Central venous catheters (CVCs) are an essential part of care for many children, but they carry the risk of bloodstream infection and serious mechanical complications. Because of their clinical significance, central-line–associated bloodstream infections (CLABSIs) are a recommended target of healthcare-associated infection surveillance in most jurisdictions, but these data are more commonly collected in the critical care setting. CLABSIs occurred in 1.4 per 1,000 line days in pediatric medical and surgical units in the most recent National Healthcare Safety Network (NHSN) report.Reference Dudeck, Weiner and Allen-Bridson 1 In 2011, the Canadian Nosocomial Infection Surveillance Program (CNISP) reported 1.33 CLABSI per 1,000 line days in pediatric intensive care units (PICUs) and 3.97 per 1,000 line days in neonatal intensive care units (NICUs). 2
Recommendations for the prevention of CLABSI in pediatric patients are usually extrapolated from evidence obtained from observational studies, time series, and a limited number of randomized controlled trials in adults.Reference Huang, Chen and Abdullah 3 Furthermore, research on CLABSI usually focuses on sub-populations of CVC users (eg, intensive care patients).Reference Dudeck, Weiner and Allen-Bridson 1 , Reference Dudeck, Horan and Peterson 4 , Reference Edwards, Peterson and Andrus 5 Limited data are available regarding CLABSI risk factors in children.Reference O’Grady, Alexander and Burns 6
In response to perceived high CLABSI rates and a lack of robust data, our institution established a program in 1994 to improve CVC care. This program included a dedicated resource nurse specialist, review and standardization of all policies and procedures related to CVC care, and a standardized data collection process. The objective of this study was (1) to analyze trends in rates of CLABSI over time and (2) to evaluate risk factors for infection in a diverse group of children followed from the time of line insertion to removal.
METHODS
This cohort study included children admitted to a pediatric secondary and tertiary care referral facility for the 3 Maritime provinces of Canada (population 2.3 million) with 265 beds and approximately 210,000 ambulatory visits in 2013. Children ages 0–18 years were followed from the time of insertion of their first CVC until CVC removal during the study period from January 1995 to December 2013. The study was approved by the IWK Research Ethics Board.
Data Sources
On an ongoing basis, the CVC insertion record for each patient is completed on the day of device placement by the inserter. This record captures demographic and baseline information. Follow-up data are collected daily that documents observations and interventions (eg, dressing changes, use of parenteral nutrition, drainage from the skin, etc.) made by healthcare professionals involved in the patient’s care. Complications associated with the CVC (eg, line blockages, changes in CVC position, leakage, etc.) are also recorded.
The Hospital-Acquired Infections Database captures information on targeted hospital acquired infections, including CLABSI, as they are identified by trained infection prevention and control nurses in an ongoing surveillance program. This database includes information on admission dates, underlying illness, reason for hospital admission, unit of admission, infection date, and infecting pathogen.
Databases were linked using deterministic linkage using the hospital identification number. A small proportion of patients (<1%) did not match on hospital identification number but were successfully matched through patient name, date of birth, and CVC insertion date.
CLABSI was defined as a blood culture positive for a known pathogen not related to infection at another site or, in the event of commensal organisms, 2 positive blood cultures and the presence of 1 of the following symptoms: fever (>38°C), chills, hypotension, or appearance of infection at the insertion site. To be included in this study, the patient had to have had a CVC in the 48 hours prior to this event. In patients <1 year of age, fever, hypothermia, apnea, or bradycardia were considered symptoms of infection.Reference Garner, Jarvis and Emori 7 During this observation period and in neonates, 1 blood culture positive for coagulase negative Staphylococcus in association with sepsis symptoms and no other explanation was included as CLABSI.
Potential Risk Factors
Patient Factors
The patients were categorized in the following age groups: neonate (0–28 days), infant (28 days–1 year), toddler (1–4 years), school child (5–9 years), teen (10–16 years), or young adult (≥17 years). Care groups were defined as the unit where the majority of inpatient time was spent: oncology/hematology, nephrology, surgery, general pediatrics, NICU, PICU, or other.
CVL Factors
We evaluated several different factors pertaining to CVLs. Year of insertion was considered in terms of overall CLABSI rate and trend and also in relation to the implementation of institutional improvements over the study period. Reasons for placement included oncology, difficult peripheral access, short-term monitoring, intensive care, malabsorption, prolonged access, or renal failure. Locations where insertion took place included operating room (OR), emergency room (ER), PICU, NICU, unit, and other. Timing of insertion was considered in 3 categories: elective, urgent, or emergency. Several different CVL types were considered separately: peripherally inserted central catheters (PICCs), short-term non-tunneled, long-term tunneled, totally implantable devices, and other. Several binary factors were considered: concurrent CVL (yes/no); lumen count (1 or 2); insertion side (left/right); pre-insertion antibiotics (yes/no); post-insertion antibiotics (yes/no); CVL blockage in the past 30 days (yes/no); mechanical CVL complication in the past 30 days (yes/no). Specific dressing types were considered: clear transparent, clear transparent with absorbent pad, gauze, pressure, semi-clear woven, Port-A-Cath, or other. Insertion vein categories included jugular, arm, head, leg, subclavian, and other. Tip locations included superior vena cava, right atrium, subclavian/brachiocephalic, inferior vena cava, and unknown.
Statistical Analysis
Patient and CVC characteristics are presented as counts and proportions. The central-line utilization ratio (CLUR, central-line days over patient days) was calculated by year.Reference Dudeck, Weiner and Allen-Bridson 1 CLABSI rates and associated 95% confidence intervals were calculated per 1,000 line days by year over the 18-year study period.
Kaplan-Meier curves were used to examine the relationship between each potential risk factor and CLABSI. A Cox proportional hazards model was constructed using Hosmer and Lemeshow’s purposeful selection procedureReference Hosmer and Lemeshow 8 to examine the association between patient and CVC characteristics and CLABSI. Only the first catheterization of a patient was considered in the analysis, and patients with multiple concurrent CVCs were excluded. Missing values for categorical data were considered as separate covariate categories, but their estimates are not presented. Stata/SE 13 software (Stata Corporation, College Station, TX) was used to perform the statistical analysis.
RESULTS
During the study period, 9,067 CVCs were inserted in 5,648 patients over 171,877 in-hospital line days and 896,402 total line days. The CLUR remained nearly constant at ~0.1 over time (data not shown). Patient and CVC characteristics are summarized in Table 1. The largest age group represented was neonates and the largest care group was in the NICU. More than half of children were cared for in a critical care setting (NICU or PICU, 43.8% and 38% of patients, respectively). The most common CVC type used was PICC, followed by short-term non-tunneled CVC and totally implantable devices. Most CVCs were inserted in the operating room (41.1%) or in a critical care unit (43%), and the most common insertion site was the jugular vein.
TABLE 1 Summary of Patient and CVC Characteristics for Pediatric Patients With a CVC Implanted at the IWK Health Center Between 1995 and 2013

Note. CVC, central venous catheter; PICU, pediatric intensive care unit; NICU, neonatal intensive care unit; PICC, peripherally inserted central catheter. Denominators are either total CVCs (n=9,067) or total patients (n=5,648) in the sample, as indicated in column titles.
CLABSIs occurred in 7.3% of CVCs, with crude CLABSI rates of 0.74 per 1,000 line days and 3.87 per 1,000 in-hospital line days. The ratio of in-hospital to total line days did not vary significantly over time (data not shown). The yearly CLABSI rates with 95% confidence intervals are presented in Figure 1 and show decreasing infection rates over time (84% reduction between 1995 and 2013). A test of proportions for the risk of CLABSI in 2009–2010 before the hand hygiene campaign to the risk of CLABSI in 2012–2013 shows a statistically significant difference (P<.0002).
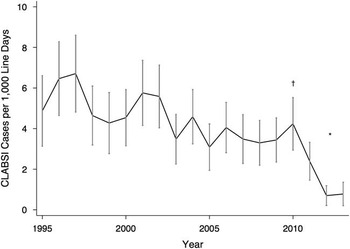
FIGURE 1 Hospital-wide CLABSI rates in children over time as cases per 1,000 in-hospital line days; vertical lines indicate 95% confidence intervals (n=9,067CVCs). CLABSI, central-line–associated bloodstream infection. †Canada’s Hand Hygiene Challenge is introduced, beginning with the Neonatal Intensive Care Unit (NICU). *A test of proportions for the risk of CLABSI in 2009/2010 before the hand hygiene campaign to the risk of CLABSI in 2012/2013 shows a statistically significant difference (P<.0002).
A decrease was observed from 4.24 of 1,000 in-hospital line days in 2010 to 0.70 of 1,000 in-hospital line days in 2012, which was maintained in 2013 at 0.78 of 1,000 in-hospital line days. CLABSI rates by CVL type are shown in Figure 2. The risk of CLABSI was highest in the first 60 days after CVC insertion (Figure 3). Gram-positive bacteria, consisting mostly of known skin commensals, were responsible for 72% of all CLABSI cases in this study. Of these, the majority were coagulase-negative Staphylococci spp. (54% of all CLABSI cases). Gram-negative bacteria were also common, accounting for 20% of CLABSI cases. A Candida sp. was the only fungal causative organism identified and was found in 3% of CLABSI cases. A summary of the organisms identified can be found in Table 2.
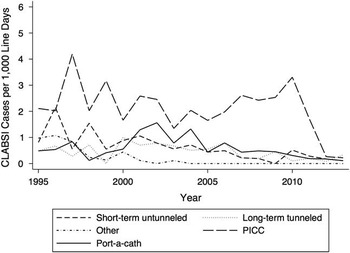
FIGURE 2 Hospital-wide CLABSI rates by device type in children (n=9,067CVCs). CLABSI, central-line–associated bloodstream infection; PICC, peripherally inserted central catheter.

FIGURE 3 Time to CLABSI after CVC insertion in children (n=5,648CVCs). CLABSI, central-line–associated bloodstream infection; CVC, central venous catheter.
TABLE 2 Summary of Organisms Causing Central-Line–Associated Bloodstream Infection in Children

The results of the unadjusted and adjusted Cox proportional hazards models can be found in Table 3. Being treated in the NICU, CVC lumen count (2 vs 1 lumen), dressing type (transparent with absorbent pad or gauze/pressure bandage), and insertion vein (subclavian) were associated with an increased risk of CLABSI in the final Cox model, whereas totally implantable CVC type was statistically significantly protective against CLABSI.
TABLE 3 Summary of Regression Analyses to Identify Risk Factors for CLABSI in Pediatric Patients with a CVC Implanted at the IWK Health Center Between 1995 and 2013

Note. CLABSI, central-line–associated bloodstream infection; CVC, central venous catheter; HR, hazard ratio; CI, confidence interval; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; ICU, intensive care unit; PICC, peripherally inserted central catheter; ER, emergency department.
All hazard ratios in the full model are controlled for year of insertion as this variable was included in the model but not subject to the backward elimination procedure.
a Includes disconnect, fracture, leakage, migration.
* indicates p<.05.
DISCUSSION
In this study, we found a consistent decrease in hospital-wide CLABSIs over almost 20 years of observation, with infection rates dropping sharply in recent years. A patient’s care group, the CVC lumen count, dressing type, insertion vein, and CVC type were statistically significantly associated with CLABSI. The risk of CLABSI appeared to be highest in the first 60 days after CVC insertion.
A clear and consistent downward trend in the hospital-wide CLABSI rate over time was observed. No changes to the definition of CLABSI or to the procedure for case ascertainment were made during the study period. Of course, several improvements in CVC care have been implemented since 1995. First, an integrated approach to CVC care was introduced at our institution, including a CVC committee, a dedicated nurse educator and program coordinator, and the review and standardization of all policies and procedures related to central lines across the institution. In 1995, a policy of maximal sterile barrier precautions was introduced.Reference Raad, Hohn and Gilbreath 9 In 1998, the institution changed from an iodophor-containing to a 0.5% chlorhexidine-containing skin antiseptic for insertion and care of all lines, and in 2006, the institution changed to a 2% chlorhexidine solution when it became commercially available in Canada. An educational initiative to improve compliance with CVAD care bundles began in 2006, and in 2010–2011 a hand hygiene campaign (Canada’s Hand Hygiene Challenge 10 ) was introduced. While this study is limited due to its before-and-after design, these interventions likely contributed to the drop in CLABSI rates, most particularly between 2010 and 2012. Such educational and quality improvement interventions have been found to reduce CLABSI rates in adult populations.Reference O’Grady, Alexander and Burns 6 , Reference Blot, Bergs, Vogelaers, Blot and Vandijck 11 Antimicrobially impregnated catheters are not used routinely at our institution, nor are antibiotic impregnated CVC site patches or antibiotic lock preparations. Our findings support the notion that infection prevention and control measures, most of which are relatively inexpensive and simple to implement, can improve patient safety and child health.
CVC type, number of lumens, dressing type and insertion vein were statistically significantly associated with CLABSI, and infants in the NICU were at the highest risk. Totally implantable devices were protective against CLABSI in this analysis, in agreement with previous studies,Reference O’Grady, Alexander and Burns 6 , Reference O’Grady, Alexander and Dellinger 12 Double-lumen CVCs were associated with increased risk compared with single-lumen CVCs, supporting the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommendation to use the lowest number of CVC lumens necessary for care.Reference O’Grady, Alexander and Burns 6 Absorbent pads or gauze dressings were associated with CLABSI in our population; HICPAC recommends that either gauze or sterile, transparent, semi-permeable dressings be used. We expect that our finding may be explained by the aggregation of absorbent pad or gauze dressing type category with pressure and “miscellaneous” dressings due to low numbers or due to (residual) confounding of dressing type with an unmeasured risk factor. HICPAC notes that either upper or lower extremities or the scalp can be used for line insertion in children.Reference O’Grady, Alexander and Burns 6 We observed a significant association of subclavian vein placement with CLABSI. Pediatric studies have found femoral CVC insertion to be associated with increased CLABSI risk compared to torso or upper-limb insertions, and with lower risk, in the NICU.Reference Hoang, Sills, Chandler, Busalani, Clifton-Koeppel and Modanlou 16 , Reference Vegunta, Loethen, Wallace, Albert and Pearl 17 It has been postulated that the high frequency of handling neonates around the head and neck compared with older children could explain this relationship.Reference Vegunta, Loethen, Wallace, Albert and Pearl 17
The quality and broad scope of the data are a major strength of this study. These data were prospectively collected, yielded a large sample size with high statistical power, included many subpopulations, and extended over the long data collection period of 18 years. Thus, we were able to capture changes in practice and potential correlations with CLABSI rate. A limitation of this study was that some variable categories had to be merged for the analysis due to small cell sizes. Additionally, underlying illness and medications used were not captured in the dataset, and we had to use the patient care group as a proxy.
The findings of this study have many implications for health care. A temporal trend of decreasing infection rates over time, particularly associated with a hand hygiene campaign, suggest that relatively simple infection prevention and control interventions and a systematic approach to central-line care can improve child health outcomes. While this observational study cannot substitute for evidence from a randomized controlled trial, it provides compelling data to support education, standards, and advocacy for best practices related to care of central lines in children.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of Lois Laroche, Martha Childs, Ann Higgins, Allana Ivany, Nadine Smith, Brenda MacDougall and Bridget Maxwell to the development of the CVAD program. Joanne Langley holds the Canadian Institutes of Health Research-GlaxoSmithKline Chair in Pediatric Vaccinology at Dalhousie University.
Financial support: Jillian Carter was supported by an entrance scholarship from the Department of Community Health and Epidemiology at Dalhousie University.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.








