Ramamurthy, Quick, Porwollik, Cheng, McClelland, Tsaprailis, Radabaugh, Hunt, Fernandez, Richter, Shah, Kilcoyne, Joshi, Nelman-Gonzalez, Hing, Parra, Dumars, Norwood, Bober, Devich, Ruggles, Goulart, Rupert, Stodieck, Stafford, Catella, Schurr, Buchanan, Morici, McCracken, Allen, Baker-Coleman, Hammond, Vogel, Nelson, Pierson, Stefanyshyn-Piper and Nickerson2007; Crabbé et al., Reference Crabbé, Pycke, Van Houdt, Monsieurs, Nickerson, Leys and Cornelis2010). Microgravity affects the core cellular processes as growth control via RNA and ribosomal biogenesis, metabolism, DNA repair, replication, response to pH, mitochondria function in yeast (Nislow et al., Reference Nislow, Lee, Allen, Giaever, Smith, Gebbia, Stodieck, Hammond, Birdsall and Hammond2015) that can contribute to increased antifungal drug resistance as well as PEF resistance. Microgravity conditions can have possible influence on the cell wall and membrane structure and integrity, as well as reparation of the hydrophilic pores after the PEF treatment. We suggested that increased general cell vitality and cellular damage reparation rate can contribute to the faster and more efficient restoration of the cell membrane integrity increasing survivability of the PEF-treated cells.
The determined PEF-induced inactivation and inability of microorganisms to form complete resistance to PEF are the major advantages, which will be responsible for the interest in the methodology. The technique may be potentially used as an effective wound decontamination method. Pseudohyphae-associated biofilm formations may alter the response of yeast to PEF; however, biofilms can be successfully eradicated by electroporation after adequate parametrical analysis. The dependence of the effect on the applied electrodes and pulse delivery system can be also highlighted as a major limitation; however, the methodology is widely spread in the area of cancer treatment and thus, all the same limitations apply, which are successfully countered. Lastly, electroporation is safe, non-toxic and the acquisition of the bureaucratic base for clinical treatment presumably will be easier.
In conclusion, our results showed that C. lusitaniae and C. guilliermondii cells grown under simulated microgravity conditions gained up to 30-fold higher resistance to amphotericin B. RCCS significantly increased survivability of both Candida species after PEF treatment as well in comparison with cells grown under routine conditions. Highly drug-resistant Candida yeast can be effectively inactivated after applying PEF higher than 15 kV cm−1.
Funding
This work was supported by the Research Council of Lithuania Towards the Future Technologies Program project ELMIGRAV (Grant number – LAT-02/2016).
Conflict of interest
None.