The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an unprecedented public health threat in the 21st century. More than 98.2 million cases of COVID-19 have been confirmed, with >2.1 million deaths globally in 1 year. 1 The overwhelming disease burden imposes a huge pressure on healthcare settings in both developed and underdeveloped countries. Insufficient isolation facilities and personal protective equipment (PPE) are major challenges to the healthcare system. 2 Healthcare workers (HCWs) have been reported to have at least 10 times increased risk for COVID-19 compared with the general population. Reference Nguyen, Drew and Graham3 In the early phase of the pandemic as of May 8, 2020, >150,000 HCW infections with 1,413 deaths occurred globally. Reference Bandyopadhyay, Baticulon and Kadhum4 Severe disease with complications developed in 5% of SARS-CoV-2–positive HCWs. Reference Gomez-Ochoa, Franco and Rojas5 By September 2, 2020, SARS-CoV-2 had infected nearly 570,000 HCWs with >2,500 deaths in the Americas. 6 Infection of HCWs and the associated mortality have detrimental effects on healthcare service as well as morale of frontline staff. Reference Cheng, Wong and Yuen7
In Hong Kong, we experienced a disastrous SARS outbreak in 2003, leading to 1,755 infections with 299 deaths 8 ; among these, 386 HCWs were infected and 8 died as a result of nosocomial acquisition of SARS-CoV-1. Since the outbreak of community-acquired pneumonia in Wuhan, Hubei province, was officially announced on December 31, 2019 (day 1), 9 our priority has been to ensure the safety of our HCWs during the COVID-19 pandemic. To achieve zero nosocomial COVID-19 cases among HCWs, we implemented a multipronged infection control strategy to minimize the risk of nosocomial COVID-19 in Hong Kong. A hospital-based approach was adopted in which symptomatic and asymptomatic COVID-19 patients were managed in airborne infection isolation rooms (AIIRs) in hospitals under the governance of Hospital Authority, and these patients were subsequently diverted to the community isolation facility (CIF) and the community treatment facility (CTF), also under the management of the Hospital Authority. Reference Wong, Leung and Tong10
Methods
Multipronged infection control strategy
All newly diagnosed COVID-19 patients were managed inside AIIRs in hospitals or were diverted to the CIF and CTF under the governance of the Hospital Authority. Laboratory diagnosis of COVID-19 is based on reverse transcription-polymerase chain reaction (RT-PCR) of SARS-CoV-2 in a clinical specimen (Supplementary File 1 online). A multipronged infection control strategy was implemented to prevent nosocomial transmission of SARS-CoV-2. In addition to the enforcement of hand hygiene and environmental cleaning, it included the following specific components: (1) stepwise enhancement of laboratory surveillance for early isolation of COVID-19 patients in AIIRs; (2) proactive screening for high-risk groups in the quarantine camp; (3) daily monitoring of the utilization of AIIRs; (4) temporary test centers, as well as the CIF and CTF, to relieve overcrowding in hospitals; (5) universal masking for HCWs and patients; (6) directly observed donning and doffing among HCWs; (7) prudent use of PPE in performing aerosol-generating procedures (AGPs); (8) special dinning arrangements for HCWs in hospitals; (9) fewer visitors in hospitals; and (10) just-in-time infection control education to the HCWs, including simulation training.
Simulation training to augment infection control practices
Simulation methodology was adopted for practices and drills to control the spread of SARS-CoV-2. The simulation training raised HCW awareness of the importance of building the competency of their entire team with better handling of COVID-19 patients. The training focused on the performance of high-risk procedures such as resuscitation and transportation of COVID-19 patients. Each 3-hour training session included a 45-minute lecture, scenario-based simulation in practice, and a debriefing session.
Outbreak investigation of nosocomial transmission of SARS-CoV-2
When COVID-19 patients were diagnosed in the non-AIIRs in hospitals, an infection control team performed an outbreak investigation and contact tracing for HCWs and hospitalized patients who met the criteria of close contact. For HCWs, a close contact was defined as one who carried out AGPs for the confirmed case without wearing appropriate PPE including surgical respirator, cap, face shield, isolation gown, and gloves. For patients, a close contact was defined as having face-to-face contact for >15 minutes or staying in the same cubicle for >2 hours with the confirmed case, regardless of whether surgical masks were worn.
Analysis of HCWs with COVID-19
Soon after the first imported case of COVID-19 was officially reported on January 23, 2020 (day 24), an electronic system of notification of infectious disease was activated for HCWs to report their illnesses, such as fever with or without any respiratory symptoms. A diagnostic test for SARS-CoV-2 by RT-PCR was offered to any symptomatic HCWs and to anyone classified as close contact with a confirmed case. Repeated testing was performed according to clinical assessment if the result of the first RT-PCR was negative for SARS-CoV-2. SARS-CoV-2 testing was also offered if a confirmed community case was living in the same residential building as our HCWs. For HCWs confirmed with COVID-19, epidemiological investigation was conducted by the infection control team to determine whether the HCW cases were hospital-acquired COVID-19 (HAC) or community-acquired COVID-19 (CAC). HAC was reported if the HCW had inappropriate PPE or any lapse in infection control procedures when caring for a confirmed case in the 14 days prior to symptom onset. Undetermined status was reported if the HCW had cared for COVID-19 cases with appropriate PPE in the 14 days prior to symptom onset and had no known source of infection in the community. CAC was reported if the HCW had a history of travel to areas with community transmission of COVID-19 or if the HCW was exposed to a person with COVID-19, such as household members of a family with a COVID-19 case, or if the HCW had had contact with staff confirmed with COVID-19 in the nonclinical setting and without appropriate PPE during patient care in the 14 days prior to symptom onset. The incidence of HCW infection per 1,000 full-time equivalent (FTE) among professional staff (ie, doctors, nurses, and allied health staff) and nonprofessional staff in the hospitals was analyzed. The FTEs of professional and nonprofessional staff were obtained from the Hospital Authority Annual Report 2019–2020. 11
Statistical analysis
The χ 2 exact test was used to compare independent categorical variables between groups. A P value of <.05 was considered statistically significant.
Results
Multipronged infection control strategy
Of 5,296 COVID-19 patients, 4,808 (90.8%) were diagnosed in the first wave (142 cases), the second wave (896 cases), and the third wave (3,770 cases), with an overall incidence of 0.71 per 1,000 population in the first 300 days in Hong Kong (Supplementary Fig. 1 online). 12,Reference To, Chan and Ip13 The median age was 43 years (range, 1 month–100 years) and 2,665 (50.3%) were female. With the exception of 1 patient who died before admission, all patients confirmed with COVID-19 were admitted to our public healthcare system. The median length of stay was 13 days (range, 1–128), with a total of 78,834 COVID-19 patient days.

Fig. 1. Number of specimens tested for SARS-CoV-2 by reverse transcription-polymerase chain reaction (RT-PCR) in the public healthcare system in Hong Kong (January–October 2020).
Learning from the previous SARS epidemic, protecting HCWs from COVID-19 was the consensus priority in our healthcare settings from the date when the outbreak of community-acquired pneumonia in Wuhan, Hubei Province was announced (December 31, 2019). Reference Cheng, Wong and Chuang14 With the implementation of a multipronged infection control strategy, we extended our infection control preparedness beyond the hospitals for early recognition and isolation of COVID-19 patients in Hong Kong. In the community, a quarantine center was set up for the close contacts of confirmed cases or returning travelers from high-risk regions or countries. A team of hospital staff composed of infection control professionals, nurses, and phlebotomists attended the quarantine center to collect nasopharyngeal swabs serially for early diagnosis of COVID-19 among 469 Hong Kong residents being evacuated from Hubei province, China, by 4 charter flights on days 65 and 66. Reference To, Cheng and Cai15 A temporary test center was also set up at the Hong Kong International Airport for rapid diagnosis of COVID-19 among inbound travelers. Reference Wong, Leung, Lee, Chung and Cheng16 In the hospitals, active surveillance evolved from testing patients with clinical and epidemiological characteristics of COVID-19 initially to universal admission screening on day 254. In total, 8 tiers of enhanced laboratory surveillance were performed step by step (Table 1). A cumulative number of 1.3 million specimens for SARS-CoV-2 were tested using RT-PCR from January to October 2020 in the public healthcare setting in Hong Kong, with an average of 3,600 specimens tested per month (Fig. 1) and an average of 250 test performed per confirmed COVID-19 case.
Table 1. Tiers of Enhanced Laboratory Surveillance for COVID-19 Patients in Hong Kong a

Note. AED, accident and emergency department; GOPC, general outpatient clinic; HKSAR, Hong Kong Special Administrative Region, China.
a Respiratory specimens, such as nasopharyngeal swab, nasopharyngeal aspirate, and sputum, are used as screening specimen. Collection of deep throat saliva is considered as an alternative screening specimen if patient can spit the sample.
b The evolving reporting criteria by Centre for Health Protection, HKSAR was illustrated in our previous publication. Reference Cheng, Wong and Chen17 Briefly, the reporting criteria include a combination of clinical (fever, acute respiratory illness, or pneumonia) and epidemiological criteria (travelling to the affected geographic areas within 14 days before onset of symptoms or with close contact to a confirmed COVID-19 patient.
c An extra specimen container would be given to asymptomatic inbound travelers for collection of deep throat saliva on day 12 of the 14-d quarantine since April 20, 2020.
d Such as staff working at the Hong Kong International Airport, residential care homes for the elderly, residential care homes for persons with disabilities, and frontline workers of bus companies, or other persons regarded as high-risk of exposure during outbreak investigation in the community.
e In effect from September 9, 2020, onward.
As the COVID-19 patients were isolated in AIIRs, the utilization of AIIR beds and rooms in the hospitals was monitored daily from the second wave onward (Fig. 2). The CIF and CTF were activated from July 24, 2020 (day 207) to September 18, 2020 (day 263), Reference Wong, Leung and Tong10 with 918 patients diverting to the CIF (243 patients) and CTF (675 patients) respectively, which admitted 918 (33.4%) of 2,747 COVID-19 patients during that period.
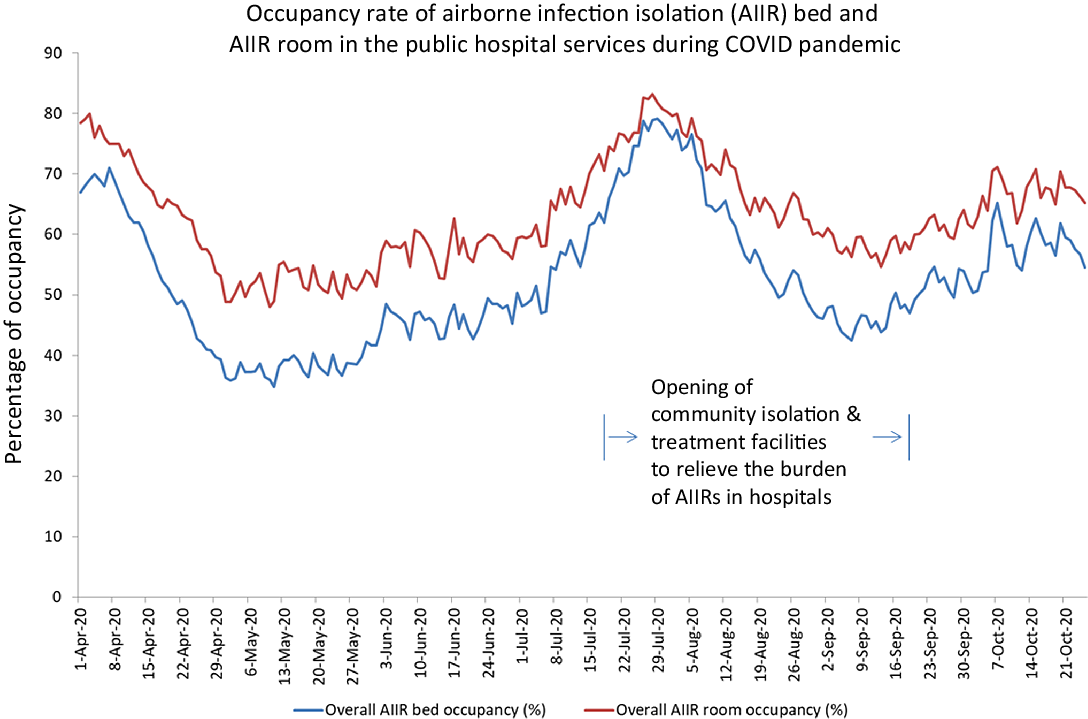
Fig. 2. Occupancy rate of airborne infection isolation (AIIR) bed and AIIR room in the public hospital services during the COVID-19 pandemic.
We adopted contact, droplet, and airborne precautions to care for the suspected and confirmed COVID-19 patients in the AIIRs with appropriate PPE, which included surgical respirator, cap, face shield, isolation gown, and gloves, as previously described. Reference Cheng, Wong and Chen17 Directly observed donning and doffing with a buddy system among HCWs was introduced in the hospitals, the CIF, and the CTF, in addition to standard infection control training. Reference Wong, Leung and Tong10,Reference Wong, Leung, Lee, Chung and Cheng16 The infection control team demonstrated and audited the appropriate use of PPE. Prudent use of PPE during AGPs was promoted to extend the use of N95 surgical respirators and face shields during serial patient encounters unless the N95 surgical respirator or face shield was damaged or soiled. 18,Reference Lynch, Davitkov and Anderson19 Hand hygiene was enforced, and staff also changed their gloves and gowns between patients. Subsequently, an alternative source of surgical respirator (a locally manufactured surgical respirator that was a European conformity certified nanofiber bactericidal surgical personal protective device) was introduced to relieve the shortage of N95 surgical respirators.
Universal masking was adopted, and the compliance of wearing surgical mask among HCWs was reported to be 100% in the clinical areas. Reference Wong, Lam and AuYeung20 Infection control measures were enforced for HCWs during activities without masks, such as meals. In the hospital canteen and pantry, chairs and tables were arranged in a unidirectional setting with partitions separating the tables and at least 1 m apart. Alcohol-based hand rub and disinfectant wipes were provided for staff use in the dining areas in the hospitals. Talking and interaction without surgical masks among HCWs were discouraged. Visitation to hospitalized patients was not allowed except for compassionate reasons, such as for terminally ill or peripartum patients, to reduce the risk of COVID-19 transmission from the community. A regular staff forum was held, and infection control teaching material with 52 updates were uploaded to the Hospital Authority website in the first 300 days of the COVID-19 pandemic.
Simulation training to augment infection control practices
During the initial phase of the COVID-19 outbreak, 5 sessions of simulation training “train-the-trainer (TTT) phase” were organized at a designated training center on January 24, 2020 (day 25), day 30, day 31, day 32, and February 13, 2020 (day 45). In total, 117 HCW participants from 7 hospital networks joined the TTT phase from medicine (26%), intensive care unit (23%), accidental and emergency department (17%), infection control teams (6%), and other clinical specialties (28%). Another 12 sessions of simulation training, “in-situ phase,” were arranged from February 18, 2020 (day 50) to March 23, 2020 (day 84). In-situ phase training was assigned for 12 groups comprising of ~30 HCWs per group from different clinical specialties of the same hospital. Of 387 HCWs trained in the in-situ phase, the ratio of senior to junior staff was 1:1. Two sets of posttraining evaluation forms were prepared for HCWs participating in the TTT and in-situ phases, and their feedback was satisfactory (Supplementary File 2 online). Those who had attended the simulation training further shared their knowledge and skills with other HCWs.
Outbreak investigation of nosocomial transmission of SARS-CoV-2
Among 5,296 patients diagnosed in the first 300 days of COVID-19 pandemic, 20 patients (0.4%) with initially unrecognized COVID-19 status were admitted to the non-AIIR of the hospitals. Eleven were men and 9 were women; their median age was 63 years (range, 4–92). Of these 20 COVID-19 patients, 10 (50.0%) stayed in medical wards and 5 (25.0%) attended accidental and emergency department or stayed in the emergency medicine ward (Table 2). Of the 10 patients (50.0%) staying in non-AIIRs for ≥2 days, the median stay was 6 days (range, 2–26 days). Furthermore, 5 cases of nosocomial transmission of COVID-19 originated from 20 patients with initially unrecognized COVID-19 status. Of 272 patients classified as close contacts, 8 (2.9%) had COVID-19. Of 41 HCWs classified as close contacts, none acquired COVID-19, which was confirmed by negative testing throughout the quarantine period.
Table 2. Exposure of Patients With Coronavirus Disease 2019 (COVID-19) in the Non–Airborne Infection Isolation Room (AIIR) of Hospitals During the First 300 Days of the COVID-19 Pandemic (December 31, 2019 to October 25, 2020) in Hong Kong

Note. AED, accidental and emergency department; CAC, community-acquired COVID-19; DTS, deep throat saliva; EMW, emergency medicine ward; HAC, hospital-acquired COVID-19; HCWs, healthcare workers; NA, not applicable; NPS, nasopharyngeal swab.
a The information is retrieved from the press release to staff and public by the Hospital Authority.
b There are 7 hospital networks under the governance of Hospital Authority, Hong Kong. All 6 of 7 hospital networks, except network B, had patients with COVID-19 in the non-airborne infection isolation room (AIIR) in the hospitals during the first 300 days of the COVID-19 pandemic.
c Epidemiological investigation was performed to determine the source of infection.
d Hospitalized patient will be transferred to airborne infection isolation room once COVID-19 is diagnosed.
e Close contact of healthcare worker is defined as those who had cared for the confirmed case without appropriate personal protective equipment for the procedures.
f Close contact of patient is defined as patients who had face-to-face contact for >15 min with the confirmed case, regardless of wearing of surgical masks, or patients who had stayed in the same cubicle for >2 h of the confirmed case, regardless of wearing of surgical masks.
g Secondary case of COVID-19 is defined as those close contacts who are positive for SARS-CoV-2 in the screening or clinical specimen by reverse transcription-polymerase chain reaction within the quarantine period of 14 d.
h The epidemiological investigation was reported previously. Reference Wong, Kwong and Wu39
i Of 9 close contacts, there were 2 secondary cases. The first was a 77-year-old female patient diagnosed with COVID-19 on July 13, 2020. As this patient was transferred to another cubicle, another 5 patients were classified as close contacts. Another was a 64-year-old female patient who was diagnosed on July 14, 2020.
j Of 10 close contacts, a 41-year-old male patient who presented with fever and was diagnosed with COVID-19 on July 19, 2020.
k Of 8 close contacts, a 75-year-old male patient had fever and cough and confirmed with COVID-19 on August 9, 2020.
l Of 9 close contacts, three male patients, aged 64, 70, and 78 years were confirmed with COVID-19 during the quarantine period.
m Of 7 close contacts, a 54-year-old male patient was diagnosed with COVID-19 during the quarantine period.
nThe concerned patient was one of the household contacts of a male patient earlier confirmed to have COVID-19.
Analysis of HCWs with COVID-19
During the first 300 days of the COVID-19 pandemic, 446 HCWs reported sick to the electronic system of notification of infectious disease, and 38 HCWs had COVID-19 (Fig. 3). The incidence of COVID-19 among HCWs was significantly lower than that of general population in Hong Kong (0.46 per 1,000 HCWs vs 0.71 per 1,000 population; P = .008). Of 38 HCWs, 5 (13.2%) were asymptomatic (9 men and 29 women). The median age was 42 years (range, 23–65). With the exception of 1 nurse with undetermined COVID-19 acquisition, and 5 nonprofessional staff acquired the infection from nonclinical settings (office, dinning place, and dormitory room), another 32 HCWs acquired COVID-19 from the community. None of the HCWs had documented transmission of SARS-CoV-2 from COVID-19 patients in the clinical setting, despite 78,834 COVID-19 patient days. Of the 38 infected HCWs, 13 were professional staff (2 doctors, 11 nurses) and 25 were nonprofessional staff (2 cleaning, 8 clerical, 4 patient care assistant, 10 supporting, and 1 security staff) (Table 3). The incidence of COVID-19 among professional staff was 0.30 per 1,000 FTE, which was significantly lower than that of nonprofessional staff (0.66 per 1,000 FTE; P = .022). Also, 34 HCWs were infected with SARS-CoV-2 in the third wave. The number of infected HCWs per 1,000 FTE was significantly higher in the third wave than in the first and second waves combined (0.41 vs 0.05; P < .001). In total, 112 HCWs were classified as close contacts of infected HCWs, with an average of 3 close contacts per index HCW, and 5 (4.5%) of these developed COVID-19 during the quarantine period.

Fig. 3. Healthcare workers with COVID-19 in Hong Kong (day 1–300 of the COVID-19 pandemic). Note. The date represented reporting date of healthcare worker infection serving in the public hospitals and clinics. The source of COVID-19 acquisition was illustrated by different colors.
Table 3. Diagnosis of Coronavirus Disease 2019 (COVID-19) Among Healthcare Workers During the First 300 Days of the COVID-19 Pandemic (December 31, 2019 to October 25, 2020) in Hong Kong a
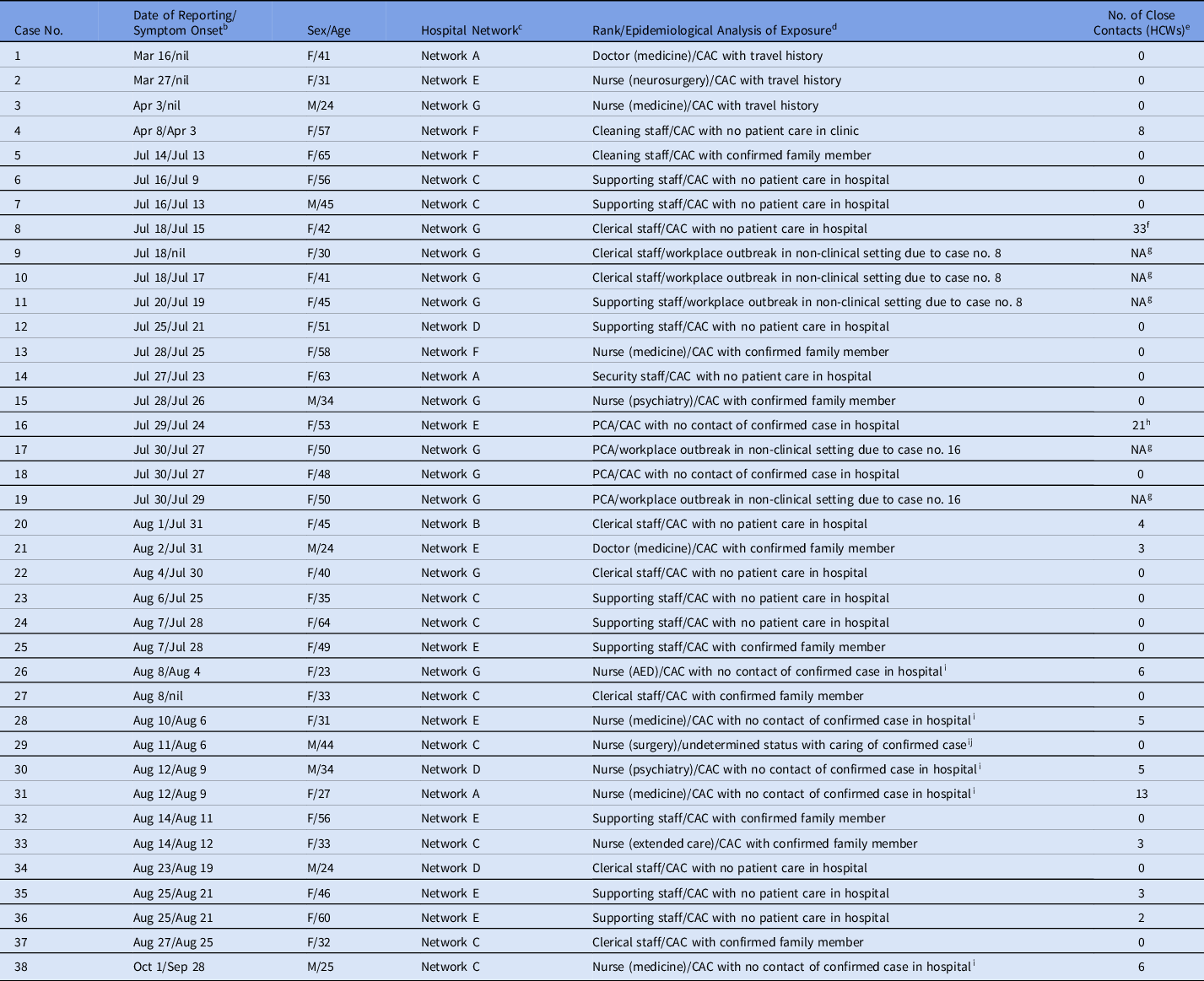
Note. AED, accidental and emergency department; CAC, community-acquired COVID-19; GOPC, general outpatient clinic; PCA, patient care assistant; HCW, healthcare worker.
a The healthcare workers are serving in the public hospitals and clinics.
b Symptom onset is described as nil if the staff had asymptomatic COVID-19.
c There are 7 hospital networks under the governance of Hospital Authority, Hong Kong. The incidence rates of healthcare workers infection per 100 COVID-19 patients managed in different hospital network were as follows: network A (0.48), network B (0.24), network C (1.38), network D (0.43), network E (1.35), network F (0.44), and network G (1.35).
d Epidemiological investigation was performed to determine the exposure from household members, healthcare workers in hospitals, patients with retrospective diagnosis of COVID-19 in non–airborne infection isolation room, and caring confirmed COVID-19 patients in airborne infection isolation room in the previous 14 d.
e As universal masking was implemented in the hospitals during COVID-19 pandemic, and appropriate personal protective equipment was worn during patient care procedure in the epidemiological investigation, no patient was defined as a close contact from the infected healthcare workers. Close contact among healthcare worker is defined as those who had face-to-face interaction without wearing surgical masks for >15 min such as dining inside or outside hospitals. During the quarantine period of 14 d, and followed by medical surveillance of another 14 d, there was no secondary case of COVID-19 among the close contacts.
f Of 33 close contacts in the office, 2 clerical and 1 supporting staff (case nos. 9–11) were diagnosed to have COVID-19 during the quarantine period.
g COVID-19 was diagnosed in the quarantine facility.
h Of 21 close contacts in the office, 2 patient care assistants (case nos. 17 and 19) were diagnosed to have COVID-19 during the quarantine period.
i Wearing appropriate personal protective equipment at work.
j Without performing high-risk procedures such as aerosol-generating procedures.
Discussion
With the increasing number of nosocomial transmissions and outbreaks of COVID-19, Reference Abbas, Robalo Nunes and Martischang21 our multipronged infection control strategy successfully prevented the nosocomial acquisition of SARS-CoV-2 among HCWs during patient care in the first 300 days of the COVID-19 pandemic in Hong Kong, despite 78,834 COVID-19 patient days. In our multipronged infection control strategy, we advocated liberal testing for early isolation of all suspected and confirmed cases in the public hospitals, Reference Cheng, Wong and Chen17 the CIF, and the CTF. Reference Wong, Leung and Tong10 This strategy contrasts with the policies of many Western countries where SARS-CoV-2 testing may not be readily accessible and most COVID-19 patients with mild-to-moderate disease have been managed at home 22,23 to protect the healthcare service from overloading and to reduce the risk of HCW infection due to fatigue with lapses in infection control procedures or depletion of PPE. Hong Kong did the exact opposite. Our hospital-based HCWs even proactively went to quarantine centers Reference To, Cheng and Cai15,Reference Hung, Cheng and Li24 and airports Reference Wong, Leung, Lee, Chung and Cheng16 to collect specimens for testing with a short turnaround time of 4–8 hours to minimize community transmission. Paradoxically, our heavily hospital-based approach spared our healthcare service from being overloaded.
When all of the confirmed COVID-19 patients were isolated, appropriate use of PPE became the most important parameter to prevent HCW infection, as illustrated in a recent analysis. Reference Chou, Dana, Buckley, Selph, Fu and Totten25 We adopted directly observed donning and doffing, a buddy system to enhance the compliance of appropriate use of PPE, especially when caring for a suspected or confirmed case of COVID-19, not only in hospitals but also in CIF and CTF. Reference Wong, Leung and Tong10 The CIF and CTF diverted one-third of COVID-19 patients to relieve overcrowding in hospitals, which may have reduced the risk to HCWs. Universal masking was mandated in the hospitals, Reference Wong, Lam and AuYeung20 associated with the enforcement of hand hygiene, Reference Wong, AuYeung and Lam26 environmental hygiene, Reference Cheng, Wong and Chan27 and standard precautions during the COVID-19 pandemic. These measures may explain why none of 41 HCWs classified as close contacts after caring for unrecognized cases acquired COVID-19.
Infection control training is associated with decreased infection risk of COVID-19, Reference Chou, Dana, Buckley, Selph, Fu and Totten25 which markedly contributed to our zero nosocomial acquisition of SARS-CoV-2 among HCWs. In Hong Kong, infection control training has been regularly provided to HCWs working in the hospitals, the CIF, and the CTF, along with PPE training, especially for those caring for COVID-19 patients. Reference Wong, Leung and Tong10,Reference Cheng, Wong and Chen17 Simulation training was organized with particular focus on infection control in the high-risk procedures, such as resuscitation and patient transfers, in the early phase of the COVID-19 pandemic. In fact, simulation training has been increasingly applied in different clinical specialties for COVID-19 prepardedness. Reference LoSavio, Eggerstedt and Tajudeen28,Reference Diaz and Dawson29 Simulation exercises have proven to be an effective strategy to increase self-efficacy, to decrease anxiety for HCWs, and to build interprofessional teamwork in response to infectious diseases. Reference Marrs, Horsley, Hackbarth and Landon30
Stringent infection control measures protected HCWs from SARS-CoV-2 in the clinical setting, but HCWs may have acquired SARS-CoV-2 from a nonclinical source, similar to the Netherlands, where most infected HCWs acquired SARS-CoV-2 in the community. Reference Kluytmans-van den Bergh, Buiting and Pas31 However, our HCWs had a significantly lower incidence of COVID-19 than the general population, in contrast with reports that patient-facing HCWs had higher odds of a positive test Reference Misra-Hebert, Jehi and Ji32 and a 3-fold increased risk of admission with COVID-19. Reference Shah, Wood and Gribben33 With our intense infection control training, our HCWs were more alert and vigilant in both hospital and community settings. However, the alertness of our nonprofessional staff was lower than that of the professional staff, resulting in HCW-to-HCW transmission in nonclinical settings when they were dining together or staying in the dormitory without masks. Activities without masks increased the risk of COVID-19 outbreaks. Reference Cheng, Wong and Chuang34,Reference Coppeta, Somma and Ippoliti35 The short-range airborne route may dominate exposure to SARS-CoV-2 during close contact, Reference Gao, Li and Wei36 especially in poorly ventilated indoor or dining places. Reference Lu, Gu and Li37 Therefore, we enforced social distancing in the hospital canteen and pantry.
With the emergence of mutant strains of COVID-19 with higher transmissibility and antibody or vaccine resistance, Reference Leung, Shum, Leung, Lam and Wu38 the risk of HCW infection will further increase. We will continue our multipronged infection control strategy to minimize the risk of nosocomial acquisition of SARS-CoV-2 among our HCWs.
Acknowledgments
We thank all the frontline and management staff of Hospital Authority for supporting and adhering to the infection control measuresduring COVID-19 pandemic. We also thank the staff involved in the setting up and maintenance of the community isolation facility at Lei Yue Mun Park and HolidayVillage, and the community treatment facility at AsiaWord-Expo.
Financial support
This study was partially supported by the Health and Medical Research Fund (HMRF) Commissioned Research on Control of Infectious Disease (Phase IV), CID-HKU1-16, Food and Health Bureau, Hong Kong SAR Government.
Conflict of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.119









