Candida auris is an emerging fungal pathogen that is often resistant to major classes of antifungal drugs. It is considered a serious global health threat because it can cause severe infections with frequent mortality in more than a dozen countries. It can survive on healthcare environmental surfaces for at least 7 days and can cause outbreaks in healthcare facilities. Clearly, infection prevention strategies, such as surface disinfection, will be essential to controlling Candida transmission. Unfortunately, data on the activity of antiseptics and disinfectants used in healthcare to inactivate this pathogen are limited.Reference Vallabhaneni, Kallen and Tsay1–Reference Piedrahita, Cadnum, Jencson, Shaikh, Ghannoum and Donskey5 In this study, we investigated 12 different disinfectants (ie, 8 low- and intermediate-level disinfectants in 2 dilutions of sodium hypochlorite and 5 high-level disinfectants/chemical sterilants) and 9 antiseptics commonly used in healthcare facilities for their antimicrobial activity against C. auris and C. albicans.
We used the disc-based quantitative carrier testing to evaluate the germicidal activity of multiple antiseptics and disinfectants against the emerging pathogen C. auris.Reference Sattar, Springthorpe, Adegbunrin, Zafer and Busa6, Reference Rutala, Peacock, Gergen, Sobsey and Weber7 We considered the carrier test to mimic disinfectant application on an inanimate surface in a clinical environment better than a suspension test commonly reported by manufacturers and the published literature. The C. auris isolate used was Antibiotic Resistance Bank no. 0385 from the Centers for Disease Control and Prevention. Based on tentative minimum inhibitory concentration (MIC) breakpoints,8 this isolate was resistant to fluconazole and was susceptible to anidulafungin, micafungin, caspofungin, and amphotericin B. To determine whether C. auris susceptibility to germicides was similar to that of other Candida species, we also tested C. albicans (ATCC strain no. 60193). In brief, 10 μL inoculum containing ∼104 with 5% fetal calf serum of C. auris or C. albicans was placed onto each stainless steel disc (1 cm diameter) and dried in a vacuum desiccator for 2 hours. After drying, each carrier was placed in a plastic vial with the inoculated side up. The dried inoculum was entirely covered by 50 μL of the test germicide for 1 minute at room temperature (∼20°C), Then 9.95 mL eluent with neutralizer (Dey/Engley neutralizing broth) was added into each carrier holder to dilute and neutralize the germicide. Serial dilutions of the eluates were filtered to evaluate the fungal viability and to achieve countable numbers. The membrane filters of appropriate serial dilutions were placed on sheep blood agar plates and incubated for 24–48 hours at 37°C, and the fungal were then counted. We performed 3 replicates for each organism and germicide. Also, 3 carrier controls were prepared during each experiment in the manner described above but without germicide exposure. Compared to mean carrier control counts, the log10 reduction of the test organism for each germicide was calculated.Reference Kanamori, Rutala, Weber, Gergen, Sickbert-Bennett and Weber9
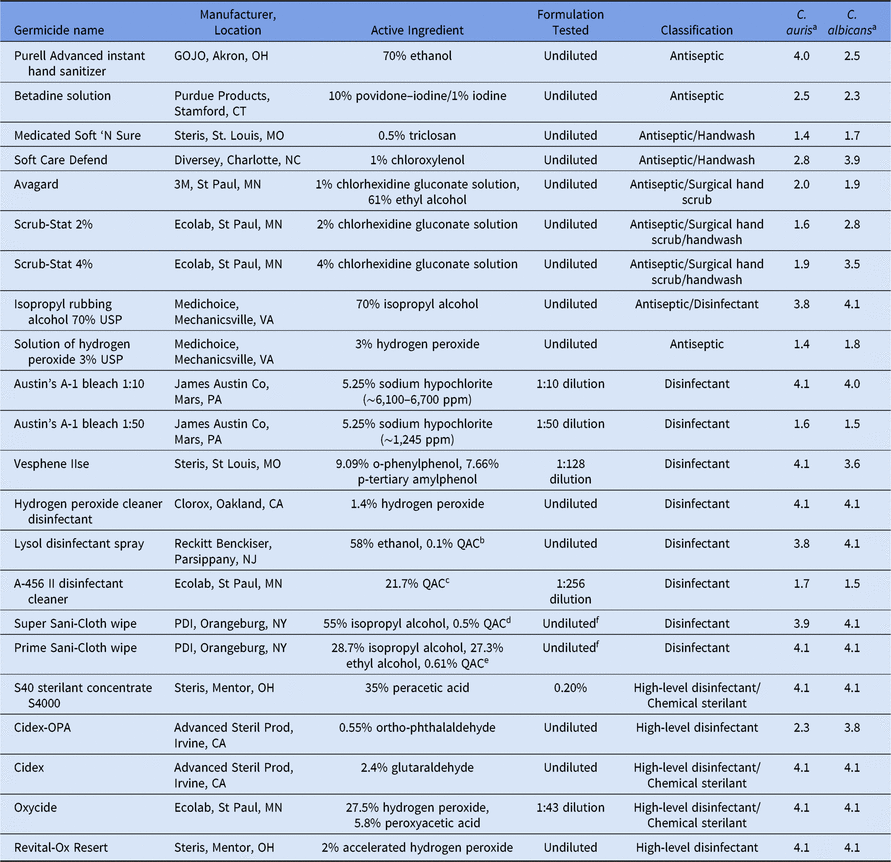
The efficacy of germicides with active ingredient, product name, manufacturer, and classification against C. auris and C. albicans are provided in Table 1. Under the challenging test conditions (ie, 5% FCS and 1 minute exposure time), 12 of 22 tested disinfectants and antiseptics (55%) demonstrated at least a 3-log10 reduction, and 16 (73%) demonstrated at least a 2-log10 reduction for C. auris. Also, 14 of these 22 (64%) demonstrated at least a 3-log10 reduction, and 17 (77%) demonstrated at least a 2-log10 reduction for C. albicans. Of the 9 antiseptics, 7 (78%) did not demonstrate a 3-log10 reduction against C. auris; these included 10% povidone-iodine, 0.5% triclosan, 1% chloroxylenol, 1% chlorhexidine gluconate (CHG) with 61% ethyl alcohol, 2% CHG, 4% CHG, and 3% hydrogen peroxide. Of 13 tested disinfectants (low-level disinfectants and high-level disinfectants), 10 (77%) demonstrated at least a 3-log10 reduction at 1 minute, with 3 exceptions: (1) a 1:50 dilution of 5.25% sodium hypochlorite (∼1,245 ppm chlorine; Chlorine Test Kit, Model CN-21P, Hach, Loveland, CO); (2) a diluted, water-based quaternary ammonium compound (QAC); and (3) a 0.55% ortho-phthalaldehyde. In general, the log10 reductions for C. auris and C. albicans were similar: 12 of 13 (92%) within a 1 log10 difference) for the 13 tested disinfectants. But 4 of 9 (44%) of the antiseptics had a >1 log10 difference in susceptibility.
Table 1. Germicidal Activity Against Candida auris and Candida albicans Using a Quantitative Carrier Test Method

Note. QAC, quaternary ammonium compound.
a Values are shown in mean log10 reductions under a test condition of 104 test organisms with 5% fetal calf serum and 1 minute contact time.
b QAC: alkyl (C14 50%, C12 40%, C16 10%) dimethyl benzyl ammonium saccharinate 0.1%.
c QAC: octyl decyl dimethyl ammonium chloride 6.51%; dioctyl dimethyl ammonium chloride 2.604%; didecyl dimethyl ammonium chlorid 3.906%; alkyl (50% C14, 40% C12, 10% C16) dimethyl benzyl ammonium chloride 8.68%
d QAC: n-alkyl (C12 68%, C14 32%) dimethyl ethylbenzyl ammonium chlorides 0.25%; n-Alkyl (C14 60%, C16 30%, C12 5%, C18 5%) dimethyl benzyl ammonium chlorides 0.25%.
e QAC: didecyl dimethyl ammonium chloride 0.61%.
f Extract from cloth.
There is no standard level of germicidal efficacy for environmental surfaces, but most of the disinfectants tested that demonstrated at least a 3-log10 reduction are likely to be clinically effective against C. auris when used appropriately. Some of the Environmental Protection Agency (EPA)–registered disinfectants used in this study have an EPA registration claim longer than the 1 minute used in this study. All of the FDA-cleared high-level disinfectants have a registration claim >1 minute (eg, 8–45 minutes). In summary, with the exception of a water-based QAC and a 1:50 dilution of sodium hypochlorite, our data demonstrate that most disinfectants (10 of 13, 77%) used in healthcare facilities are effective (>3-log10 reduction) against C. auris. Importantly, water-based QACs, which are commonly used for surface disinfectants, had limited activity and therefore should not be used for disinfection of environmental surfaces or noncritical patient equipment in rooms housing patients with C. auris.Reference Cadnum, Shaikh and Piedrahita10 In contrast, 7 of 9 antiseptics (78%) did not achieve a 3-log10 reduction of C. auris in 1 minute.
Because C. auris can persist on surfaces in healthcare environments, cleaning and disinfecting the patient care environment (daily and discharge/terminal cleaning) with effective products is essential. The CDC has recommended the use of EPA-registered hospital-grade disinfectants effective against Clostridium difficile spores (primarily chlorine-based products).8 Our data demonstrate that several other commonly used surface disinfectants (ie, a phenolic, 1.4% improved hydrogen peroxide, and alcohol-quaternary ammonium compounds) are as effective against C. auris as chlorine-based products. Other infection prevention strategies to minimize the contribution of the environment to C. auris transmission are “no-touch” room decontamination technologiesReference Cadnum, Shaikh and Piedrahita10 and improved thoroughness of cleaning/disinfecting environmental surfaces using thoroughness indicators (eg, fluorescent markers). Further studies are needed to evaluate other test surfaces (eg, polymer) and to identify infection prevention strategies that prevent contaminated surfaces from being a source of acquisition by patients of this globally emerging pathogen.
Acknowledgements
None.
Financial support
This study was supported by UNC Health Care.
Conflicts of interest
Dr Rutala is a consultant for PDI and Advanced Sterilization Products. Dr Weber is a consultant for PDI. The other authors report no conflicts of interest relevant to this article.