Clostridium difficile infection (CDI) is the leading cause of hospital-associated infectious diarrhea.Reference Dubberke and Olsen 1 Diarrhea is the most common presentation; however, CDI may result in more serious sequelae including pseudomembranous colitis, toxic megacolon, and death,Reference Freeman, Bauer and Baines 2 , Reference Dubberke, Butler and Reske 3 and mortality ranges from 5% to 10%.Reference Dubberke, Butler and Reske 3 Frequent recurrence, affecting ~20% of treated participants, is particularly challenging for CDI management.Reference Johnson 4 Most CDI cases occur in higher-income countries,Reference Lessa, Mu and Bamberg 5 but data from middle- and low-income countries are lacking. Surveillance data suggest that the incidence density ranges between 2.45 and 7.5 per 10,000 patient days or between 9 and 80 per 10,000 patient admissions.Reference Freeman, Bauer and Baines 2 , Reference Slimings and Riley 6 – Reference Lessa, Gould and McDonald 8 An individual patient’s risk of developing CDI differs based on numerous host and environmental factors,Reference Freeman, Bauer and Baines 2 , Reference Slimings and Riley 6 , Reference Louie, Miller and Crook 9 , Reference Vesteinsdottir, Gudlaugsdottir, Einarsdottir, Kalaitzakis, Sigurdardottir and Bjornsson 10 most importantly antibiotic exposure, which is thought to disrupt the intestinal microbiota, allowing C. difficile to proliferate.Reference Slimings and Riley 6
Probiotics, defined as live microorganisms that, when administered in adequate amounts, may confer a health benefit on the host, are a potential CDI prevention strategy.Reference Hill, Guarner and Reid 11 A 2013 Cochrane meta-analysis of 23 randomized controlled trials (RCTs) demonstrated a 64% decrease (95% confidence interval [CI], 49%–73%) in CDI incidence with probiotic prophylaxis.Reference Goldenberg, Ma and Saxton 12 The results of a subsequent large RCT (n=2,941) were indeterminate; however, CDI events were less frequent than anticipated. An updated aggregate data meta-analysis incorporating the study showed that the beneficial effect of the probiotic remained.Reference Goldenberg, Yap, Lytvyn, Lo, Beardsley, Mertz and Johnston 13 A recent review of clinical practice guidelines on CDI preventionReference Lytvyn, Mertz, Sadeghirad, Alaklobi, Selva, Alonso-Coello and Johnston 14 found that recommendations mainly revolved around core strategies (eg, education of healthcare staff on CDI, patient isolation, antimicrobial stewardship, and utilization of disinfectants), but none recommend probiotics for prophylaxis.Reference Carrico, Bryant and Lessa 15 – Reference Hawkey, Bain and Borriello 19 Guidelines cited concerns regarding insufficient evidence,Reference Surawicz, Brandt and Binion 17 , Reference Debast, Bauer and Kuijper 20 too much weight given to studies with high baseline CDI risk,Reference Dubberke, Carling and Carrico 16 and safety concerns.Reference Dubberke, Carling and Carrico 16 , Reference Debast, Bauer and Kuijper 20
To address some of these concerns and to further investigate the effectiveness and safety of probiotics on populations and interventions with varying characteristics, we conducted an individual participant data (IPD) meta-analysis. Our objective was to determine whether adding probiotics to an antibiotic regimen reduces the incidence of CDI among children and adults, when adjusting for age, sex, hospitalization status, the number of antibiotics taken, and the administration of high-risk antibiotics.
METHODS
We registered the protocol for this IPD meta-analysis in the PROSPERO registry in January 2015.Reference Lytvyn, Mertz and Thabane 21
Searches
We considered all studies deemed eligible in a previous comprehensive systematic review on probiotics for the prevention of CDI (Appendix Methods 1),Reference Goldenberg, Ma and Saxton 12 and we updated the search on April 11, 2016 (Appendix Tables 1 and 2).
TABLE 1 Characteristics of Included Studies

NOTE. B, Bifidobacterium; CDI, Clostridium difficile infection; CFU, colony-forming units; d, day; L, Lactobacillus; P, placebo; SAE, serious adverse events; SD, standard deviation; SC, standard care.
a The timing of administration and the duration of probiotics ranged from providing probiotics for the duration of antibiotic exposure and up to 7 d following completion of antibiotics. Timing of administration ranged from immediately when starting antibiotics and up to 7 d following antibiotics.
b High-risk antibiotics were third- and fourth-generation cephalosporins, lincosamides, and fluoroquinolones.
c Median and interquartile range.
d B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. bulgaricus, and S. thermophiles.
TABLE 2 Summary Data From Trials Providing Individual Patient Data
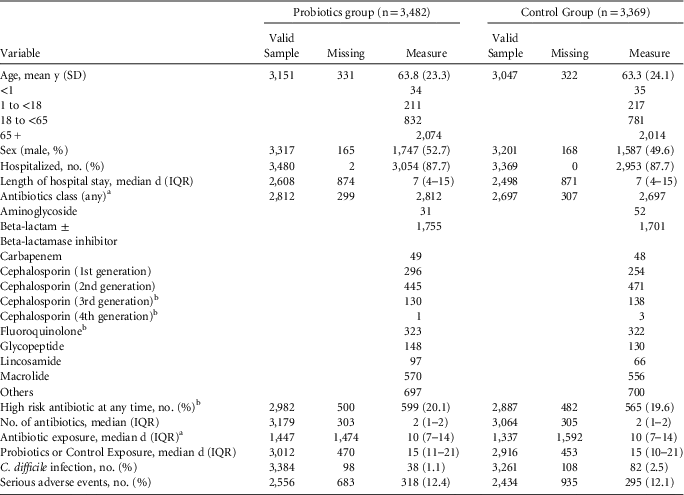
a Values for antibiotic class and median, IQR for antibiotic exposure were computed based on studies who had these measures available within data (43–45 were not included).
b High-risk antibiotics were considered 3rd- and 4th-generation cephalosporins, lincosamides, and fluoroquinolones.
Study Selection
Two reviewers independently screened titles, abstracts, and full-text articles for eligibility and resolved differences through consensus. We included RCTs with children (0 to <18 years) or adults administered antibiotics (ie, any reason or duration) with concomitant probiotics (ie, any dose, species, strain, or duration), compared to placebo, alternative prophylaxis, or no treatment (standard care), and that reported CDI as an outcome. The latter was our primary outcome, defined as either diarrhea with laboratory confirmation of C. difficile, presence of pseudomembranes on sigmoidoscopy or colonoscopy, histological diagnosis of C. difficile, or diagnosis of toxic megacolon.Reference Bartlett and Gerding 22 Our secondary outcome was the incidence of serious adverse events (SAEs).
Data Extraction and Quality Assessment
We contacted the authors of all eligible studies and requested ethics-approved, deidentified data including participants’ allocated treatment, age, length of hospital stay, CDI history, antibiotics given, probiotics given, presence of diarrhea, CDI diagnosis, and SAEs. We also requested any information on missing outcome data, such as participants lost to follow up and participants who had diarrhea but no C. difficile test.
Reviewer pairs (B.C.J., J.G., L.L.) independently assessed risk of bias based on the Cochrane Handbook for Systematic Reviews of Interventions.Reference Higgins, Altman and Gøtzsche 23 For studies from the previous review, we used the previous assessment,Reference Goldenberg, Ma and Saxton 12 with 6 modifications (Appendix Methods 2). For the overall certainty in estimates for each outcome, we used Grading of Recommendations, Assessment, Development and Evaluation (GRADE), independently and in duplicate.Reference Guyatt, Oxman and Vist 24 Publication bias was evaluated with a funnel plot.Reference Egger, Smith, Schneider and Minder 25
Data Synthesis and Analysis
We pooled IPD across trials and analyzed it using a generalized linear mixed model using the SAS GLIMMIX procedure using SAS/STAT version 9.4 software (SAS Institute, Cary, NC) and calculated the odds ratio (OR) and 95% CIs. We considered the study level as a random effect and the participant variables as fixed. For our adjusted analysis, based on known CDI risk factors, variables available across datasets, and data provided by authors, we developed a model adjusting for 5 variables: age (years),Reference Vesteinsdottir, Gudlaugsdottir, Einarsdottir, Kalaitzakis, Sigurdardottir and Bjornsson 10 sex, hospitalization status, use of multiple antibiotics, and exposure to high-risk antibiotics (third- and fourth-generation cephalosporins, lincosamides, and fluoroquinolones).Reference Slimings and Riley 6 Only hospitalized participants had SAEs; thus, we did not adjust for this variable.
Statistical heterogeneity was evaluated using the I2 value, where an I2 value of 0 to 40% represented low heterogeneity and an I2 value of 30%–60% represented moderate heterogeneity.Reference Deeks, Higgins and Altman 26 We used Review Manager version 5.3 software (Copenhagen, Denmark) for aggregate data meta-analyses and funnel plots. We used SPSS version 20 software (IBM Armonk, NY) and SAS/STAT version 9.4 software for data cleaning and analysis, respectively. We used Stata version 13 software (StataCorp, College Station, TX) to graph the IPD meta-analysis forest plots. Given the low event rate for CDI and SAEs (<10%), the OR approximates the relative risk (RR). Thus, we reported relative risk reduction (RRR) for ease of interpretation.
Subgroup Analyses
We conducted 4 a priori subgroup analyses. First, as an approximation of baseline CDI incidence, we examined studies with low (<5%) control-group event rates versus those with rates ≥5%.Reference Dubberke, Carling and Carrico 16 Second, we compared no probiotics (ie, control group) to single-species probiotics. Third, we compared no probiotics to multispecies or multistrain probiotics.Reference Goldenberg, Yap, Lytvyn, Lo, Beardsley, Mertz and Johnston 13 Fourth, we looked at probiotic dose, where participants in the control group had zero colony-forming units (CFU) per day (ie, control group), and we examined the effect of increasing the dose by 1 billion CFU per day in the intervention group.Reference Johnston, Goldenberg, Vandvik, Sun and Guyatt 27
Sensitivity Analyses
We conducted 4 a priori sensitivity analyses on our primary outcome. First, we compared the unadjusted analysis (18 studies, n=6,645) with the pooled estimate of effect based on aggregate data (18 studies, n=6,447). Second, we compared the adjusted analysis (13 studies; n=5,074) with the pooled estimate of effect based on aggregate data (13 studies; n=5,341). Third, for the adjusted analysis of CDI, we categorized age (infants 0 to <1 year, children 1 to <18 years, adults 18 to <65 years, older adults 65+ years) to determine whether age groups are more predictive of CDI than continuous linear age. We discerned infants from children because before 1 year of age, C. difficile colonization is common and does not reflect CDI.Reference Gerding, Olson and Peterson 28 Fourth, for the adjusted analysis, we accounted for clustering using generalized estimation equations (GEE) using the SAS GENMOD procedure.
Handling Missing Participant Data
Participants with no reported outcome for CDI were considered as having missing outcome data. If participants with missing CDI outcome data had reported no diarrhea, we assumed they did not have CDI. If participants had reported diarrhea but no CDI test result, we considered this missing outcome data. We conducted a sensitivity analysis using the PROC MI procedure in SAS to impute missing data to create 5 imputed data sets. We examined the effect of multiple imputation on regression estimates using PROC MIANALYZE.
RESULTS
Data Selection and Data Obtained
Of 2,021 articles, we obtained IPD from 18 of 32 confirmed eligible trialsReference Gao, Mubasher, Fang, Reifer and Miller 29 – Reference Ruszczyński, Radzikowski and Szajewska 46 with IPD for 6,851 of 8,713 participants (78.6% of all available data). Among our sample of 6,851, participants had 120 CDI events and 613 SAEs (Table 1). There were 11 formulations of probiotics given, with doses ranging from 0.1 to 900 billion CFUs per day. Most studies (88.9%) used placebo as a control and were conducted in adult hospitalized participants (72.2%) (Table 1). The proportion of participants on high risk antibiotics at any given time ranged from 0%Reference Duman, Bor and Özütemiz 30 , Reference Bravo, Bunout and Leiva 31 to 85.4%Reference Ehrhardt, Guo and Hinz 44 (Table 2). For the outcome CDI, 2 studies (11.1%) did not report participant level data on antibiotics taken,Reference Plummer, Weaver, Harris, Dee and Hunter 38 , Reference Psaradellis, Sampalis and Rampakakis 39 and 3 studies (16.7%) did not report age.Reference Miller 36 , Reference Miller 37 , Reference Georgieva, Pancheva, Rasheva, Usheva, Ivanova and Koleva 43 Thus, 1,777 of 6,851 participants (26%) were excluded from the adjusted CDI model (n=5,074). The length of hospital stay and CDI history were not available for most datasets and were not included in the model. In terms of missing outcome data for CDI, study results ranged from zeroReference Gao, Mubasher, Fang, Reifer and Miller 29 , Reference Kotowska, Albrecht and Szajewska 32 , Reference Klarin, Wullt, Palmquist, Molin, Larsson and Jeppsson 35 , Reference Plummer, Weaver, Harris, Dee and Hunter 38 , Reference Selinger, Bell, Cairns, Lockett, Sebastian and Haslam 40 , Reference Allen, Wareham and Wang 41 , Reference Ehrhardt, Guo and Hinz 44 – Reference Ruszczyński, Radzikowski and Szajewska 46 to 25.8%.Reference Pozzoni, Riva and Bellatorre 33 For our secondary outcome, 14 of 18 studies provided IPD for SAEs.Reference Gao, Mubasher, Fang, Reifer and Miller 29 – Reference Pozzoni, Riva and Bellatorre 33 , Reference Klarin, Wullt, Palmquist, Molin, Larsson and Jeppsson 35 – Reference Plummer, Weaver, Harris, Dee and Hunter 38 , Reference Selinger, Bell, Cairns, Lockett, Sebastian and Haslam 40 , Reference Allen, Wareham and Wang 41 , Reference Ehrhardt, Guo and Hinz 44 – Reference Ruszczyński, Radzikowski and Szajewska 46 Our adjusted model was based on 11 of these 14 studies because 3 studies did not report age (16.7%).Reference Miller 36 , Reference Miller 37 , Reference Georgieva, Pancheva, Rasheva, Usheva, Ivanova and Koleva 43 Thus, 272 of 4,990 participants (5.5%) were excluded from this model (n=4,718).
Risk of Bias Assessment
For the primary outcome CDI, 4 studies were at high risk of bias for incomplete outcome data because >10% of participants either had no data provided or had diarrhea but no CDI testReference Duman, Bor and Özütemiz 30 , Reference Bravo, Bunout and Leiva 31 , Reference Pozzoni, Riva and Bellatorre 33 , Reference Psaradellis, Sampalis and Rampakakis 39 (Appendix Figure 1). Another study was at high risk of bias for blinding of participants and personnel along with blinding for outcome assessment.Reference Wong, Jamous and O’Driscoll 42 For SAEs, 1 study was at high risk of bias for incomplete outcome data because authors reported SAEs but did not provide the data.Reference Hickson, D’Souza and Muthu 34 We identified 2 studies that were at high risk of bias for blinding both of participants and personnel and of outcome data.Reference Duman, Bor and Özütemiz 30 , Reference Wong, Jamous and O’Driscoll 42 Overall, for both CDI and SAEs, 2 studies were at high risk for selective reportingReference Duman, Bor and Özütemiz 30 , Reference Hickson, D’Souza and Muthu 34 and 5 studies were at high risk of other bias for potential conflict of interest due to industry funding.Reference Klarin, Wullt, Palmquist, Molin, Larsson and Jeppsson 35 – Reference Plummer, Weaver, Harris, Dee and Hunter 38 , Reference Lönnermark, Friman, Lappas, Sandberg, Berggren and Adlerberth 45
There was no suspected publication bias for CDI among the included studies, which was similar for all eligible studies and studies with data available (Appendix Figure 2). (Figure 1)

FIGURE 1 Flow of eligible studies providing and not providing individual patient data.
Primary Outcome: Clostridium difficile Infection
Of the 18 studies reporting on CDI outcomes, 38 of 3,384 of cases (1.1%) were in the intervention group, and 82 of 3,262 (2.5%) were in the control group (Table 2). Probiotic prophylaxis reduced the unadjusted odds of CDI (OR, 0.37; 95% CI, 0.25–0.55; P<.0001; n=6,645; Figure 2), similar to the pooled estimate for the 14 studies for which IPD was not obtained (OR, 0.30; 95% CI, 0.19–0.47; P<.0001; n=2,266; Appendix Figure 3). Of the 13 studies included in the adjusted model, probiotics significantly reduced the CDI (OR, 0.35; 95% CI, 0.23–0.55; P<.0001; n=5,074; Figure 2). The use of 2 or more antibiotics significantly increased the risk of CDI (OR, 2.20; 95% CI, 1.11–4.37; P=.0243), whereas age, sex, hospitalization status, and high-risk antibiotic exposure did not.

FIGURE 2 Incidence of Clostridium difficile infection.
Multiple imputation sensitivity analysis provided similar regression estimates to the adjusted analysis (OR, 0.37; 95% CI, 0.25–0.56; P<.001).
Subgroup Analyses
A control group event rate of ≥5% was significantly associated with CDI in the adjusted model (OR, 16.33; 95% CI, 7.79–34.26; P<.0001; n=5074) (Figure 2) and had significant interaction with the treatment effect (P=.007). Compared to no probiotics, multispecies probiotics (OR, 0.33; 95% CI, 0.20–0.56; P<.0001; n=5,074) (Figure 2) significantly reduced CDI, whereas the effect of single-species probiotics was not statistically significant (OR, 0.41; 95% CI, 0.17–1.00; P=.051; n=5,074) (Figure 2). Compared to no probiotics, a 1 billion CFU per day increase in dose did not significantly reduce CDI risk (OR, 0.99; 95% CI, 0.99–1.00; P=.108; n=5,074) (Figure 2).
Post hoc, we conducted a subgroup analysis of 5 trials using S. boulardii (n=1,473), the probiotic most commonly used among RCTs for CDI prevention, and compared it with trials using other strains (n=5,381). We found no significant difference (OR, 0.86; 95% CI, 0.10–7.04) (Figure 2).
Sensitivity Analyses
When treating age as a categorical variable in the multivariate analysis, the effect of probiotics remained similar (OR, 0.35; 95% CI, 0.22–0.55; P<.0001) (Figure 2). In a post hoc analysis, we removed infants <1 years of age, which resulted in a similar summary estimate (OR, 0.37; 95% CI, 0.23–0.59; P<.0001) (Figure 2). Results remained similar for the 18 studies in the unadjusted model regarding random-effects aggregate-data meta-analysis (n=6,447 participants; OR, 0.45; 95% CI, 0.30–0.68; P<.0001; I2=8%), for the 13 studies included in the adjusted model (n=5,341 participants; OR, 0.42; 95% CI, 0.24–0.72; P=.002; I2=21%), and when using GEE for the adjusted analysis (OR, 0.47; 95% CI, 0.29–0.76; P=.0022) (Figure 2).
Secondary Outcome: Serious Adverse Events
Of the 14 studies reporting on the incidence of SAEs, 318 of 2,556 cases (12.4%) were in the intervention group and 295 of 2,434 cases (12.1%) were in the control group (Table 1). There was no significant difference between groups (OR 1.06; 95% CI, 0.89–1.26; P=.536) (Figure 3). There were no cases of bacteremia or fungemia, and none of the SAEs were deemed attributable to probiotics, based on published findings and correspondence with investigators. Similarly, there were no differences among the 11 studies with data available for the adjusted model (OR, 1.06; 95% CI, 0.89–1.28; P=.503) (Figure 3). Increasing age (OR, 1.03; 95% CI, 1.02–1.04; P<.0001), multiple antibiotic usage (OR, 1.60; 95% CI, 1.25–2.05; P=.0002), and being treated with high-risk antibiotics (OR, 1.36; 95% CI, 1.06–1.74; P=.015) were significantly associated with SAEs, whereas sex was not (Figure 3).

FIGURE 3 Incidence of serious adverse events.
DISCUSSION
Our IPD meta-analysis of 18 trials with data on 6,851 participants found that probiotics reduced the risk of CDI by two-thirds. The risk of SAEs was not significantly different between probiotics and control groups. Importantly, none of the SAEs were attributable to probiotics. The quality (certainty) in the effect estimates for CDI was moderate, downgraded due to a low number of events, while for SAEs the certainty was moderate, downgraded for risk of publication bias (Table 3).
TABLE 3 Summary of Findings Table Based on Adjusted AnalysisFootnote a

NOTE. CI, confidence interval; OR, odds ratio; GRADE, working group grades of evidence; CDI, Clostridium difficile infection; SAE, severe adverse event; IPD, individual participant data; RCT, randomized controlled trial.
a Patient or population: adults and children exposed to antibiotics. Settings: inpatient and outpatient. Intervention: probiotics.
b Risk for the control group represents mean rate across studies. Risk for probiotics group is calculated by applying the relative effect rate to the control risk.
c High quality: further research is very unlikely to change our certainty in the estimate of effect. Moderate quality: further research is likely to have an important impact on our certainty in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our certainty in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate.
d We rated CDI down for imprecision because of a low number of total events (<300).
e We rated SAEs down for risk of publication bias as we were only able to obtain IPD for 18 of 32 RCTs and among the IPD trials only 11 trials (n=4,718) could be included in our adjusted analysis.
Our results corroborate the findings in a previous aggregate-data meta-analysis.Reference Goldenberg, Ma and Saxton 12 , Reference Hempel, Newberry and Ruelaz 47 Recent clinical practice guidelines currently do not recommend probiotic prophylaxis, even though probiotics have the highest quality evidence among cited prophylactic therapies, including antibiotic stewardship, hypochlorite solutions, and bundle strategies.Reference Lytvyn, Mertz, Sadeghirad, Alaklobi, Selva, Alonso-Coello and Johnston 14
Some suggest that probiotics are most likely to be of benefit in high CDI incidence settings.Reference Colli, Pozzoni, Conte and Casazza 48 , Reference Brown, Fisman, Moineddin and Daneman 49 Data on disease pressure were not available; thus, we approximated the baseline CDI risk using the control group event rates of individual trials. This analysis suggested that CDI incidence ≥5% interacts with the overall group effect, suggesting that probiotics may be more effective when the baseline CDI risk is moderate to high. Considering the absolute risk reduction (ARR) and corresponding number needed to treat (NNT), if the baseline CDI risk is 1.6% (median of all studies), the observed effect (OR, 0.35) corresponds to an ARR of ~1.0% and an NNT of 96. Using the same effect estimate, given the mean baseline, CDI risk across all studies is 4.34%, and the ARR will be 2.8% (NNT, 35).
Combining different probiotic species and strains in meta-analyses is a contentious issue. Some suggested that pooling different probiotic agents is problematic,Reference Szajewska 50 while others suggested that pooling probiotics and subsequently assessing any differences using a priori subgroup analysis based on species, strain, and dose is in accordance with standard meta-analysis methods.Reference Goldenberg, Ma and Saxton 12 As with previous aggregate-data meta-analyses, we found no significant difference between species or strains, suggesting that specific effects based on the type of probiotic may not be an important factor to consider when choosing a probiotic. Instead, it is probably more important to consider the quality of the product and credibility of the manufacturerReference Zawistowska-Rojek, Zareba, Mrówka and Tyski 51 and to use a product that has been investigated in RCTs, particularly a multistrain product given our findings. However, as is typical of subgroup assessments, our analysis is likely underpowered. For example, we rated downward for precision for each of our outcomes. For single species and multispecies probiotics, our data suggest that while both reduce CDI compared to no probiotics, multispecies probiotics demonstrated a statistically significant treatment effect compared to no probiotics, whereas single-strain probiotics did not. One hypothesis is that multiple species may have an additive effect or may be associated with a higher dose. Among studies of multistrain probiotics,Reference Gao, Mubasher, Fang, Reifer and Miller 29 , Reference Hickson, D’Souza and Muthu 34 , Reference Plummer, Weaver, Harris, Dee and Hunter 38 – Reference Allen, Wareham and Wang 41 , Reference Ruszczyński, Radzikowski and Szajewska 46 the daily median value was 50 billion CFU per day; in studies with single-strain probiotics,Reference Duman, Bor and Özütemiz 30 – Reference Pozzoni, Riva and Bellatorre 33 , Reference Klarin, Wullt, Palmquist, Molin, Larsson and Jeppsson 35 – Reference Miller 37 , Reference Wong, Jamous and O’Driscoll 42 – Reference Lönnermark, Friman, Lappas, Sandberg, Berggren and Adlerberth 45 the daily median value was 15.6 billion CFU per day. However, in exploring the impact of dose response, we found no statistically significant trend. Failure to find a dose effect may represent the variability in dose administered (viable organisms per capsule) as well as the potential variability between studies with respect to whether the consumed dose survived gastric passage (viable organisms arriving in small and subsequently large bowel). Both are important areas of inquiry for future studies.
Our study has several strengths. First, we used a comprehensive search; we had a high response rate from study authorsReference Thomas, Radji and Benedetti 52 ; and we were able to obtain 18 of 32 trials, including the 2 of the largest trials to date,Reference Allen, Wareham and Wang 41 , Reference Ehrhardt, Guo and Hinz 44 one of which enrolled almost 3,000 participants.Reference Allen, Wareham and Wang 41 Our unadjusted effect estimates across primary and secondary outcomes were similar to studies for which we did not have IPD available, making the presence of a selection bias unlikely. Our analyses were robust to sensitivity analyses using different analytic methods, including categorization of age, aggregate-data meta-analyses, GEE analysis, and missing participant outcome data.
Several limitations must be emphasized. First, data for adjusted models were not available in ~25% of participants. However, as the estimates were similar for the unadjusted model and adjusted IPD model, and the aggregate-data meta-analysis, this is unlikely to have introduced a significant bias. Second, data were too limited to allow an examination of each class of antibiotic separately; thus, we categorized antibiotics as high risk versus not high risk.Reference Brown, Valenta, Fisman, Simor and Daneman 53 For the same reason, we were unable to follow our a priori protocol to adjust for other potentially important confounding factors such as length of antibiotic exposure and length of hospitalization stay. Third, missing participant outcome data was a common issue across trials. Our multiple imputation analysis, however, suggested that outcomes were similar for participants with and without missing data. The incidence of CDI may be underestimated, given that 71 of 760 participants (9%) with diarrhea were not tested for CDI. This affected participants in the intervention and control groups equally; thus, it is unlikely to have biased the results.
Moderate quality (ie, certainty) evidence suggests that probiotic prophylaxis is a useful CDI prevention strategy, particularly among participants taking 2 or more antibiotics and in hospital settings where the risk of CDI is ≥5%. There was no evidence for concern regarding serious adverse events related to the preparations evaluated. Patients may value the absolute risk reduction (1.0%; NNT, 96) we found, specifically among those probiotics with the largest body of evidence for efficacy and safety. The observation that probiotic prophylaxis is of greater benefit in populations with a moderate-to-high baseline risk should inform site selection in future studies, as should the use of probiotics in clinical settings.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: S.J.A. reports other support from Cultech Ltd, United Kingdom, grants and other from Yakult, United Kingdom, and personal fees from Astellas Pharma, outside the submitted work. H.S. reports grants from Arla and Biogaia, personal fees from Biogaia, Biocodex, Danone/Nutricia, Hipp, Nestle, Nestle Nutrition Institute, Sequoia, and Yakult, as well as nonfinancial support from Dicofarm and Sequoia, outside the submitted work. M.M. is an employee at BioMerieux. C.P.S. reports unrestricted grants from Ferring Pharmaceuticals during the conduct of the study as well as grants and personal fees from Warner Chilcott, grants and personal fees from Abbvie, personal fees from Dr Falk, Takeda, and M.A.S., outside the submitted work. B.C.J. reports a grant from BioK+ International for work outside the submitted work. All other authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.84.








