Central-line–associated bloodstream infection (CLABSI) is an important quality measure included in the Centers for Medicare and Medicaid Services pay-for-performance programs and public reporting.1, 2 Most neonatal intensive care units (NICUs) aim to eliminate CLABSIs,Reference Erdei, McAvoy, Gupta, Pereira and McGowan3–Reference Bizzarro, Sabo and Noonan5 but these may only represent a subset of all healthcare-associated bloodstream infections (HABSIs).Reference Dantes, Abbo and Anderson6, Reference Dantes, Rock and Milstone7 With recent reductions in CLABSIs and improved survival of premature babies, HABSIs are becoming more evident in some NICUs.Reference Verstraete, Boelens and De Coen8 Hence, hospitals that only track CLABSI rates may not attempt further prevention efforts due to the ceiling effect and a false sense that no opportunity for improvement exists.Reference Rock, Thom and Harris9The NICU population, particularly very low birthweight infants and those born prematurely, are at significant risk for HABSIs secondary to nutritional and immunologic deficits, underlying comorbidities, invasive procedures, prolonged intravenous access, mechanical ventilation, impaired gastrointestinal motility, and increased permeability, all of which promote bacterial translocation.Reference Papoff, Ceccarelli and d’Ettorre10, Reference Gatt, Reddy and MacFie11, Reference Collins, Weitkamp and Wynn12 Better understanding of the prevalence, epidemiology and risk factors for all HABSIs in the NICU will help direct prevention efforts. National Healthcare Safety Network (NHSN) definitions used for source attribution of CLABSIs could be used for all HABSIs. However, the current NHSN site-specific definitions have some limitations with respect to clinical criteria and gut translocation in neonates that may lead to potential misclassification and inaccurate reporting.Reference Papoff, Ceccarelli and d’Ettorre10, Reference Urao, Moy, Van Camp, Drongowski, Altabba and Coran13, Reference Dahan, O’Donnell and Hebert14 As part of our efforts to understand risk factors for HABSIs in the NICU, we performed surveillance and collected epidemiologic data on all HABSIs in the NICU over a 6-year period. We also systematically compared NHSN site-specific definitions to clinical definitions for source attribution of all HABSIs. Our objectives were (1) to review the epidemiology of HABSIs in our NICU and (2) to identify opportunities in which the NHSN site-specific definitions might be modified to better suit the NICU population and avoid misclassification.
Methods
Study design
We conducted a retrospective descriptive study of neonates admitted to Yale-New Haven Children’s Hospital with a HABSI between January 1, 2013 and December 31, 2018. This study was approved by the Yale University Human Investigation Committee (protocol no 2000022831).
Study setting and patients
The study was conducted at a 54-bed level IV NICU at Yale New Haven Children’s Hospital in New Haven, Connecticut. All infants in our NICU were eligible for inclusion.
Definitions
Healthcare-associated infections (HAIs) were defined according to NHSN criteria. HABSI was defined as a positive blood culture growing an organism not considered a contaminant on or after day 3 of life. CLABSI was defined as a positive blood culture in a patient with a central line in the absence of secondary site of infection using 2018 NHSN criteria.15 Non–central venous catheter–related bloodstream infection (non-CVC BSI) was defined as HABSI in a patient without a central line. A single blood culture positive for Staphylococcus spp (not S. aureus), Micrococcus spp, Bacillus spp (not B. cereus or B. anthracis), Corynebacterium spp (not C. jeikeium), or Propionibacterium spp, was considered a contaminant. A patent ductus arteriosus (PDA) was identified via echocardiogram interpreted by a pediatric cardiologist and was considered clinically significant by the NICU team. Respiratory distress syndrome (RDS) was defined based on the presence of respiratory distress and chest radiograph findings. Necrotizing enterocolitis (NEC) was defined according to the modified Bell’s criteria and included only cases at stage IIA or above.Reference Walsh and Kliegman16 Bronchopulmonary dysplasia (BPD) was defined as need for supplemental oxygen at 36 weeks corrected gestational age.Reference Trembath and Laughon17 Severe intraventricular hemorrhage (IVH) was defined using the Papile classification and included grades III and IV.Reference Papile, Burstein, Burstein and Koffler18 Neutropenia was defined as an absolute neutrophil count <1,500 neutrophils/μL.19 Sepsis-related mortality was calculated with the numerator representing the number of episodes of sepsis resulting in death and the denominator representing the total number of episodes of sepsis.Reference Bizzarro, Shabanova, Baltimore, Dembry, Ehrenkranz and Gallagher20
Data collection
Trained infection preventionists performed CLABSI surveillance. The catheter days, patient days, and culture data were extracted from our hospital’s infection prevention database. Additional variables were collected from the hospital’s NICU database, including (1) demographic data such as gestational age, birthweight, gender, Apgar score; (2) potential risk factors including total parenteral nutrition (TPN), H2 blockers, steroids, previous surgery, neutropenia; and (3) outcomes and comorbidities associated with HABSI. Chart reviews of neonates with HABSIs were performed by an infectious disease physician and a neonatologist. NHSN site-specific definitions were used by reviewers for source attribution of HABSIs. Differences in bloodstream infection (BSI) attribution between reviewers were reconciled by discussion with a pediatric infection preventionist.
Blood culture collection
The blood culture collection policy did not change during the study period. Blood cultures are collected by NICU nursing staff with the goal of obtaining 2 sets of blood cultures from 2 separate peripheral sites (a peripheral artery or vein) with no less than 1 mL blood in each. Cutaneous antisepsis is performed using povidone iodine swabs with a 30-second scrub time per swab. The povidone must completely air dry before each subsequent swab is applied. Once the third povidone scrub and dry time are completed, an alcohol swab is applied and left to air dry before the culture is obtained. Blood cultures are incubated using the BACTEC FX blood culture system (Becton Dickinson, Franklin Lakes, NJ), and organisms from positive cultures are identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS; bioMérieux, Marcy-l’Étoile, France).
Analysis
Rates of HABSI and CLABSI were reported per 1,000 patient days and 1,000 catheter days, respectively. Comparisons of patient-related variables between CLABSIs and non-CVC BSIs were done using the Z test and the Student t test as appropriate. Clinical definitions per treating NICU team and NHSN site-specific definitions were compared for source attribution of HABSIs using the McNemar χ2 test. Analyses were conducted using Stata version 15 software (Statcorp, College Station, TX).
Results
We identified 86 HABSIs during the 6-year study period, with an overall rate of 0.8 HABSIs per 1,000 patient days. Most of these infections (n = 75, 87%) were non-CVC BSIs. CLABSI rates remained low during the study period from 2013 to 2018 (0.47–0.29 per 1,000 catheter days). Patient-related variables and risk factors for CLABSIs and non-CVC BSIs are compared in Table 1. Both CLABSIs and non-CVC BSIs occurred primarily in preterm, low-birth-weight neonates and were associated with a long duration of hospitalization and high sepsis-related mortality (P > .05) (Table 1). However, non-CVC BSIs were associated with a significantly higher incidence of comorbidities like PDA, RDS, and BPD, and they occurred in infants who were more likely to be receiving respiratory support (P < .05) (Table 1). Most HABSIs were caused by gram-positive bacteria, mainly Staphylococcus aureus (n = 29, 33.7%). Only a minority of HABSIs were secondary to coagulase-negative staphylococci (n = 4). A large proportion of HABSIs (60.5%) were caused by a mucosal barrier injury (MBI) organism.
Table 1. Patient Related Variables for HABSIs (CLABSI and non-CVC BSI) in Neonates
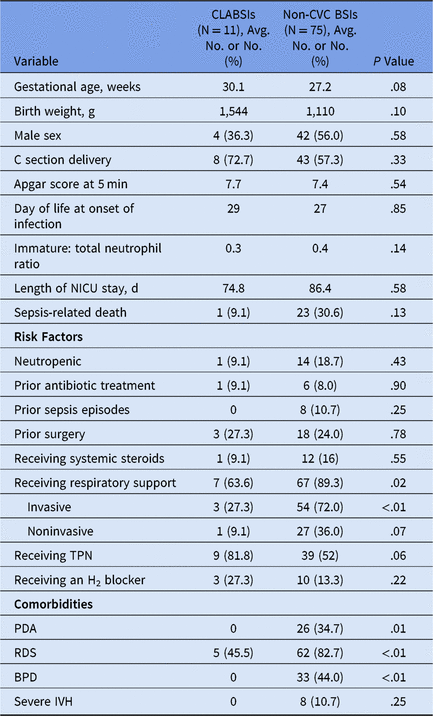
Note: NICU, neonatal intensive care unit, TPN, total parenteral nutrition; PDA, patent ductus arteriosus; RDS, respiratory distress syndrome; BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage.
Based on NHSN definitions, most HABSIs were secondary to NEC (24%), pneumonia (16%) or an unidentified source (28%). Comparing NHSN definitions to clinical definitions, we found overall good concordance for infections like NEC (n = 21), osteomyelitis (n = 1), endocarditis (n = 5), extracorporeal membrane oxygenation–related BSIs (n = 2), and urinary tract infections (n = 3). However, NHSN definitions were significantly less likely than clinical definitions to identify a skin soft-tissue infection (2 vs 8 infections; P = .04), and they showed a trend toward diagnosing fewer intra-abdominal infections (2 vs 6 infections; P = .48), and meningitis (4 vs 6 infections; P = .25) compared to our clinical definitions. Interestingly, NHSN definitions showed a trend toward diagnosing more pneumonia in neonates compared with clinical definitions (14 vs 10 infections; P = .37). Overall, the NHSN definitions were less likely to identify a source compared with clinical definitions used by the NICU treating team (22 vs 10 HABSIs without a source; P < .01). In 50% of neonates who did not have a source of infection identified by NHSN definitions, BSIs occurred secondary to an MBI organism, likely from gut translocation.
Discussion
We found that CLABSIs represent a small subset of BSIs over the entire NICU hospitalization. Both CLABSIs and non-CVC BSIs occurred in preterm, low-birth-weight infants, and they were associated with a long duration of hospitalization and high mortality. Non-CVC BSIs were more likely to be seen in neonates with underlying comorbidities and those receiving respiratory support. As CLABSI rates decline, surveillance and source investigation of non-CVC BSIs is becoming just as pertinent, suggesting that all HABSIs warrant investigation. NHSN site-specific definitions can be used for source attribution of all HABSIs, but criteria specific to the NICU population have significant room for improvement.
Although CLABSI and HABSI rates are related, HABSIs are very significant in some NICUs.Reference Folgori, Bielicki and Sharland22 Previous studies document incidence rates of HABSIs ranging from 5% to 30%.Reference Stoll, Hansen and Fanaroff23–Reference Horan, Andrus and Dudeck25 Risk factors for HABSIs reported in the literature include lower birth weight, younger gestational age, need for mechanical ventilation, vascular access, and TPN, similar to our study, but other studies report coagulase-negative staphylococci as a major cause of HABSIs in the NICU.Reference Gray24, Reference Brodie, Sands and Gray26–Reference Perlman, Saiman and Larson30 Rates of coagulase-negative staphylococci bacteremia in our NICU were very low, likely due to our CLABSI prevention efforts, ongoing HABSI surveillance, and blood culture collection techniques.Reference Bizzarro, Sabo and Noonan5, Reference Bizzarro, Shabanova, Baltimore, Dembry, Ehrenkranz and Gallagher20
Other studies have shown that NHSN definitions may overestimate CLABSIs by 30%.Reference Farrell, Gilman, Teszner, Coffin and Sammons31, Reference Hammadi, Ostrosky-Zeichner, Boston, McInnis-Cole and Butler32 For specific infections like skin soft-tissue infections, meningitis, and intra-abdominal infections, NHSN criteria could not be reliably applied to our NICU population. The NICU population includes a high percentage of premature infants, who may present with different physiologic norms and clinical signs of infection compared to healthy infants and in whom symptoms (eg, nausea) cannot be expressed. For example, if we utilize the same site-specific definition for pneumonia in all infants <1 year of age, then we assume that the same symptom and radiologic criteria can be applied to a 22-week gestational age neonate intubated in the NICU as to a previously healthy 10-month-old infant. The challenges with applying site-specific NHSN definitions to the NICU population are described in the following sections.
Skin and soft-tissue infection definitions (SSTI)
The NHSN skin criteria 2a and soft-tissue infection criteria 1 (SSTI) can be used for secondary BSI attribution. To meet these definitions, both clinical and microbiologic criteria (matching organism in blood and site-specific cultures) should be met during the secondary BSI attribution period (infection window period + repeat infection timeframe [RIT]). In 2 neonates, cultures from site-specific infection grew matching organisms but were obtained a few days outside the infection window and therefore could not be used to meet criteria. Another neonate developed Staphylococcus aureus bacteremia from cellulitis of a peripheral intravenous catheter site, but the microbiologic criteria could not be met due to inability to obtain site-specific cultures. In 3 neonates, clinical criteria could not be met as specific signs of inflammation could not be elicited either due to breakdown of extremely premature skin or delayed appearance of some of these signs. Interestingly, 2 of these neonates met the NHSN pneumonia definition due to concurrent development of respiratory symptoms and persistent radiographic opacities from underlying lung disease, even in the absence of clinically diagnosed pneumonia.
Meningitis (MEN) definition
To meet the NHSN meningitis (MEN) criteria 3 for infants <1 year of age, microbiologic criteria plus 2 of the following 3 clinical criteria need to be met: (1) fever, apnea, bradycardia, or irritability, (2) meningeal signs, and (3) cranial nerve signs. In addition, meningeal signs and cranial nerve signs cannot be used if a plausible alternative diagnosis exists. Neonates with meningitis may often have comorbidities like posthemorrhagic hydrocephalus (PHH), but clinical signs of worsening PHH cannot be used to meet the NHSN MEN definition. In addition, severe PHH often necessitates placement of intracranial shunts and reservoirs, which increase the likelihood of meningitis. In our study cohort, 2 premature neonates with underlying severe PHH did not meet the NHSN definition due to inability to meet clinical criteria (meningeal signs and cranial nerve signs). The NICU treating team, in both cases, opted to treat for meningitis based on clinical signs, cerebrospinal fluid pleocytosis, the presence of intracranial hardware, and exclusion of other sources of infection.
Intra-abdominal (IAB) infection definition
There are no clinical criteria specific to the pediatric or neonatal populations for IAB site-specific infections (IAB criteria 2b or 3b), with the exclusion of NEC. In critically ill neonates with an IAB infection, it is not possible to elicit some NHSN clinical criteria (eg, nausea), and it is difficult to separate others (eg, vomiting, abdominal tenderness, elevated transaminase level, jaundice) from an alternative cause. In our cohort, 2 neonates with clinically diagnosed spontaneous intestinal perforation (SIP), 1 with Candida parapsilosis in the setting of open abdomen after surgery (outside RIT) and 1 baby with stricture after surgery for NEC did not meet NHSN IAB or NEC definitions. SIP is a separate clinical entity from NEC and represents a localized area of perforation with isolated mucosal ulceration and inflammation, submucosal edema, and serosal inflammation.Reference Jawad, Al-Rabie and Hadi21
Pneumonia definition (PNEU) and criteria (PNU criteria)
The criteria within the pneumonia definition (PNU criteria 2 or 3) that can be used for BSI attribution requires clinical, radiologic and microbiologic criteria (positive blood culture) within the infection window. Although PNU criteria 1 has more restrictive criteria with respect to neonates, it cannot be used for secondary BSI attribution except when within the RIT of this infection. A large proportion of neonates have underlying respiratory disease, but these criteria (despite need for 2 images) are not specific enough for premature neonates because consolidation due to atelectasis is very frequent in both RDS and BPD and cystic changes in severe BPD can be difficult to differentiate from pneumatoceles. In addition, infants with BPD often have exacerbations of their underlying disease that can result in periods of worsening gas exchange, and tachypnea, retractions, rales, and rhonchi may be part of their baseline examination. Hence, the NHSN definition identified pneumonia in 4 infants that were not clinically diagnosed or treated for pneumonia.
Maternally or externally acquired infection definition
No NHSN definitions exist for maternally acquired infections. In our study population, 1 premature neonate was diagnosed with candidemia on day 5 of life. The neonate was born in the setting of maternal chorioamnionitis and had evidence of cutaneous candidiasis at birth, and the umbilical cord and placental pathology revealed the presence of candida. Due to limitations of the infection window and lack of a NHSN definition for maternally acquired infection, no site-specific definitions were met, and the infection was reported as a CLABSI. Another neonate developed Lactobacillus-related bacteremia, likely due to probiotic use, but there are no NHSN criteria to capture infections from external sources like blood products or supplements.
Mucosal barrier injury, laboratory-confirmed bloodstream infection (MBI-LCBI)
The MBI-LCBI definition applies to eligible pathogens in specific patient populations (eg, stem cell transplant recipients with compromised skin or gastrointestinal tract integrity or neutropenia). Despite the high risk of gut translocation in preterm neonates due to impaired immune function, poor intestinal motility, increased intestinal permeability, and an altered gastrointestinal microbiome, this definition cannot be applied to neonates.Reference Collins, Weitkamp and Wynn12 In our cohort, 46% of neonates with a BSI secondary to a MBI organism were clinically diagnosed with an IAB pathology, compared with 2.9% of neonates with a non-MBI organism (P < 0.05).
We propose modifications to the NHSN site-specific definitions for the NICU population to improve the classification of site-specific infections (Table 2). In terms of the MEN and SSTI definition, we recommend expanding the clinical criteria to include more signs or adding criteria for physician documentation and antimicrobial therapy of these infections. Due to higher risks of sampling and smaller sampling yield, it may help to eliminate the microbiologic criteria in neonates for SSTI or to expand the infection window to allow for safer specimen collection. The PNEU site-specific definition may benefit from using clinical criteria in PNU1 for infants (as opposed to clinical criteria in PNU2), with the addition of specific radiologic criteria and clinical parameters for preterm neonates. The NHSN IAB definition could be modified to make the clinical criteria less restrictive given the difficulty of eliciting nausea in neonates, and including additional criteria for spontaneous intestinal perforation, recent abdominal surgery and procedures, especially in cases with bacteremia from an MBI organism. We also propose adding a definition for maternally acquired infections if a matching organism is isolated from blood cultures in neonate and site-specific placental or blood cultures in the mother within a defined infection window.
Table 2. Proposed Changes to National Health Safety Network (NHSN) Site-Specific Definitions for Neonates
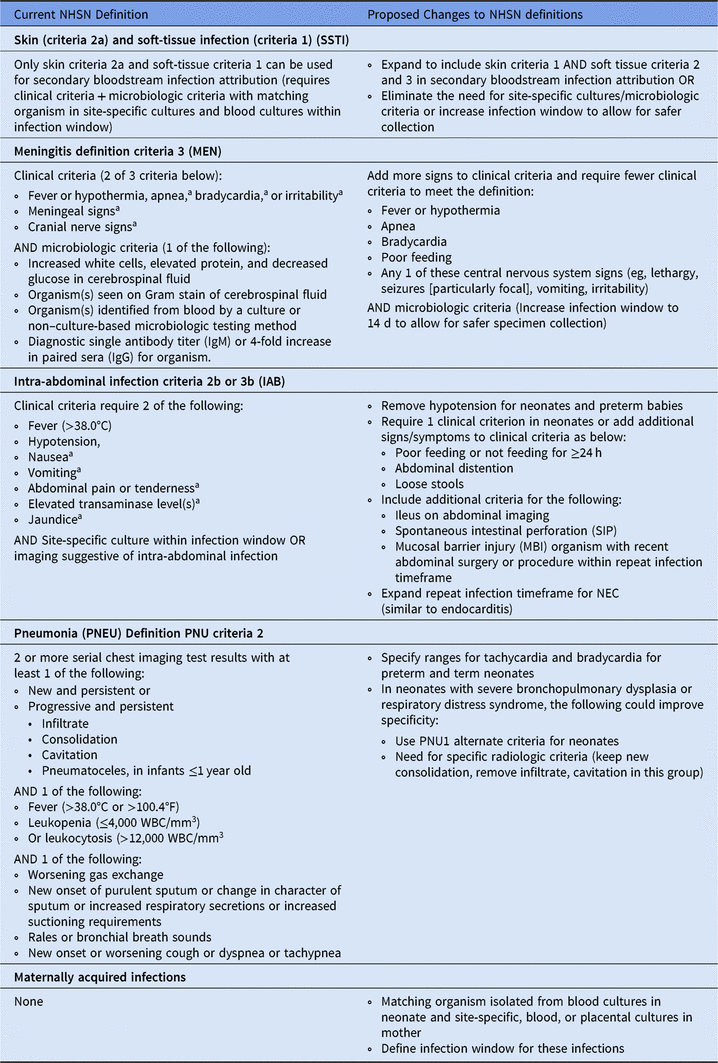
a With no other recognized cause.
A large proportion of HABSIs in our NICU were secondary to MBI organisms, and about half of these neonates had an underlying gastrointestinal condition. Previous NICU studies have shown an increased risk of BSIs secondary to bacterial translocation in neonates.Reference Dahan, O’Donnell and Hebert14, Reference Blanchard, Fortin and Rocher33 Bacterial translocation has also been described in patients with recent abdominal surgery, bowel obstruction, malnutrition, those requiring parenteral nutrition and following ischemia-reperfusion injury shock.Reference Gatt, Reddy and MacFie11, Reference Sherman34, Reference MacFie, Reddy, Gatt, Jain, Sowdi and Mitchell35 Most NICUs aim to eliminate CLABSIs using bundles for insertion and maintenance.Reference Shepherd, Kelly and Vinsel4, Reference Bizzarro, Sabo and Noonan5 However, BSIs secondary to gastrointestinal bacterial translocation may not be prevented by prevention strategies such as hand hygiene, CLABSI bundles, and decreasing device use.Reference Papoff, Ceccarelli and d’Ettorre10, Reference Urao, Moy, Van Camp, Drongowski, Altabba and Coran13 Previous studies have proposed definitions for MBI-LCBI in neonates with an eligible gastrointestinal condition.Reference Dahan, O’Donnell and Hebert14, Reference Coffin, Klieger and Duggan36 Such a definition could assist with attribution of neonates with gastrointestinal conditions that do not meet the IAB definition.
Our findings have some limitations. This was a single-center study at a large academic medical center, which limits generalizability. The CLABSI rate in our NICU is very low due to our CLABSI prevention efforts, appropriate culturing practices, and avoidance of central cultures. Hence, our ratio of HABSIs to CLABSIs may be much higher than other NICUs. Our low rates of coagulase-negative staphylococcal bacteremia compared to other studies could be explained in part due to the variability in the definitions used in the literature.Reference Stoll and Hansen37 We were unable to perform a regression analysis due to smaller number of events. Another limitation is the subjectivity and poor consistency related to clinical diagnoses. Although our retrospective design introduces some subjectivity and hindsight bias, CLABSI attribution for surveillance purposes requires retrospective review with some level of subjectivity.
In conclusion, NICUs should participate in national HABSI surveillance to facilitate benchmarking and facilitate prevention efforts.Reference Verstraete, Boelens and De Coen8, Reference Folgori, Bielicki and Sharland22 This strategy has been suggested as an alternative to CLABSI surveillance and could potentially reveal other preventable BSIs that need novel prevention efforts. If NHSN definitions are to be used for source attribution of all HABSIs, then some modifications are needed to better suit the NICU population. It is important for surveillance definitions to align with clinical definitions to capture preventable events and direct quality improvement efforts. Although multicenter data may be needed for NHSN definition changes, our findings represent a starting point. Future efforts should focus on developing uniform case definitions for NICU HABSI surveillance.
Acknowledgments
We thank the Neonatal Intensive Care Unit staff at Yale New Haven Children’s Hospital for their support of this project.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




