Transmission of pathogens to and from patients is associated with the spread of antimicrobial resistance and healthcare-associated infections. Transmission usually occurs indirectly via healthcare providers (HCPs) or via mobile or immobile fomites. 1 Hands are universally recognized as the most important vector,Reference Pittet, Dharan, Touveneau, Sauvan and Perneger 2 but equipment (eg, stethoscopes) may also act as a vector for pathogen transfer.Reference Schabrun and Chipchase 3 For successful transfer, pathogens must first be transmitted from a surface of origin (eg, a patient) to an intermediate surface (ie, a vector) and must then survive there long enough to be finally transmitted to the next patient or to the next intermediate vector.Reference Longtin, Schneider and Tschopp 4 The likelihood of transfer depends on multiple factors, including number and type of pathogens present, surface structure,Reference Ali, Moore and Wilson 5 contact time, lag time,Reference Bardell 6 humidity,Reference Sattar, Springthorpe and Mani 7 and pressure.Reference Mbithi, Springthorpe, Boulet and Sattar 8
A patient encounters many different HCPs during a stay in a healthcare institution. Each patient is visited by a median of 3.5 HCPs per hour, mostly nurses, for a median of 3 minutes per visit. The patient environment, intact skin, and body fluids are touched in 33%, 27%, and 18% of visits, respectively.Reference Cohen, Hyman, Rosenberg and Larson 9 If infection prevention measures are not followed, each contact poses a risk of pathogen transfer.
Prevention policies such as standard and isolation precautions, particularly hand hygiene and the use of gloves and gowns, aim to prevent pathogen transmission.Reference Labeau, Vandijck and Rello 10 Detailed understanding of transmission dynamics during patient care, however, is pivotal to tailoring preventive measures and policies. To date, no systematic review has addressed the extent to which HCP behavior results in transfer of pathogens.
Therefore, we performed a systematic review and meta-analysis (1) to summarize and describe the current knowledge on pathogen transfer associated with care activity, including the frequency and quantity of pathogen transfer between patients, environmental surfaces, HCPs, their clothing, and medical devices, and (2) to identify the factors associated with increased transmission risk.
Methods
Data sources and searches
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) recommendations when conducting this systematic review.Reference Moher, Altman, Liberati and Tetzlaff 11 We searched Medline/Ovid, EMBASE and the Cochrane Library using a combination of subject headings and free text search terms (see Appendix: Search Strategy). Studies published before July 7, 2017 in English, French, and German were included. Additional articles were identified by a reference list search of articles included in full text review.
Study selection
We retained studies that met the following criteria:
1. The study occurred in a defined healthcare setting (eg, inpatient and outpatient settings of hospitals, nursing homes, and medical practices) and included common behavior in healthcare institutions (eg, handshakes, physical examinations, and phone calls).
2. The study reported transfer of bacteria, viruses, or fungi by contact from a defined origin surface to a defined destination surface through quantification of contamination by frequency (ie, percentage of contaminated destination surfaces) or by quantity (ie, colony forming units (CFU) on destination surface).
3. The HCP activity associated with pathogen transfer was described.
4. Transfer to destination surface was ascertained by genomic or pulsed-field gel electrophoresis typing of the pathogen or ascertaining a sterile destination surface.
5. Origin and destination surfaces were either the patient’s body sites; surfaces in the patient’s immediate environment; critical, semicritical, or noncritical medical devices; or HCP body sites, attire, or personal protective equipment.
6. The microbiological sampling method (eg, contact plate, swab, or glove juice methodReference Larson, Strom and Evans 12 ) was accurately described.
We excluded studies reporting laboratory simulations or using artificial contamination or tracers to evaluate transfer. We also excluded investigations of transfer by droplet or airborne route and pathogen transfer during surgical procedures.
One researcher (A.W.) screened all titles and abstracts for potentially relevant studies. To check interrater reliability, 2 independent researchers (L.C. and S.P.) screened a subset of titles and abstracts, then 2 authors (A.W. and L.C.) independently reviewed the full texts of all potentially eligible articles. Discrepancies regarding eligibility were resolved by consensus or by decision of a third reviewer (H.S.).
Data extraction and quality assessment
Using a standardized template, the following variables were abstracted: study setting, microorganism transferred, ‘origin’ surface (surface from which pathogen was transferred), ‘destination’ surface (surface to which pathogen was transferred), interaction (activity) leading to transfer, number of interactions, frequency of transfer (percentage of positive destination surfaces), quantity of transfer (number of transferred CFU), and microbiological sampling method. All retained articles were assessed for quality using the Downs and Black checklist,Reference Downs and Black 13 which was modified to fit the noninterventional characteristics of included studies (Appendix Table 1).
Data synthesis and analyses
For studies reporting raw transfer frequency data, rates were calculated by dividing the number of positive destinations by the total number of exposed destination surfaces. The Freeman-Tukey double arcsine transformation for data with a binomial distribution was applied to stabilize the variances. Transformed proportions were pooled using random-effects meta-analysis with exact confidence intervals, and results were displayed in forest plots. All meta-analyses were conducted using the metaprop command in Stata software. Also, 2 studies that did not report raw rates were excluded from meta-analysis but were retained in the systematic review.Reference Ludlam, Swayne and Kearns 14 , Reference Ojajarvi 15 An a priori subgroup analysis was performed for studies examining pathogenic bacteria examining destination surface, surface of origin, HCP behavior (standardized vs nonstandardized), microorganism, and microbiological sampling method. Studies that reported transfer quantities and risk factor analysis were not pooled, and the findings are summarized descriptively.
Data synthesis was done using Stata version 13.1 software (StataCorp, College Station, TX). Statistical heterogeneity was initially inspected graphically (forest plot); the degree of heterogeneity was quantified using the I2 statistic. We defined heterogeneity as I2>60%. Subgroup analyses for differences between subgroups were foreseen if the criterion for heterogeneity was not met. P values <.05 were considered statistically significant. This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 16
Results
After removal of duplicates, 13,121 articles were identified by database and manual reference search. We excluded 12,932 articles after title and abstract screening. Interrater reliability of title and abstract screening of a subset of articles (n=2,000) calculated using the Cohen κ was 0.85 (CI 95%, 0.75–0.94) indicating excellent interrater agreement.Reference McHugh 17 After full-text reviews of 189 articles, 32 articles (17%) met eligibility criteria and were included in the overall review (Fig. 1).

Fig. 1 Study inclusion flow diagram.
Tables 1–3 detail the study characteristics. All studies were conducted in high-income settings. The sample size ranged from 3 to several hundred (mean, n=124). Surface of origin was the patient or his environment in 94% of the studies (n=30). Also, 1 study dealt with telephones as the surface of origin,Reference Jeske, Tiefenthaler, Hohlrieder, Hinterberger and Benzer 18 and 1 dealt with the patient environment exclusively.Reference Ray, Hoyen, Taub, Eckstein and Donskey 19 Common destination surfaces were hands (n=18, 56%), gloves (n=15, 47%), and gowns or uniforms (n=7, 22%). Rarely, surfaces such as ultrasound probes,Reference Frazee, Fahimi, Lambert and Nagdev 20 , Reference Ohara, Itoh and Itoh 21 stethoscopes,Reference Longtin, Schneider and Tschopp 4 , Reference Zachary, Bayne, Morrison, Ford, Silver and Hooper 22 , Reference Tschopp, Schneider, Longtin, Renzi, Schrenzel and Pittet 23 or the patient and his environment were evaluated as destination surfaces.Reference Ludlam, Swayne and Kearns 14 , Reference Duckro, Blom, Lyle, Weinstein and Hayden 24 Overall, 30 articles (94%) reported transfer frequency, 9 (28%) reported transfer quantity. Transferred pathogens were bacteria in all but 3 articles: 1 article each focused on dermatophytes, 1 on human papillomavirus (HPV), and 1 on both fungi and bacteria. A multitude of clinical care activities were studied, ranging from a brief touch or handshake to well-defined care tasks (eg, dressing change) to more complex medical or nursing activity (eg, morning care). In 31% of studies (n=10), the interaction was standardized by role playing with real patients.
Table 1 Summary and Quality Assessment for Studies Reporting Frequency of Contamination
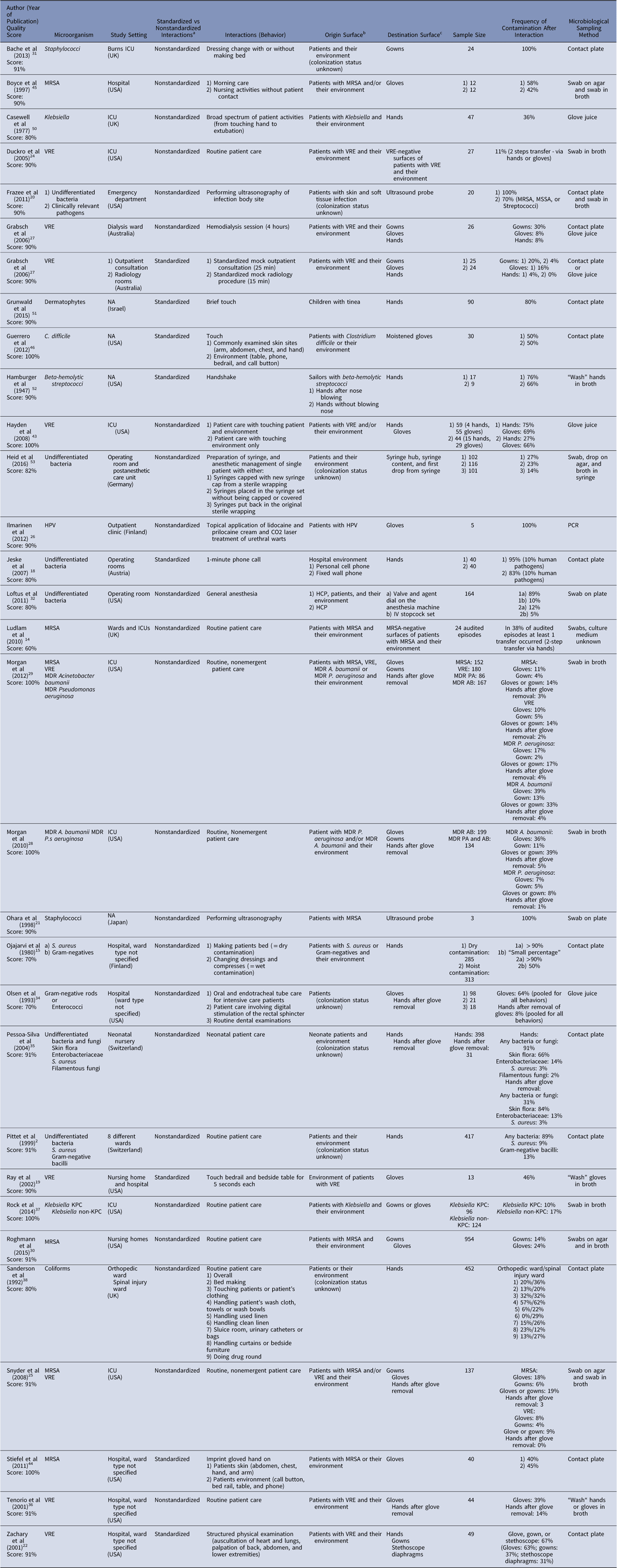
Note. HCP, healthcare practitioner; HPV, human papilloma virus; ICU, intensive care unit; KPC, Klebsiella pneumonia carbapenemase; NA, not applicable; MDR, multidrug resistant; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; UK, United Kingdom; USA, United States of America; VRE, vancomycin-resistant Enterococci.
aIn standardized interactions, the interaction was standardized by role playing; in nonstandardized interactions, the interactions were observed in real life.
bSurface from which pathogen was transferred.
cSurface to which pathogen was transferred.
Table 2 Summary and Quality Assessment for Studies Reporting Quantity of Contamination

Note. CFU, colony-forming unit; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; UK, United Kingdom; USA, United States of America.
a In standardized interactions, the interaction was standardized by role playing; in nonstandardized interactions, the interactions were observed in real life.
b Surface from which pathogen was transferred.
c Surface to which pathogen was transferred.
Table 3 Summary and Quality Assessment for Studies Reporting Risk Factor Analysis

Note. ICU, intensive care unit; KPC, Klebsiella pneumonia carbapenemase; NA, not applicable; MDR, multidrug resistant; MRSA, methicillin-resistant Staphylococcus aureus; UK, United Kingdom; USA, United States of America; VRE, vancomycin-resistant Enterococci.
a Surface from which pathogen was transferred.
b Surface to which pathogen was transferred.
Various microbiological sampling techniques were used. Swabbing was applied in 12 studies (38%), contact plates in 14 studies (44%), the glove juice method in 4 studies (13%),Reference Larson, Strom and Evans 12 and washing hands or gloves in broth in 3 studies (9%). Polymerase chain reaction (PCR) was used to detect viral transfer in 1 study.
Transfer frequency
Most studies examined transfer frequency during nonstandardized tasks, that is, in real-life situations (n=23, 76%) (Table 1). These interactions lasted up to 4 hours. In 8 studies, the transfer frequency after standardized interactions was examined. In 6 of these studies, interactions were simple, such as a handshake, briefly touching a patient, or taking a phone call. In 2 studies, the transfer frequency after multistep interactions, such as an outpatient consultation or a radiology procedure, was examined. Hand and glove contamination ranged from 0% with vancomycin-resistant enterococci (VRE) after a standardized mock radiology procedureReference Snyder, Thom and Furuno 25 to 100% with HPV after treatment of urethral warts.Reference Ilmarinen, Auvinen, Hiltunen-Back, Ranki, Aaltonen and Pitkaranta 26 Gown contamination was between 2% and 37% for VRE, methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant (MDR) Pseudomonas aeruginosa and Acinetobacter baumanii,Reference Zachary, Bayne, Morrison, Ford, Silver and Hooper 22 , Reference Snyder, Thom and Furuno 25 , Reference Grabsch, Burrell, Padiglione, O’Keeffe, Ballard and Grayson 27 – Reference Roghmann, Johnson and Sorkin 30 and 100% for staphylococci.Reference Bache, Maclean, Gettinby, Anderson, MacGregor and Taggart 31 Ultrasound probes were contaminated to 100% with skin bacteria,Reference Frazee, Fahimi, Lambert and Nagdev 20 , Reference Ohara, Itoh and Itoh 21 transfer of undifferentiated bacteria to IV stopcocks and syringes occurred in 5%–26% of anesthesia procedures.Reference Loftus, Muffly and Brown 32 , Reference Heid, Bender, Gervais, Schmeck, Kohnen and Noppens 33
The pooled proportions of transfer frequency of bacteria to hands and gloves were 33% (95% CI, 12%–57%; 9 studies) and 30% (95% CI, 23%–38%; 21 studies) with overall heterogeneities of I2=90.95% and 92.03%, respectively (Appendix Fig. 1). The estimated proportions of transfer frequency to gowns and to ‘hands after glove removal’ were 10% (95% CI, 6%–14%; 13 studies) and 3% (95% CI, 1%–5%; 9 studies), respectively (Appendix Figs. 2 and 3). These results remained unchanged when performing a sensitivity analysis including only studies with a quality score of 100% (Appendix Figs. 4–6). Estimated transfer frequencies stratified according to type of behavior (ie, standardized vs nonstandardized), surface of origin, microorganism, and microbiological sampling method are displayed in Appendix Figs. 7–10.
Figures 2 and 3 provide an overview of transfer frequency for the 2 most commonly evaluated pathogens, VRE and MRSA, depending on origin and destination surfaces.

Fig. 2 Transfer frequency of VRE. Abbreviations: ICU, intensive care unit; VRE, vancomycin resistant enterococci; black circle, surface of origin, patient, and the patient environment; grey circle, surface of origin, patient environment, or inanimate objects; dotted circle, transfer surface; dashed circle, destination surface. Percentage is the transfer frequency or percentage of destination sites colonized or contaminated with corresponding microbe.

Fig. 3 Transfer frequency of MRSA. Abbreviations: ICU, intensive care unit; MRSA, methicillin-resistant S. aureus; black circle, surface of origin, patient, and the patient environment; grey circle, surface of origin, patient environment, or inanimate objects; dotted circle, transfer surface; dashed circle, destination surface. Percentage is the transfer frequency or percentage of destination sites colonized or contaminated with corresponding microorganism.
Transfer quantity
The burden of contamination from pathogen transfer was reported in 9 studies (Table 2). Eight authors reported CFU counts of 10–1000 transferred to 5 fingertips or to the hand. Only 1 study reported considerably higher CFU counts of up to 2×106 related to ‘moist interactions.’Reference Olsen, Lynch, Coyle, Cummings, Bokete and Stamm 34
Risk factor analysis
Behavior leading to contamination was identified by 12 authors through univariable or multivariable analysis (Table 3). Among the studies, 5 identified ‘duration of care’ as a risk factor for higher frequency of contamination.Reference Pittet, Dharan, Touveneau, Sauvan and Perneger 2 , Reference Morgan, Liang and Smith 28 , Reference Morgan, Rogawski and Thom 29 , Reference Pessoa-Silva, Dharan and Hugonnet 35 , Reference Tenorio, Badri and Sahgal 36 Furthermore, 7 authors described ‘contact with moist body sites or invasive devices’ (eg, wounds and ventilators) as a risk factor for contamination,Reference Snyder, Thom and Furuno 25 , Reference Morgan, Liang and Smith 28 – Reference Roghmann, Johnson and Sorkin 30 , Reference Pessoa-Silva, Dharan and Hugonnet 35 – Reference Rock, Thom, Masnick, Johnson, Harris and Morgan 37 and 3 authors described the sheer ‘presence of an invasive device’ (eg, urinary catheter, tracheostomy, and ileostomy).Reference Zachary, Bayne, Morrison, Ford, Silver and Hooper 22 , Reference Snyder, Thom and Furuno 25 , Reference Rock, Thom, Masnick, Johnson, Harris and Morgan 37
Quality of included articles
Quality scores of the studies ranged from 60% to 100% (Tables 1–3), with a mean of 88% and median of 90%. Overall, 26 articles scored >80%, 1 scored 60%,Reference Ludlam, Swayne and Kearns 14 2 scored 70%,Reference Ojajarvi 15 , Reference Olsen, Lynch, Coyle, Cummings, Bokete and Stamm 34 and 3 scored 80%.Reference Jeske, Tiefenthaler, Hohlrieder, Hinterberger and Benzer 18 , Reference Loftus, Muffly and Brown 32 , Reference Sanderson and Weissler 38 Appendix Table 1 displays the proportion of studies meeting each quality item. The most common reasons for poor scores included failing to report estimates of the random variability in the data (n=19, 59%) and colonization status of the surface of origin (n=10, 31%).
Discussion
This systematic review on the frequency and quantity of pathogen transfer associated with HCP activities shows substantial variability in studied settings, pathogens, and care activities, as well as application of diverse microbiologic sampling methods. Most studies focused on transfer from patients and their environment to HCP hands and gloves, whereas only 2 investigated the transfer from HCP to patients. Despite this variability, the following statements have been substantiated: (1) Transfer of pathogens between patients or their environments and HCP or medical devices occurs frequently during patient care, with transfer to hands and gloves occurring more often than transfer to gowns. (2) Higher frequencies of transfer are associated with moist body sites, invasive devices, and longer duration of care. (3) Higher CFU counts are found in contacts with moist surfaces.
Hands are commonly cited as the main vector for transfer of pathogens in the hospital. 39 Gloved hands also act as vectors if gloves are not changed according to guidelines or hands are not disinfected after glove use. In 2006, Pittet et alReference Pittet, Allegranzi and Sax 40 provided an evidence-based model for hand transmission during patient care and identified 5 steps in patient-to-patient pathogen transmission via HCP hands: (1) organisms present on patients’ skin or environment, (2) organism transfer to hands, (3) organism survival on hands, (4) defective hand cleansing resulting in hands remaining contaminated, and (5) contaminated hands cross transmitting organisms to the next patient. Our review examines this sequence and systematically summarizes the existing literature. Unsurprisingly, in concordance with the prevailing perception that hands are “the” vector in healthcare settings, most papers focus on pathogen transfer to hands or gloves. Hand and glove contamination after contact with patients and their environment occur in an estimated 33% and 30% of interactions, respectively. Strikingly, only 2 studies addressed the transfer to the patient (step 5 in the aforementioned concept paper), which is immediately relevant for patient safety.Reference Ludlam, Swayne and Kearns 14 , Reference Duckro, Blom, Lyle, Weinstein and Hayden 24 Duckro et alReference Duckro, Blom, Lyle, Weinstein and Hayden 24 examined the transfer of VRE from positive to negative body sites of patients and surfaces in their immediate environment via hands and demonstrated a transfer frequency of 11%. Ludlam et alReference Ludlam, Swayne and Kearns 14 investigated the transfer of MRSA in a similar study and found that in 38% of audited care episodes at least 1 destination surface was contaminated. With poor hand hygiene compliance before patient contact,Reference Grayson, Russo and Cruickshank 41 the question of transfer frequency to a patient is of highest interest. We suspect that due to patient safety concerns, studies on pathogen transfer from one patient to another might be limited to laboratory studies not covered by this review.
However, 4 studies did address the question of contamination frequencies of hands after glove removal. An estimated contamination frequency of 3% shows that gloves do not provide full protection against pathogens. Contamination may occur through glove microperforation or during doffing, which highlights the importance of hand hygiene after glove removal.
The hospital environment and contaminated surfaces make important contributions to the transmission of nosocomial pathogens,Reference Otter, Yezli and French 42 and patients are considered the main source for environmental contamination. This review identified 2 studies investigating the transfer of VRE and MRSA to the patient environment via HCP hands.Reference Ludlam, Swayne and Kearns 14 , Reference Duckro, Blom, Lyle, Weinstein and Hayden 24 Additionally, contamination of anesthesia machines, stopcocks, and syringes via HCP hands was studied.Reference Loftus, Muffly and Brown 32 , Reference Heid, Bender, Gervais, Schmeck, Kohnen and Noppens 33 The acquisition of pathogens from the patient environment to HCP hands, gloves, or gowns was the subject of many reports: HCP behaviors entailing only environmental contact led to transfer frequencies >40% for VRE, MRSA, and Clostridium difficile.Reference Ray, Hoyen, Taub, Eckstein and Donskey 19 , Reference Hayden, Blom, Lyle, Moore and Weinstein 43 – Reference Guerrero, Nerandzic, Jury, Jinno, Chang and Donskey 46 In conclusion, pathogen transfer to and from patient environments and noncritical medical devices occurs often. These contaminated surfaces can then act as intermediate vectors and may play an important role in transmission to patients.
Whether HCP attire plays a role in the transfer of pathogens is a topic of considerable debate and controversy. While we identified numerous studies addressing pathogen transfer to uniforms or gowns, we did not identify any study reporting transfer pathogens from gowns and uniforms to patients. In general, gowns became contaminated at a lower percentage than gloves or hands, but estimated proportions were still significant at 11%. Notably, a 2007 review was also not able to find conclusive evidence that contaminated uniforms act as vehicles to transfer pathogens to patients.Reference WIlson, Loveday, Hoffman and Pratt 47
The quantity of contamination was reported to be ~10–1000 CFU per 5 fingertips or hand. The single study reporting considerably higher CFU counts investigated moist interactions.Reference Olsen, Lynch, Coyle, Cummings, Bokete and Stamm 34 This finding is in line with the finding of the risk-factor analyses showing that contacts with moist body sites lead to higher frequency of transfer of pathogens.Reference Snyder, Thom and Furuno 25 , Reference Morgan, Liang and Smith 28 – Reference Roghmann, Johnson and Sorkin 30 , Reference Pessoa-Silva, Dharan and Hugonnet 35 – Reference Rock, Thom, Masnick, Johnson, Harris and Morgan 37 Sampling technique may impact on CFU counts when studying transmission. The 2 studies reporting the highest CFU counts differed from the others by applying glove juice or “broth wash” sampling instead of the contact plate method.
Our review has several limitations. First, the studies in this review are very heterogeneous. This is partly explained by the fact that a multitude of factors influences the transfer of microorganisms such as the type of pathogen, surface characteristics (eg, moisture), frequency and intensity of contact between surfaces, and inoculum size. The number of organisms present on intact areas of patient skin is known to vary from 102 to 106 CFU/cm.Reference Pittet, Allegranzi and Sax 40 Moreover, several factors influence the detection of pathogens on the sampled destination surface such as microbial sampling, culture technique, and the size of the sampled surface area. A systematic review by Jullian et alReference Jullian-Desayes, Landelle, Mallaret, Brun-Buisson and Barbut 48 on hand contamination with C. difficile also attributed the wide range of values to the heterogeneity of study designs. Second, in 10 studies the origin surface was not sampled, in others only the colonization status of the patient with MDR pathogens was indicated but not the exact colonized body sites or the contamination status of the inanimate surfaces. Such study methods could lead to an underestimated transfer frequency because no contact with contaminated surfaces is possible in the study scenarios. Third, during multistep prolonged interactions, the exact origin of transferred microorganisms remains unknown. When the observed interaction involved patients and the environment, both can act as origin surfaces. The HCP himself may even be the origin of pathogens; for example, touching the nose is a common habit and can contaminate hands.Reference Kwok, Gralton and McLaws 49 These 3 issues preclude a generalizable statement about exact transfer frequencies from the data of the 32 studies, and estimated proportions must be considered carefully. However, despite these limitations, every study provided an estimate of the transfer frequency or the level of contamination after a defined interaction in a real care setting. Furthermore, the studies closely mirrored clinical reality, where the colonization status usually remains unknown.
To the best of our knowledge, this is the first systematic review and meta-analysis to address the transfer of pathogens associated with HCP behavior. This is surprising considering that precise knowledge about pathogen transmission impacts the nature and extent of required preventive measures. In the absence of such knowledge, prevention policies must accept large safety margins to safeguard the system against the spread of MDR pathogens. Beyond binding unnecessary resources, this ambiguity from weak scientific evidence jeopardizes HCP acceptance and motivation to adhere to preventive policies.
In summary, this systematic review of behavior-related transmission of pathogens in healthcare settings unveiled a lacunar knowledge base coming from very heterogeneous studies. Often, the exact HCP behavior leading to pathogen transfer remains hidden in complex prolonged care sequences. Despite these uncertainties, the included studies each provide unique insight in the risk associated with contact between HCP and patients, their immediate environment, and mobile objects in real-life care settings. These commonalities allow the general conclusion that pathogen transfer is very frequent in healthcare settings. Risk factors for transmission are moist surfaces, the manipulation of invasive devices, and prolonged care activity. Higher CFU transfer is associated with moist surfaces. More systematic and well-reported research in this crucial area at the crossroads between microbiology and HCP behavior is urgently warranted to support optimal design of preventive policies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.156
Acknowledgments
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support
The study was funded by the Swiss National Science Foundation grant “Human Factors Analysis of Infectious Risk Moments” (grant no. 32003B_149474 to H.S.) and by the United States Centers for Disease Control and Prevention (grant no. BAA 200-2016-91954 to L.M.).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.








