Nonventilator hospital-associated pneumonia (HAP) is a prevalent healthcare-associated infection (HAI) that is twice as frequent as ventilator-associated pneumonia and that has greater total costs overall than ventilator-associated pneumonia.Reference Davis and Finley 1 HAP is associated with substantially increased morbidity and mortality, longer hospital stays, and increased costs of >$40,000 per patient.Reference Magill, Edwards and Bamberg 2 – Reference Leu, Kaiser, Mori, Woolson and Wenzel 4 Also, HAP excludes ventilator-associated pneumonia (VAP) and community-acquired pneumonia. The existing literature primarily addresses VAP, with few reports addressing non-ventilator-related HAP in adults.Reference Passaro, Harbarth and Landelle 5 – Reference Kazaure, Martin, Yoon and Wren 7 Thus limited evidence is available to guide prevention efforts for HAP. McAuley et alReference McAuley, Price and Phillips 8 attempted to perform a meta-analysis, but of 5,101 potential sutdies, only 2 were methodicallogically adequate for inclusion. Consequently, key questions about the incidence, risk factors, attributable mortality, and preventability of nonventilator HAP have barely been studied.Reference Klompas 9 Factors that may complicate the process of answering these questions include errors inherent in diagnosing pneumoniaReference Roulson, Benbow and Hasleton 10 , Reference Russell, Koch, Laurenson, O’Shea, Sutherland and Mackintosh 11 and controversy regarding microbial etiologies.Reference Rotstein, Evans and Born 12 – Reference Schreiber, Chan and Shorr 15 The National Healthcare Safety Network (NHSN) defines HAP, in part, by onset >48 hours after admission, but other aspects of the definition may be nonspecific and subjective.Reference Russell, Koch, Laurenson, O’Shea, Sutherland and Mackintosh 11 , Reference Rotstein, Evans and Born 12 , Reference Horan, Andrus and Dudeck 16 , Reference Wipf, Lipsky and Hirschmann 17 Wolfensberger et alReference Wolfensberger, Meier, Kuster, Mehra, Meier and Sax 18 reported a specificity of 0.35 using only codes from the International Classification of Disease, Tenth Revision (ICD-10).
Although scant, several elements address key points in the pathogenesis of pneumonia related to aerodigestive tract colonization and bacterial aspiration. In a meta-analysis of 29 trials including >5,000 patients, oropharyngeal decontamination was associated with a relative risk (RR) of HAP of 0.56 (95% confidence interval [CI], 0.46–0.69), compared to standard oral care.Reference Roquilly, Marret, Abraham and Asehnoune 19 Factors leading to bacterial aspiration include sedation.Reference Obiora, Hubbard, Sanders and Myles 20 In observational studies of adults with community-acquired pneumonia, benzodiazepine use was associated with a 22%–28% higher risk ofpneumonia.Reference Obiora, Hubbard, Sanders and Myles 20 , Reference Taipale, Tolppanen and Koponen 21 Gastroesophageal aspiration is also promoted by a supine body position.Reference Alexiou, Ierodiakonou, Dimopoulos and Falagas 22 A meta-analysis of 3 randomized controlled trials including 337 patients found that a semirecumbent position with the head of the bed elevated to 45° was associated with a substantially reduced risk of VAP (odds ratio, 0.47; 95% CI, 0.27–0.82).Reference Alexiou, Ierodiakonou, Dimopoulos and Falagas 22 Inhibition of respiratory defense mechanisms can lead to HAP. Smoking interferes with these mechanisms; a meta-analysis of 7 observational studies of preoperative smoking cessation found a significantly decreased risk of postoperative pulmonary complications among former smokers compared with current smokers (RR, 0.81; 95% CI, 0.70–0.93).Reference Lawrence, Cornell and Smetana 23 Postoperative patients, particularly those with upper abdominal or thoracic procedures, may not inflate their lungs optimally due to discomfort. In a systematic review, incentive spirometry reduced postoperative pulmonary complications among patients undergoing noncardiothoracic surgery and may be the least labor-intensive strategy for promoting lung expansion after surgery.Reference Lawrence, Cornell and Smetana 23 , Reference Qaseem, Snow and Fitterman 24 Inactivity results in reduced clearance of pulmonary secretions that contribute to HAP.Reference Stolbrink, McGowan and Saman 25 A prospective study among inpatients examined the impact of an early mobility program after surgery; the hazard ratio for HAP among patients who received the intervention was 0.39 (95% CI, 0.22–0.68) compared with patients who did not receive it.Reference Stolbrink, McGowan and Saman 25 Mobilization can also improve gastric emptying, reducing the likelihood of aspiration of gastric contents.Reference Lipp, Schnedl, Hammer, Kotanko, Leb and Krejs 26 Monitoring residual tube feeding volumes and marking feeding tubes at the nares to ensure that feeding ports are below the esophagus can also reduce the risk of aspiration.Reference Metheny 27 , Reference Metheny, Meert and Clouse 28
Prevention bundles for VAP are broadly implemented even though rigorous evidence supporting individual bundle elements is scant.Reference Klompas 29 A meta-analysis by Roquilly et alReference Roquilly, Marret, Abraham and Asehnoune 19 found limited support for prevention bundles in pneumonia in intensive care settings, mainly addressing VAP. Despite concerns about the validity of possible prevention bundles, the mortality rate among patients with HAP made it imperative that we attempt to reduce its incidence.
In 2008, Kaiser Permanente Northern California (KPNC) conducted a mortality review that identified HAP as a prominent contribution to potentially avoidable mortality. The high impact in morbidity and mortality led to this quality improvement project to reduce the incidence of HAP. Attempting to reduce HAP required implementing both effective surveillance as well as prevention strategies. We developed a definition enabling accurate programmatic surveillance and implemented a HAP prevention bundle.
Methods
This quality improvement project consisted of a longitudinal observational design with multiple interventions. It was conducted in Kaiser Permanente Northern California, a not-for-profit integrated healthcare delivery system, caring for 4.4 million members who receive care at 21 medical centers in the Northern California region. The demographics of the population served reflect those of the general community of Northern California.Reference Koebnick, Langer-Gould and Gould 30
This project had strong support from KPNC clinical and operational executive leaders and was conducted under the oversight of the regional HAI committee, which included infection preventionists, infectious disease and other physicians, operational leaders, nurses, respiratory therapists, pharmacists, and facilities management and environmental services leaders.
We developed an electronic surveillance system to permit real-time and reproducible findings across the expansive system. Two physician members of the multidisciplinary team reviewed the charts of 100 patients from 3 medical centers with disparate characteristics who had a discharge diagnosis of HAP. Patients were excluded if they were on comfort care in the first 24 hours of their hospital stay, if they had pneumonia present on admission, or if they had VAP.
Of these 100 patients, 31 were identified as having HAP with high likelihood. Their charts were further reviewed to determine whether elements of the National Healthcare Safety Network (NHSN) clinical algorithm for identifying pneumonia were consistently present, were reproducible, and were automatically extractable from the electronic health record (EHR) to facilitate surveillance.Reference Horan, Andrus and Dudeck 16 Fever, leukocytosis, and new sputum characteristics were inconsistently documented or transient in nature. Sputum characteristics were particularly transient and had little value for identifying patients with HAP. Microbiology, including Gram stain and culture, and histopathologic exams were inconsistently available. In addition, imaging abnormalities identifying pneumonia were described in numerous ways.
However, new imaging abnormalities persisting for >24 hours were consistently recorded in patients with verified HAP. To address this, we developed a natural language processing system to search imaging reports for opacity descriptors consistent with pneumonia. In the end, HAP was identified by a discharge diagnosis of pneumonia occurring >48 hours after admission with associated new imaging abnormalities that persisted for >24 hours.
The development of prevention activities was initiated with a review of the literature to identify interventions with the potential to prevent HAP. These included elevating the head of the bed,Reference Drakulovic, Torres, Bauer, Nicolas, Nogue and Ferrer 31 , Reference Niel-Weise, Gastmeier, Kola, Vonberg, Willie and van der Broek 32 oral care,Reference Roquilly, Marret, Abraham and Asehnoune 19 , Reference Fourrier, Dubois and Pronnier 33 , Reference DeRiso, Ladowski, Dillon, Justice and Peterson 34 , Reference Li, Ai, Li, Zheng and Jie 35 nasogastric and other feeding tube care,Reference Metheny, Schnelker and McGinnis 36 reducing proton pump inhibitor use,Reference Herzig, Howell, Ngo and Marcantonio 37 , Reference Eom, Jeon, Lim, Cho, Park and Lee 38 reducing sedation,Reference Loeb, McGeer, McArthur, Walter and Simor 39 , Reference Obiora, Hubbard, Sanders and Myles 20 incentive spirometry,Reference Rosière, Vader and Cavin 40 and smoking cessation.Reference Lindstrom, Sadr Azodi and Wladis 41 , Reference Mills, Eyawo, Lockhart, Kelly, Wu and Ebbert 42 The quality of evidence and ability to extrapolate to HAP varied widely. We also identified KPNC medical centers that had low HAP rates. Notably, the units with lowest rate had populations likely to be at increased risk for HAP. They included an acute rehabilitation center and bariatric surgery centers. Moreover, 7 interventions were consistently implemented across these latter settings: (1) aggressive mobilization, (2) upright posture for meals, (3) careful swallowing evaluation before any feeding, (4) attention to sedation levels (particularly near mealtimes), (5) elevated head of bed (HOB) for sleep, (6) rigorous oral care, and (7) feeding tube care.
To examine acceptability and potential effectiveness of some of these potential interventions, a pilot study was initiated at a single medical center and focused on an initial bundle of 3 elements: oral care, up in chair, and tube care (creating the acronym “OUT”). The pilot clarified a need to identify the population at risk when nursing and medical staff expressed interest in applying the bundle to all inpatients.
Consequently, the physician reviewers examined the charts of patients with verified HAP and controls and compared the prevalence of several factors: smoking status, comorbidities (obesity, altered mental status, neurologic symptoms, pulmonary disease, cardiovascular disease, history of stroke, and hypoalbuminemia [<3 g/dL]), current medication use (corticosteroids, proton pump inhibitors, and H2-receptor antagonists), and clinical events (operative procedures, readmission within 7 days of a previous hospital discharge, bilevel positive airway pressure or mechanical ventilation within the first 72 hours of hospitalization, an intensive care unit stay before HAP diagnosis, NPO status, and feeding tube use). Based on the chart review, four prominent risk factors for HAP were identified: (1) postoperative patients, (2) a chart notation of altered mental status, (3) serum albumin <3 g/dL, and (4) tube feedings. In addition, patients for whom aspiration precautions were ordered were included in the risk definition at the discretion of the attending physician.
During the pilot study, overall HAP incidence decreased 38% (32 cases per month to 20 cases per month), and incidence among nonsurgical patients decreased by 50% (14 cases/ month to 7 cases per month). At the conclusion of the pilot study, the bundle consisting of respiratory care, oral care, up in chair, progressive ambulation, tube care, and patient education (leading to the acronym “ROUTE”) was finalized.
Information technology supports were added to the electronic health record to support the identification of patients at risk. Patients were automatically flagged in the electronic health record as being high risk for HAP if they were postoperative, were on aspiration precautions, had confusion or altered mental status, had a serum albumin <3 g/dL, had a nasogastric or feeding tube, or had HAP prevention orders placed by the attending physician. Interventions requiring physician orders were added to pre- and postoperative order sets, and a pneumonia prevention order set was added to the EHR. Nursing documentation tools were created for bundle elements, and a mobility protocol was added to the EHR.
A pilot study of preoperative education for pneumonia prevention began at preoperative medicine clinics and was then extended to all 21 centers. Patients received education about incentive spirometry, smoking cessation, mobility, and oral care and bags containing a toothbrush and toothpaste, chlorhexidine mouthwash, and an incentive spirometer were dispensed to these patients prior to their admission for surgery. Separately, an agreement on the appropriate use of proton pump inhibitors by the chiefs of service in cardiology, gastroenterology, critical care, and infectious diseases was incorporated into clinical guidelines.
Immediately prior to implementation at all medical centers in 2015, multidisciplinary summits were held with staff from all remaining medical centers. These included quality directors, chief nursing executives or designees, hospitalists, surgeons, infection preventionists, infectious disease specialists, respiratory therapists, and improvement advisors. Summit presentations addressed the magnitude of the problem, provided patient stories, described the rationale and evidence for the solution, presented the HAP prevention bundle and related EHR supports and tools, outlined the implementation and performance improvement strategies, and described data to be collected and reported each month.
In addition, HAP prevention was added to the portfolio of initiatives of a well-established KPNC performance improvement collaborative group. Hospital and Emergency Department Reliability and Operational Excellence for Safety (“HEROES”) is a comprehensive initiative with the goal of improving the culture of safety in acute care and emergency departments in all 21 current KPNC medical centers.
Monthly data were collected by KPNC quality staff and were distributed to all hospital and medical staff leadership. These data were used as a proportion of all inpatient medical-surgical days. The data dashboard included HAP rates, process measures for ambulation, preoperative chlorhexidine rinses, patient education, and benzodiazepine use (associated with our effort to decrease delirium but felt to be valuable for HAP reduction as well). These data were used to compare hospital performance. Benzodiazepine use was measured as percentage of inpatient days on which benzodiazepines were administered.
The primary outcome metrics in this study were the rates of HAP per 1,000 hospital admissions and HAP cases per 100,000 members, to normalize for rapid growth and increasing utilization of outpatient facilities for procedures previously done in the hospital. Virtually all care delivered to patients occurs at a KPNC facility and is captured in the electronic medical record. Secondary outcome metrics included the use of broad-spectrum antibiotics, measured as days of therapy per 100,000 members.
Linear regression was used for each measure to compare outcomes (SAS Enterprise Guide, SAS Institute, Cary, NC; MS Excel for Office 365 MSO, Analysis ToolPak add-on, Microsoft, Redmond, WA).
Results
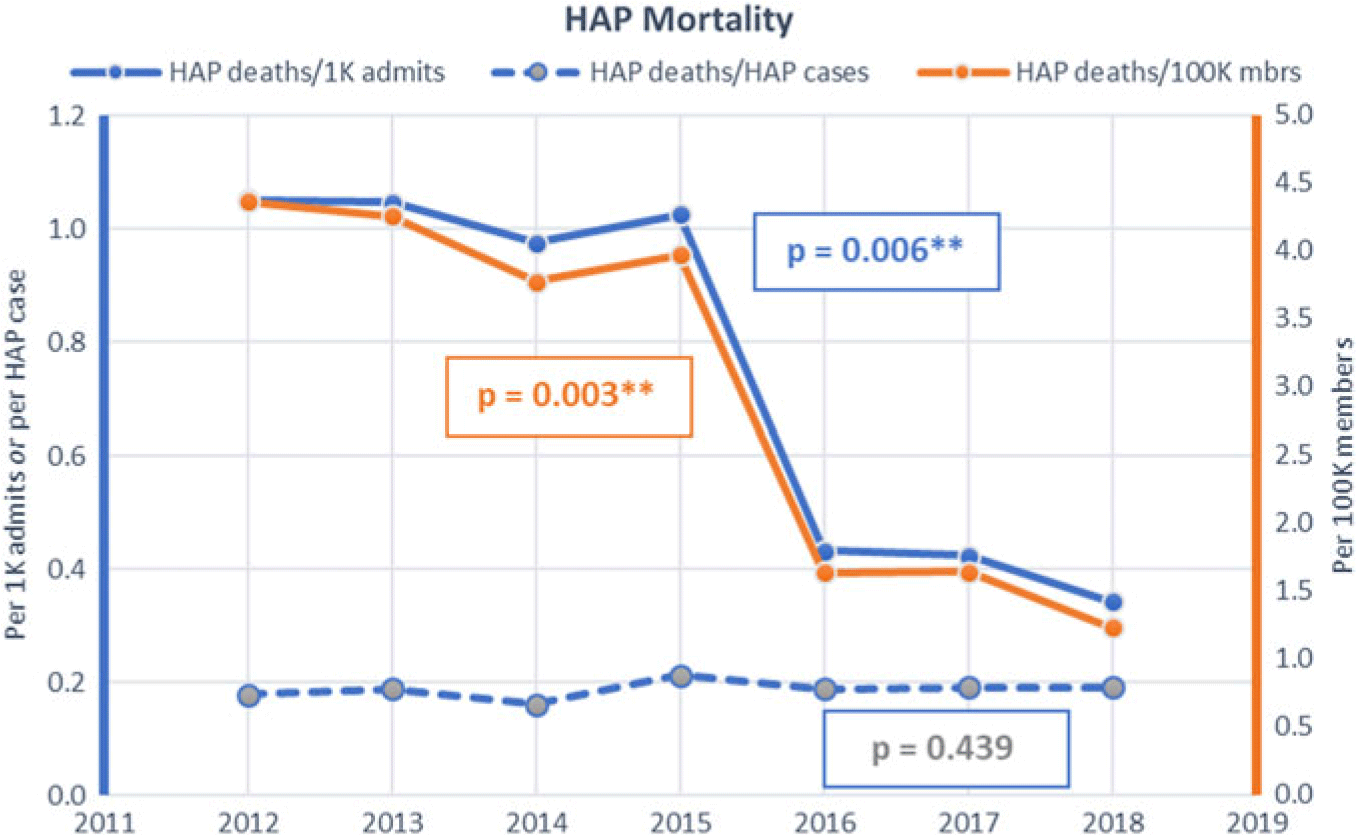
The HAP rates remained substantially unchanged from a baseline of 5.92 per 1,000 admissions and 24.57 per 100,000 members in 2012 until 2015, when all facilities initiated the prevention program and incorporated process metrics as well as HAP rates in hospital performance dashboards. Thereafter, the HAP rate decreased to 1.79 per 1,000 admissions (P = .0031) and 6.49 per 100,000 members (P = .0014) in 2018 (Fig. 1). HAP mortality decreased from 1.05 to 0.34 per 1,000 admits and from 4.37 to 1.24 per 100,000 members (Fig. 2).
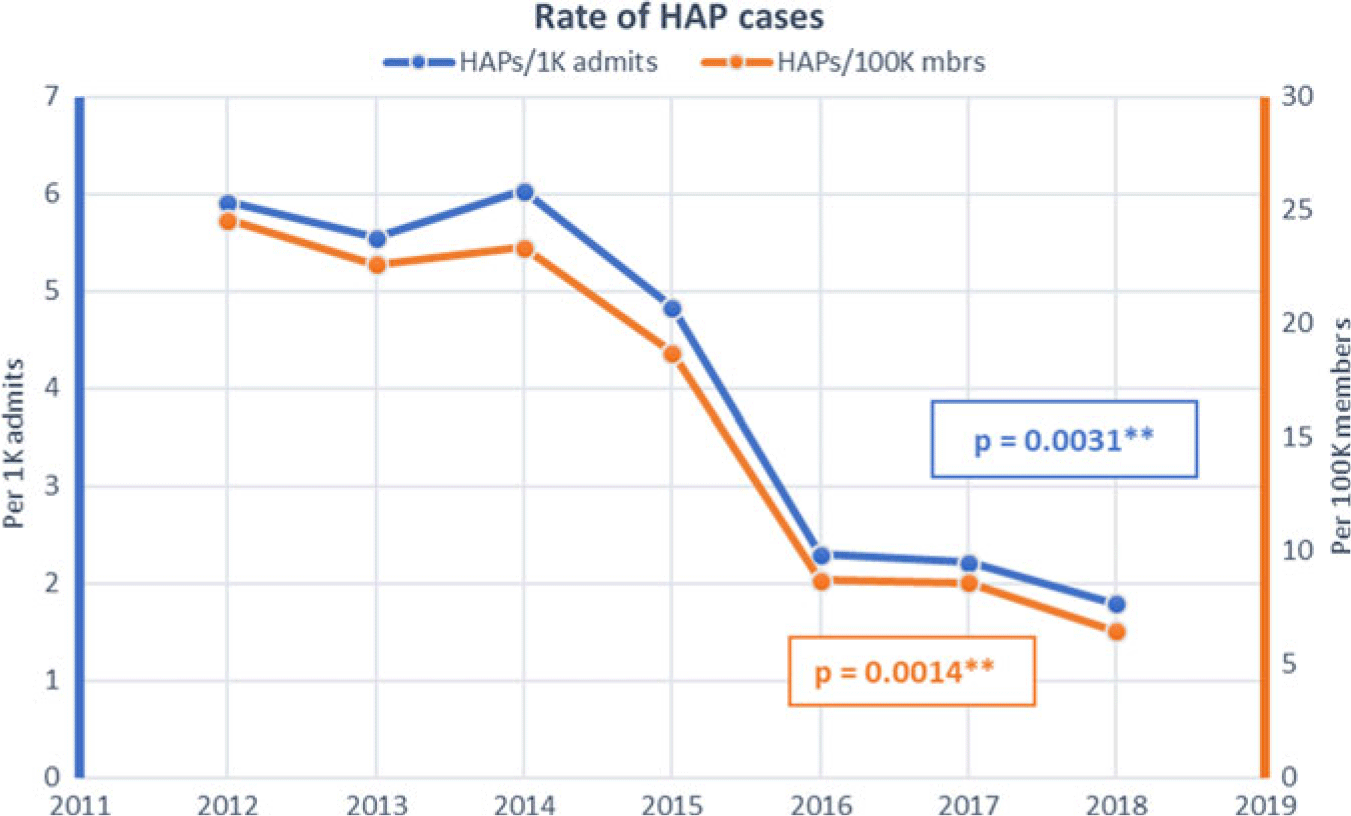
Fig. 1. Hospital-acquired pneumonia (HAP) rates by hospitalizations and population.
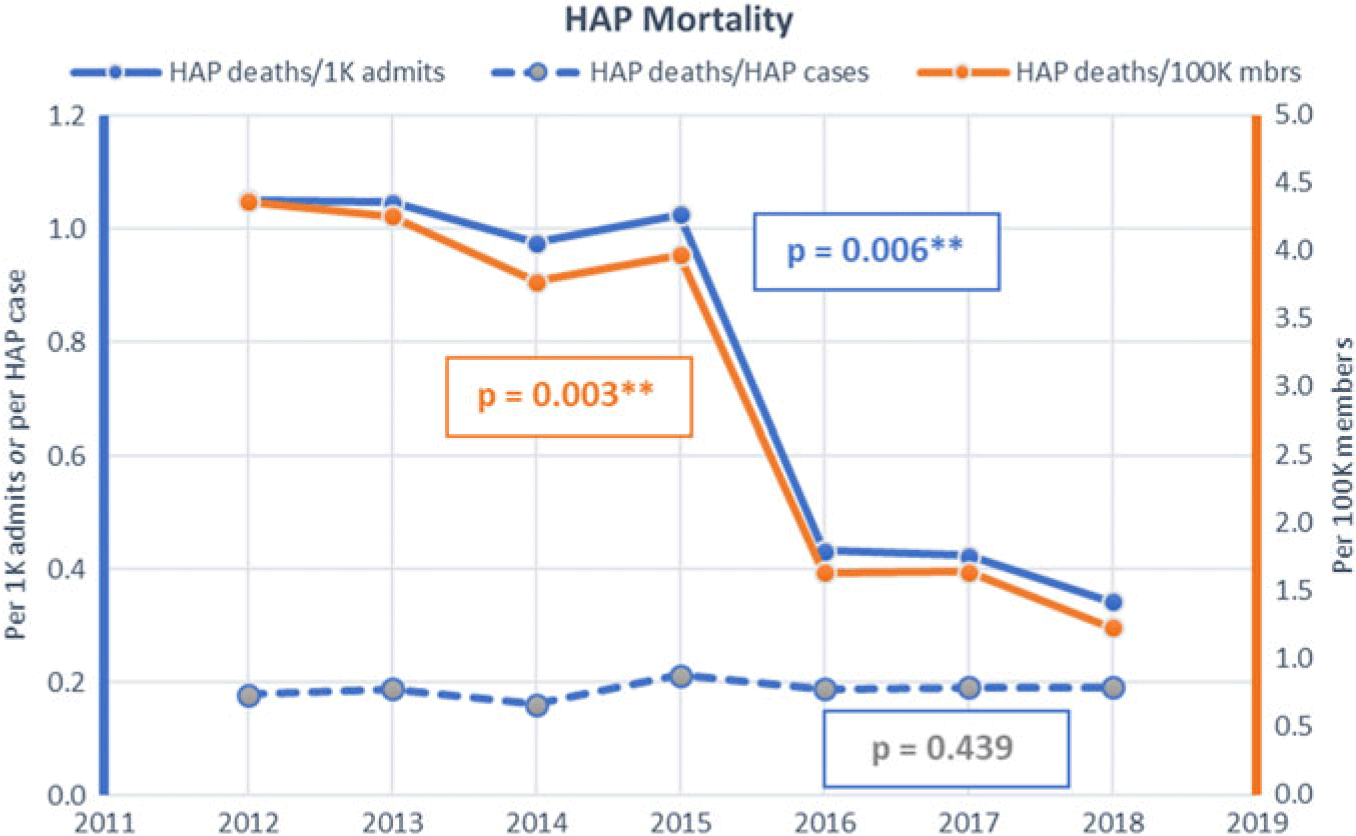
Fig. 2. Hospital-acquired pneumonia (HAP) mortality rates by hospitalizations and population.
Antibiotic utilization metrics demonstrated significant reductions of broad-spectrum antibiotic use. Days of antibiotic therapy significantly decreased over the same period (2012– 2018). Reductions were observed among the carbapenem days (694 to 463 per 100,000 members; P = .0020), aminoglycoside days (154 to 61 per 100,000 members; P = .0165), vancomycin days (2087 to 1,783 per 100,000 members; P = .002), and quinolone days (2,162 to 1,287 per 100,000 members; P < .0001). The only antibiotic class that increased was cephalosporins, and this increase was driven by the change from broader-spectrum antibiotics to ceftriaxone (264 to 460 per 100,000 members; P = .0009) (Fig. 3). Benzodiazepine use decreased from use on 10.4% of all inpatient medical-surgical days in 2014 to 8.8% of inpatient days in 2016, the years for which this metric was available. The mortality rates for patients with an HAP diagnosis were 18% in 2012% and 19% in 2016 (P = .439).
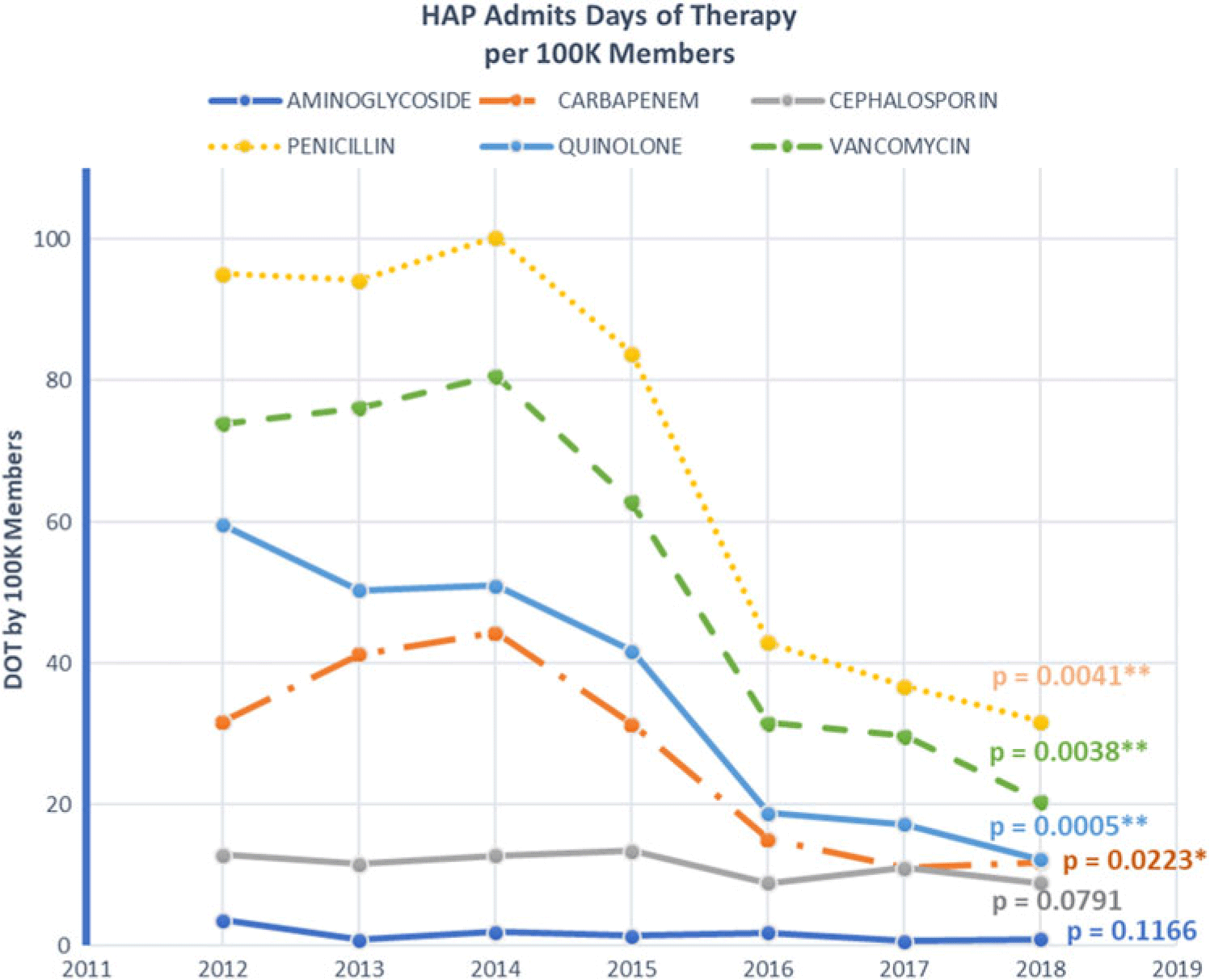
Fig. 3. Days of therapy by antibiotic class by population.
Discussion
In this study, we demonstrated a substantial reduction of incidence of HAP in multiple facilities in a large healthcare system. Efforts on either this scale and the sustained effectiveness has not been previously reported. We have presented a practical model for implementation of a prevention bundle. It provides for a real-world implementation, one that does not require a large staff for surveillance and validation of HAP.
The number of HAP cases and the mortality associated with this diagnosis significantly decreased. Various secondary benefits were also realized, including a significant reduction of broad-spectrum antibiotic and all antibiotic use, reduced use of sedation, and improved oral hygiene and ambulation for these patients. Although several interventions had limited support in the literature, most of them augmented basic nursing care. None had risks of serious adverse consequences.
Comparing our findings to the small number of other reports is challenging due to differences in populations and reported outcomes. Wren et alReference Wren, Martin, Yoon and Bech 6 and Kazaure et alReference Kazaure, Martin, Yoon and Wren 7 reported early and long-term results of a program for postoperative pneumonia prevention in nonmechanically ventilated patients. Implemented on a single surgical ward, their program included the following interventions: (1) nursing staff education, (2) incentive spirometry, (3) twice-daily oral hygiene with chlorhexidine, (4) ambulation, (5) head-of-bed elevation to ≥30° and sitting up for all meals, (6) quarterly review of program results with nursing staff, (7) nursing documentation of the pneumonia bundle, and (8) pneumonia prevention order set in physician order entry system in the EHR.Reference Kazaure, Martin, Yoon and Wren 7 Using the Veterans’ Administration National Surgical Quality Improvement Program (NSQIP) criteria, the rate of HAP decreased from 7.8 per 1,000 noncardiac surgical patients admitted to the ward to 1.8 per 1,000 noncardiac surgical patients.Reference Wren, Martin, Yoon and Bech 6 Over the following 5 years, the average HAP rate was 4.4 per 1,000 noncardiac surgical patients admitted to that ward. A third report examined the incidence of HAP at a single medical center before and after the implementation of a prevention program consisting of (1) incentive spirometry, (2) coughing and deep breathing, (3) oral care (brushing teeth and using mouthwash twice daily), (4) patient and family education, (5) getting out of bed ≥ 3 times daily, and (6) head of bed elevation.Reference Cassidy, Rosenkranz, McCabe, Rosen and McAneny 43 Using the NSQIP definition for postoperative pneumonia among the population consisting of patients who underwent an operation on general and vascular surgery services and were admitted to either ICU or non-ICU wards, the incidence of pneumonia decreased from 2.6% to 1.6% (P = .09).Reference Cassidy, Rosenkranz, McCabe, Rosen and McAneny 43
The strengths of our findings include the scale of the population examined, the marked reduction of HAP rates and the ability to examine related measures that support the findings, primarily antibiotic use profiles. The reproducibility of programmatic identification of HAP and the model for implementation of a large-scale intervention provides an additional point of value for this study.
Our study has several limitations. Our observational design limited our ability to attribute outcomes solely to the prevention bundle. The nonstandard definition of the HAP target is a drawback, but we chose it in response to the absence of reliable and readily extractable definitions in existence. Downcoding of HAP is a consideration, but reduction of antibiotic use refutes this as significant. The generalizability of our experience is unknown but should be broadly applicable.
In conclusion, our study demonstrated significant reductions in HAP rates, mortality and broad-spectrum antibiotic use. The structure of the project provides a framework for hospitals to reduce a significant hospital acquired infection. These results also support the need to examine practices to improve care despite limited literature and even more so, support a need to study and define improvement opportunities for these difficult nebulous areas of care.
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.