According to the 2019 Centers for Disease Control and Prevention (CDC),1 Acinetobacter baumannii has become an urgent threat in healthcare facilities in the United States,Reference Consales, Gramigni, Zamidei, Bettocchi and De Gaudio2–Reference Wybo, Blommaert and De Beer10 with contaminated environment or contaminated healthcare workers hands playing a major role in the spread of this organism.Reference Harris, Johnson, Pineles, O’Hara, Bonomo and Thom11–Reference Ng, Marimuthu and Lee14 Facilities have implemented infection control measures, such as surveillance, to control its spread.Reference Munoz-Price and Quinn15–Reference Enfield, Huq and Gosseling21 Although surveillance aims to identify asymptomatic carriers,Reference Wybo, Blommaert and De Beer10–Reference Martins, Martins and de Freitas12,Reference Ng, Marimuthu and Lee14 uncertainty remains regarding how long after an outbreak this surveillance should be performed.
In 2018, an outbreak of carbapenem-resistant A. baumannii (CRAB) was detected across multiple facilities in Wisconsin, involving predominantly postacute care facilities.Reference Florek, Wagner and Lasure22 In response to this outbreak, and based on the initial epidemiology, our healthcare system instituted surveillance among patients transferred from postacute care facilities. For this study, we leveraged 9 months of surveillance (1) to assess the CRAB positivity rate among patients screened over time, (2) to determine the positivity rate based on body site, and (3) to characterize the longitudinal changes of surveillance results among CRAB patients.
Methods
Setting
This observational study was conducted from December 5, 2018, to September 6, 2019, at Froedtert Hospital, which has 607 licensed beds, 6 intensive care units (ICUs), and 150 ICU beds. This study was granted a waiver of informed consent by the Medical College of Wisconsin Institutional Review Board (IRB no. PRO00035267).
Point-prevalence surveys
Surveillance for CRAB was performed among consecutive admissions from postacute care facilities. Additionally, weekly surveillance was performed on any patient housed in the same inpatient unit as a patient positive for CRAB during the entire stay of the positive patient and for at least a week after unit discharge. Specimens collected included tracheostomy secretions for all patients with tracheostomy tubes or intubated, skin swabs obtained from the inguinal region, and unpreserved stool or rectal swabs. Inguinal samples were obtained by bedside nurses using BBL culture swabs (Copan Italia, Italy) that were premoistened with sterile saline prior to sampling a 10×10-cm area in the groin. Respiratory secretions and stool samples were collected using sterile containers.
Validated surveillance cultures
Surveillance samples were processed within 24 hours of collection. Swabs were broken off into a trypticase soy broth (TSB) suspension with a final concentration of 4.5 µg/mL meropenem and briefly mixed in a vortexer. Specimens in containers were sampled using a swab, transferred into the TSB suspension, and briefly mixed in a vortexer. Inoculated broths were incubated at 35°C in ambient air for 18–24 hours, mixed in a vortexer, and streaked (10 µL) to a MacConkey agar plate. A 10-µg meropenem disk was placed onto the inoculated plates in the area of specimen inoculation and cultures were incubated at 35°C in ambient air for 18–24 hours. Any bacterial colonies within a zone ≤18 mm from the meropenem disk were further characterized using matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS) (Bruker Diagnostics, Billerica, MA) for definitive identification and Etest meropenem (bioMèrieux, Marcy-l'Étoile, France) to confirm phenotypic minimum inhibitory concentration.
Real-time polymerase chain reaction
CRAB isolates were sent to the Wisconsin State Laboratory of Hygiene for carbapenemase gene detection. Real-time polymerase chain reaction (PCR) was performed to detect bla KPC, bla NDM, bla VIM, bla IMP, bla OXA-23-like, bla OXA-24/40-like, and bla OXA-58-like carbapenemase genes. Isolates were extracted using a thermal NaOH method for preparation of bacterial lysates. PCR primers and probes sequences were obtained from CDC protocols.
Each sample was tested in 20-µL volumes using an optical 96-well plate with optical cap strip tubes (Applied Biosystems, Foster City, CA). Each mixture contained 2x Quantifast Probe PCR Kit (Qiagen, Germany), primer-probe mix for each target, and molecular grade water with 2 µL extracted DNA. PCR was performed on the ABI 7500 Fast system (Applied Biosystems, Foster City, CA) with the following thermal cycling conditions: 95°C for 3 minutes, 40 cycles of 95°C for 3 seconds, 60°C for 30 seconds. A crossing threshold of <30 for any 1 of the markers (carbapenemase genes) was interpreted as a positive result.
Definitions
A patient was considered positive for CRAB if any surveillance test yielded CRAB. A surveillance culture set was defined as the group of surveillance cultures (skin, stool, or respiratory) performed on the same day (±1 day). The positivity rate of surveillance cultures was defined as the proportion of patients with a positive surveillance culture divided by the total number of patients screened using the same body source.
Epidemiologic data collection
Demographics were collected using electronic medical records for the following variables: age, gender, race, ethnicity, mechanical ventilation status, use of other long-term invasive devices (ie, Foley catheter or feeding tube) for 30 days or longer, prior hospitalization in the past 30 days, length of stay in the first hospitalization, underlying conditions, and residency at a postacute care facility.
Infection control interventions
All CRAB patients were placed on enhanced contact precautions (ie, gloves, gowns, and shoe covers) and placed in a cohort next to other positive patients.Reference Florek, Wagner and Lasure22 Whenever feasible, nursing staff were also placed in a cohort of care providers for A. baumannii patients. Surfaces in the patient’s room were disinfected with peroxyacetic acid and hydrogen peroxide. Disposable stethoscopes were used, and communal objects were avoided whenever feasible.
Statistical analysis
Characteristics of the study population were determined using proportions for categorical variables and mean and standard deviation or median and interquartile range for continuous variables. The Pearson χReference Consales, Gramigni, Zamidei, Bettocchi and De Gaudio2 test was used for comparing categorical variables. The Student t test was used for means, and the Mann-Whitney U test was used for medians. Multivariate analyses were performed to determine the association between CRAB positivity and variables found to be statistically significant in the univariate analysis (P < .05). Tests were 2-tailed, and an α of .05 was considered statistically significant. Analyses were conducted using SPSS version 24.0 software (SPSS Inc, Chicago, IL).
Results
Overall cohort
During the 9 months of observation, 1,817 surveillance cultures were performed among 682 patients (Table 1). Of these 682 patients, 354 were male (51.9%), and the cohort had a mean age of 61.6 years (standard deviation [SD], 16.9). Nearly two-thirds were white (n = 425, 62.3%), and most were self-reported non-Hispanic (n = 661, 96.9%). Approximately 80% (n = 531) of patients reported living in their private residence and the remainder (n = 151, 22.1%) resided in postacute care facilities. The most frequent comorbidities were renal disease (21.4%), solid tumors (20.2%), congestive heart failure (17.3%), and chronic pulmonary obstructive disease (15.5%) (Table 1).
Table 1. Demographic and Clinical Characteristics of Patients Screened for Carbapenem-Resistant Acinetobacter baumannii Status

Note. SD, standard deviation; IQR, interquartile range; LOS, length of stay; CVA/TIA, cerebrovascular accident or transient ischemic attack; DM, diabetes mellitus.
a White race was the reference category.
b P < .05.
The distribution of cultures by body site, regardless of results, was as follows: 768 (42.6%) from skin, 743 (41.2%) from stool, and 291 (16.2%) from the respiratory tract (255 tracheal secretions, 23 sputum, and 13 bronchoalveolar-lavage). In total, 16 patients (2.3%) were identified as positive for CRAB throughout the surveillance period (Supplementary Fig. 1 online). Among the samples from 16 CRAB-positive patients, 13 (82%) positive results were from stool samples, 9 (56%) were from skin, and 6 (38%) were from respiratory secretions. The median number of days from admission to first surveillance culture was 5 days (interquartile range [IQR], 1–9) for patients admitted from their private residency and 1.5 days (IQR, 1–4) for patients admitted from a postacute care facility. Also, 11 patients had >1 surveillance culture set (range, 1–9 sets). As of September 2020, none of the CRAB patients had developed invasive infections or required antibiotic treatment with coverage for these isolates.
Of the 16 CRAB patients, 10 (62.5%) were positive on their initial surveillance culture set, and 5 (31%) of these patients were positive in >1 body site. Also, 3 patients had only 1 surveillance set performed. Furthermore, 4 patients were only transiently positive with negative culture results in subsequent tests (Fig. 1). However, 7 patients (43.8%) were persistently positive on all subsequent culture sets (performed for up to 4 weeks).
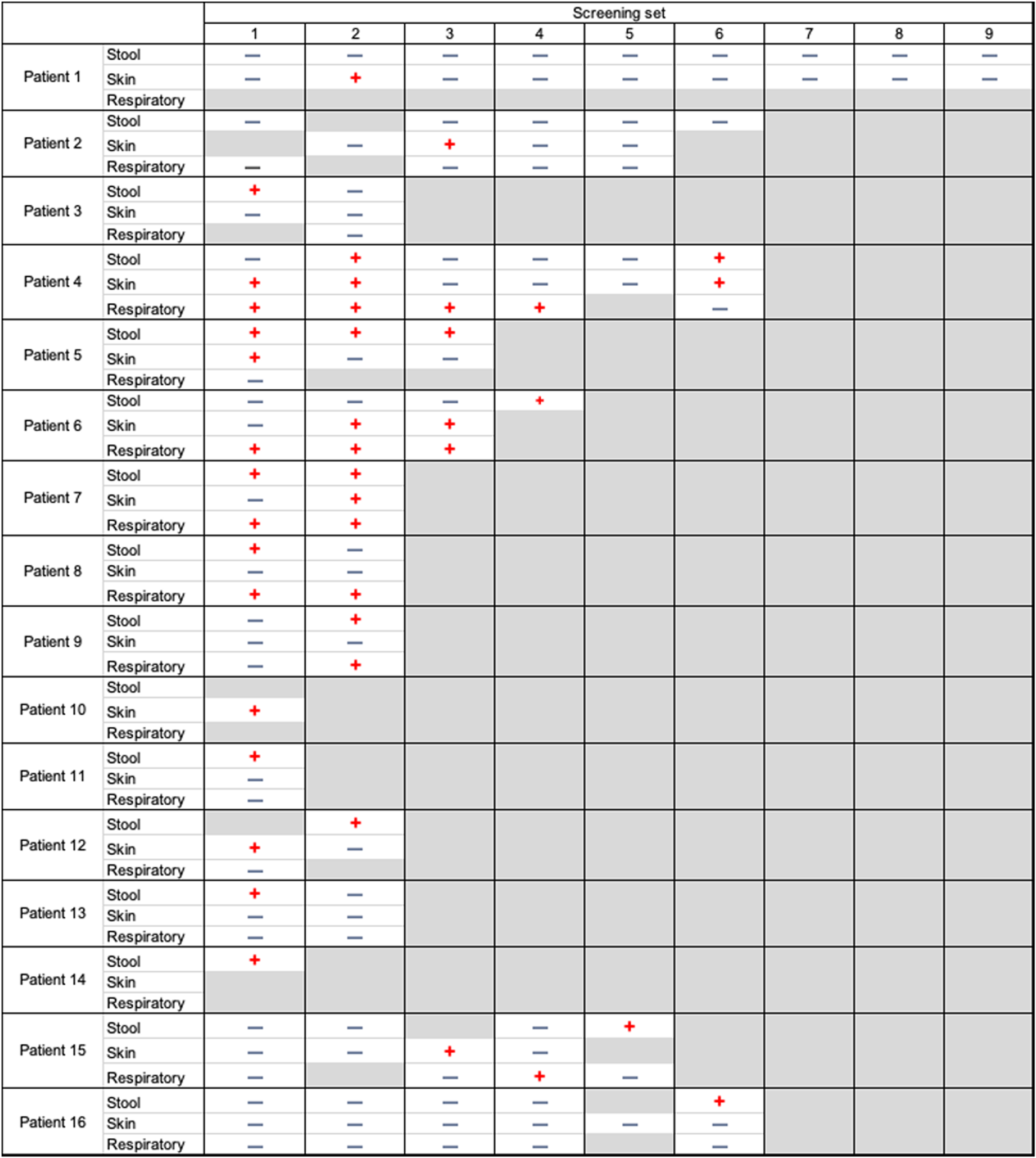
Fig. 1. Longitudinal changes in the carbapenem-resistant Acinetobacter baumannii status among positive patients. Note. Negative blue signs show negative cultures. Positive red signs show positive cultures. Gray boxes mean cultures not performed.
As depicted in Table 1, CRAB patients were more likely to be African American (11 [68.8%] vs 209 [31.4%]; P = .002), to live in a postacute care facility (14 [87.5%] vs 137 [20.6%]; P < .001), to have long-term tracheostomy or ventilation dependence (10 [62.5%] vs 27 [4.1%]; P < .001), to use invasive devices such as Foley catheters, feeding tubes, or tracheostomies (12 [75%] vs 83 [12.5%]; P < .001), to have chronic wounds (8 [50%] vs 43 [6.5%]; P < .001), or to be quadriplegic (5 [31.3%] vs 13 [2%]; P < .001). Multivariate analysis found that ventilator dependence (odds ratio [OR], 7.07; 95% confidence interval [CI], 1.57–31.75; P = .011) and the presence of chronic wounds (OR, 6.69; 95% CI: 1.60–27.95; P = .009) were both associated with a greater likelihood of positive surveillance results.
Residents from postacute care facilities
In total, 151 patients were admissions from postacute care facilities (Table 2). Among them, 78 (51.7%) were male, and the cohort had a mean age of 66.2 years (SD, ±15.4). Also, 37 (5.4%) were ventilator dependent, 30 (19.9%) had a Foley catheter, and 46 (30.5%) had a feeding tube. The most frequent comorbidities among these patients were renal disease (42, 27.8%), cerebrovascular accident or transient ischemic attack (36, 23.8%), and chronic pulmonary obstructive disease (34, 22.5%). Of the 16 CRAB patients, 14 (87.5%) were residents from postacute care facilities. Compared to noncolonized patients, CRAB patients from postacute care facilities were younger (55 years [SD, ±13] vs 67 years [SD, ±15]; P = .003), more often had a Foley catheter (9 [64.3%] vs 21 [15.3%]; P < .001) or a feeding tube (11 [78.6%] vs 35 [25.5%]; P < .001), and were more often ventilator dependent (10 [71.4%] vs 19 [13.9%]; P < .001) than patients who tested negative on surveillance (Supplementary Table 1 online).
Table 2. Demographic and Clinical Characteristics of Patients Screened for Carbapenem-Resistant Acinetobacter baumannii Status Based on Residency

Note. SD, standard deviation; DM, diabetes mellitus; LOS, length of stay; IQR, interquartile range; CVA/TIA, cerebrovascular accident or transient ischemic attack.
All 2019 isolates were tested for the presence of carbapenemases as outlined in Methods. Only 2 of these isolates (August 2019) were positive for OXA-24/40-like β-lactamase OXA-72 (Supplementary Fig. 1 online).
Discussion
During 9 months of surveillance following a CRAB multifacility regional outbreak, we observed a very low positivity rate, with stool showing the most positive results. The likelihood of being positive for CRAB was significantly higher among patients with chronic wounds and mechanical ventilator dependence. Patients from postacute care facilities had higher risk of testing positive, although this association disappeared after adjusting for chronic wounds and mechanical ventilator dependence. Only 2 isolates, observed among postacute care patients, were detected to carry OXA-24/40–like β-lactamase OXA-72 compatible with the initial regional outbreak.Reference Florek, Wagner and Lasure22 Given our results, surveillance after a regional outbreak involving patients from postacute care facilities should probably be geared to screening postacute care patients requiring mechanical ventilation or with chronic wounds. Although not statistically significantly during multivariate analyses, CRAB patients were more frequently African American. In a highly segregated city such as Milwaukee, this finding may suggest the influence of racial or socioeconomic determinants on exposure and colonization by CRAB.
Previous studies have described the prevalence of A. baumannii among postacute care patients ranging between 15% and 63%.Reference Sengstock, Thyagarajan, Apalara, Mira, Chopra and Kaye6,Reference Mody, Gibson and Horcher9,Reference Wybo, Blommaert and De Beer10,Reference Thom, Maragakis and Richards23 However, only a few studies have performed surveillance studies and have investigated antibiotic resistance in this population.Reference Munoz-Price and Quinn15 Mortensen et alReference Mortensen, Trivedi and Rosenberg8 found that 4 (12.1%) of 33 residents in a long-term care facility were positive for CRAB.Reference Mortensen, Trivedi and Rosenberg8 In that cross-sectional study, ventilator use was independently associated with A. baumannii colonization (adjusted OR, 4.24; 95% CI, 1.06–16.93). In our study, 6 of 16 patients with an initially positive CRAB culture continued to test positive for up to 4 weeks after the initially positive surveillance culture. Only a few studies have longitudinally tested patients to determine the duration of colonization. In these studies, CRAB colonization ranged from 285 days to 16 months,Reference Nutman, Lerner, Fallach, Schwartz and Carmeli24,Reference Marchaim, Navon-Venezia and Schwartz25 and duration of colonization has been associated with being admitted from postacute care.Reference Nutman, Lerner, Fallach, Schwartz and Carmeli24
Our study has several limitations. It was based on a single-center experience, and although a large number of patients and samples were processed, the percentage of positivity was low. In addition, not all patients underwent weekly samples or had all body sites cultured, and only the 2019 isolates underwent confirmatory testing for carbapenemase production. Given that we did not have access to nursing-home records, we were unable to accurately obtain antibiotic exposures for all patients; thus, this variable was not used for the analyses. Finally, whole-genome sequencing of all isolates was not performed; thus, we were not able to compare their clonality with the original regional outbreak.
In summary, we found a low number of positive surveillance cultures following a regional outbreak involving postacute care facilities. Not surprisingly, surveillance results were positive mainly among postacute care patients, and stool was the source with highest positivity. Based on our experience, screening high-risk patients (ie, postacute care facility residents with chronic wounds or with mechanical ventilation dependence) upon hospital admission is an intervention that should be considered following a regional CRAB outbreak. Finally, social disparities as they relate to exposure and colonization of long-term care residents should be explored in future studies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.162
Acknowledgments
We thank all the staff at the Froedtert Memorial Lutheran Hospital Infection Control Department for their help ensuring that the surveillance tasks were performed.
Financial support
This study was performed with financial support by the Medical College of Wisconsin Department of Medicine.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.






