Pneumonia is the most common healthcare-associated infection (HAI) in the United States, and it accounts for almost 25% of all hospital infections.Reference Magill, Edwards and Bamberg1, Reference Magill, O’Leary and Janelle2 Healthcare-associated pneumonia poses a substantial burden on the healthcare system, with 14% mortality and an estimated cost of >$3 billion per year.Reference Klevens, Edwards and Richards3, Reference Zimlichman, Henderson and Tamir4 In the 1990s, 83% of healthcare-associated pneumonia cases were ventilator-associated (VAP); thus, the US Centers for Disease Control and Prevention (CDC) has traditionally focused surveillance efforts on VAP.Reference Klevens, Edwards and Richards3, Reference Calfee and Farr5
Recent studies have reported dramatic decreases in VAP rates over the past 15 yearsReference Klompas, Branson and Eichenwald6; however, the rate of non–device-associated pneumonia (ND pneumonia) remained stagnant during this period, and ND pneumonia now accounts for the majority of nosocomial pneumonia in hospitals settings.Reference Magill, Edwards and Bamberg1, Reference Weber, Sickbert-Bennett, Brown and Rutala7–Reference See, Chang and Gualandi12 Despite this growing body of literature on the burden and importance of ND pneumonia in hospitals, little is known about the risk factors for infection, and there are currently no evidence-based guidelines for ND pneumonia prevention.Reference Klompas13, Reference Passaro, Harbarth and Landelle14 In this study, we aimed to update current estimates of ND pneumonia rates and their frequency relative to VAP, to assess trends over time, and to identify potential risk factors.
Methods
Data sources and study population
Electronic medical records (EMRs) from adults (≥18 years old) admitted to the University of North Carolina (UNC) Hospitals between January 1, 2013, and December 31, 2017, were obtained from the Carolina Data Warehouse for Health (CDW-H), a central repository for clinical and administrative data from the UNC Healthcare System. Prisoners were excluded from analysis. Patients may have had multiple hospitalizations during the study period. HAIs were identified through the UNC Hospitals’ Epidemiology database, which included both device and non–device-associated HAIs, captured through comprehensive, hospital-wide active surveillance, in accordance with CDC case definitions and methodology.Reference Weber, Sickbert-Bennett, Brown and Rutala7, Reference Kanamori, Weber and DiBiase15 In July 2014, the UNC Hospital Department of Epidemiology began also capturing ventilator-associated events, a new surveillance concept that CDC developed as an alternative to traditional VAP surveillance.Reference Klompas16 The 2 databases were then deterministically linked using admission date, medical record numbers, and full name. This study was approved by the UNC Institutional Review Board.
Incidence of ND pneumonia
Quarterly incidence rates, per 10,000 hospitalization days, between 2013 and 2017 were calculated, and Poisson regression was used to assess a potential change in ND pneumonia rates over time. The proportion of pneumonia cases that were non–device related each year were also calculated. Cochran-Armitage trend tests (2-sided) were used to test the null hypothesis that the proportion of ND pneumonia cases did not change between 2015 and 2017 (after new definitions were implemented).
Risk factors for ND pneumonia
Only hospitalizations between 2015 and 2017 with a length of stay (LOS) >2 days were included in risk-factor analyses. Potential risk factors of interest included patient sex, age, comorbidities, body mass index (BMI), immunosuppression (including neutropenia), modified early warning score (MEWS), Morse fall scale, and inpatient medication usage (specifically, anesthetics, antibiotics, antipsychotics, benzodiazepines, opioids, and acid suppressing medications), total parenteral nutrition (TPN), chlorhexidine mouthwash, prior endotracheal ventilation, prior tracheostomy, intensive care unit (ICU) stay, and trauma admission.
Patient comorbidities were captured using the discharge diagnosis codes from the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) (January 2015–September 2015) and the ICD-10-CM (October 2015–December 2017) on each record. Deyo et al (1992)Reference Deyo, Cherkin and Ciol17 and Quan et al (2005)Reference Quan, Sundararajan and Halfon18 algorithms were adapted to identify components of the Charlson comorbidity index (CCI) score (Supplemental Table 1 online). Diagnosis codes for incident events (eg, acute myocardial infarction) were removed from all component definitions.Reference Deyo, Cherkin and Ciol17 Peripheral vascular disease (PVD) was also excluded due to low incidence (n = 201), and both malignancy (solid tumor or metastatic disease) and human immunodeficiency viruses (HIV) were incorporated into the broader classification of immunocompromised. Additionally, chronic pulmonary diseases were broken into more discrete categories: chronic bronchitis/emphysema, asthma, bronchiectasis, and other/unspecified chronic obstructive pulmonary diseases (COPD).
Immunocompromised patients were identified using the Advisory Committee on Immunization Practices (ACIP) and Infectious Diseases Society of America (IDSA) guidelines on which persons cannot receive live-attenuated vaccinations.19 Diagnoses of immunosuppressive conditions, which included HIV, neutropenia, organ transplant, and malignancy, were identified using discharge diagnosis codes, and relevant ICD-9-CM codes were identified using the Greenberg et al (2016)Reference Greenberg, Hohmann, Hall, Kress and David20 algorithm. The ICD-10-CM codes were identified using Centers for Medicare and Medicaid Services (CMS) generalized equivalence mapping (Supplemental Table 1 online). Patients receiving chemotherapeutic agents, corticosteroids, or immune-modulating agents within the first 2 days of their hospitalization were identified using inpatient medications (Supplemental Table 2 online). Neutropenia was identified using both diagnosis codes and laboratory blood test results within the first 2 hospitalization days (defined as ≥2 white blood cell [WBC] counts <500 cells/mm3).
The severity of illness and frailty were measured using the MEWSReference Subbe, Kruger, Rutherford and Gemmel21 and Morse fall scale,Reference Morse, Morse and Tylko22, Reference Morse, Black, Oberle and Donahue23 respectively. These metrics are both automatically calculated within the UNC Hospitals EMR. For the MEWS, specific vital signs are used to identify patients at risk for imminent deterioration: systolic blood pressure, heart rate, respiratory rate, temperature, conscious level, and hourly urine output (for 2 hours). The Morse fall scale includes history of falling, number of secondary diagnoses, whether ambulatory aid is needed, intravenous therapy/heparin lock, gait, and mental status. It is used to predict future falls in hospitalized patients. For each patient, the first MEWS and Morse fall scale score within the first 2 days of admission was captured and categorized using the clinically relevant cutoff points: MEWS, <1 (reference), 2, 3, ≥4 and Morse fall scale, 0 (reference), 1–24, 25–45, >45. A MEWS ≥4 and a Morse fall scale score >45 were both considered indicators of severe illness.
We treated ICU stay, inpatient medications, chlorohexidine mouthwash, TPN, and mechanical ventilation as time-varying exposures, and we considered the patient exposed for the remainder of the hospitalization. For example, once a patient received antibiotics on day 4, they would be considered exposed from day 4 until discharge and classified as unexposed on days 1–3. All medications were identified using orders captured in the EMR, and receipt was confirmed using the medication administration record (Supplemental Table 2 online).
Multivariable Cox proportional hazards regression was used to simultaneously estimate the association between each potential risk factor and the incidence of ND pneumonia. Correlation between repeat hospitalizations of the same patients were taken into account by utilizing robust sandwich covariance matrix estimates as described by Lee et al (1992).Reference Lee, Wei, Amato, Leurgans, Klein and Goel24 Both VAP and inpatient mortality were treated as competing risks using the Fine and Gray (1999)Reference Fine and Gray25 model.
Due to missing BMI values (n = 15,146, 17%), MEWS (n = 18,761, 21%), Morse fall scale (n = 8,571, 10%), and location/discharge disposition (n = 8,482, 10%), inverse probability of missing weights (IPMW) were calculated.Reference Seaman and White26 Weights were estimated using multivariable logistic regression, which modeled the probability of being a complete case as a function of the year and season of admission, cause of admission, patient age, sex, Charlson score comorbidities (excluding HIV and cancer), immunosuppression, TPN, medication usage anytime during hospitalization (antibiotics, antipsychotics, local anesthetics, general anesthetics, benzodiazepines, opioids, alpha-2 agonists, nonsteroidal anti-inflammatory drugs [NSAIDs], calcium channel blockers, statins, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARBs], histamine-2 agonists, proton pump inhibitors), device use (urinary catheter, ventilator, central venous catheter, peripheral venous catheter), whether they underwent any surgery (CPT 10021 – 69990), and LOS, as well as interaction terms between admission timing and both cause of admission and LOS. Age and LOS were modeled as restricted quadratic splines.Reference Howe, Cole, Westreich, Greenland, Napravnik and Eron27 Because 99% of hospitalizations from January through March 2015 were missing MEWS (28% of all missing), all hospitalizations in this time period were excluded.
Our statistical analysis strategy is consistent with the American Statistical Association’s statements on P values.Reference Wasserstein and Lazar28, Reference Wasserstein, Schirm and Lazar29 All statistical computations were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
From 2013 to 2017, there were 163,386 hospitalizations (97,485 unique patients) and 771 cases of healthcare-associated pneumonia (520 ND pneumonia and 191 VAP) during 666 unique hospitalizations at UNC Hospitals. Of the 771 pneumonia cases, 768 (99.6%) were successfully linked to a hospitalization record. Of hospitalizations, 87% (n = 142,836) were >2 days (median, 5 days; interquartile range [IQR], 3–8 days). Patient demographics and causes of admission are described in Table 1.
Table 1. Hospitalization Characteristics
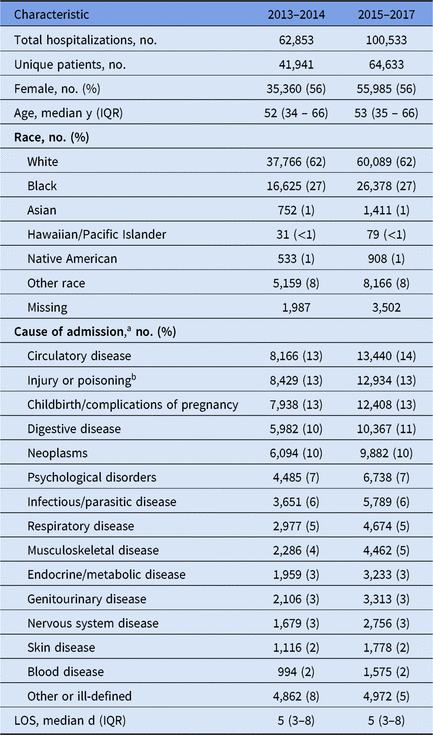
Note. IQR, interquartile range; LOS, length of stay.
a Classified using primary diagnosis on each hospitalization; 2,341 hospitalizations (1%) were unable to be linked to their diagnosis codes
b A subset of these codes were used to identify trauma admissions.
Median time to diagnosis was 9 days for ND pneumonia (IQR, 5–19) and 11 days for VAP (IQR, 6–21). Between 2013 and 2017, the rate of ND pneumonia was stable, with 4.15 ND pneumonia cases per 10,000 hospitalization days in 2013 and 4.54 ND pneumonia cases per 10,000 hospitalization days in 2017 (P = .65) (Fig. 1A). In 2013–2014 (prior to the implementation of the CDC’s updated definition for VAP), >80% of pneumonia cases were non–device associated. Between 2015 and 2017 (after the CDC ventilator-associated event definition was implemented), the proportion of non–device infections ranged from 64% to 74% (P = .09) (Fig. 1B). From 2015 to 2017, only 36% (n = 107) of ND pneumonia cases occurred in the ICU; 45% (n = 137) occurred on a floor; and 18% occurred on a stepdown unit (n = 55) (25 events could not be attributed to a specific location due to missing location data). In comparison, 96% of all VAPs occurred in an ICU (n = 140); however, this represents only 57% of all pneumonia cases in ICUs. The 30-day and 60-day cumulative incidences of ND pneumonia were 19.7 and 41.7 infections per 10,000 patients, respectively (Fig. 2).
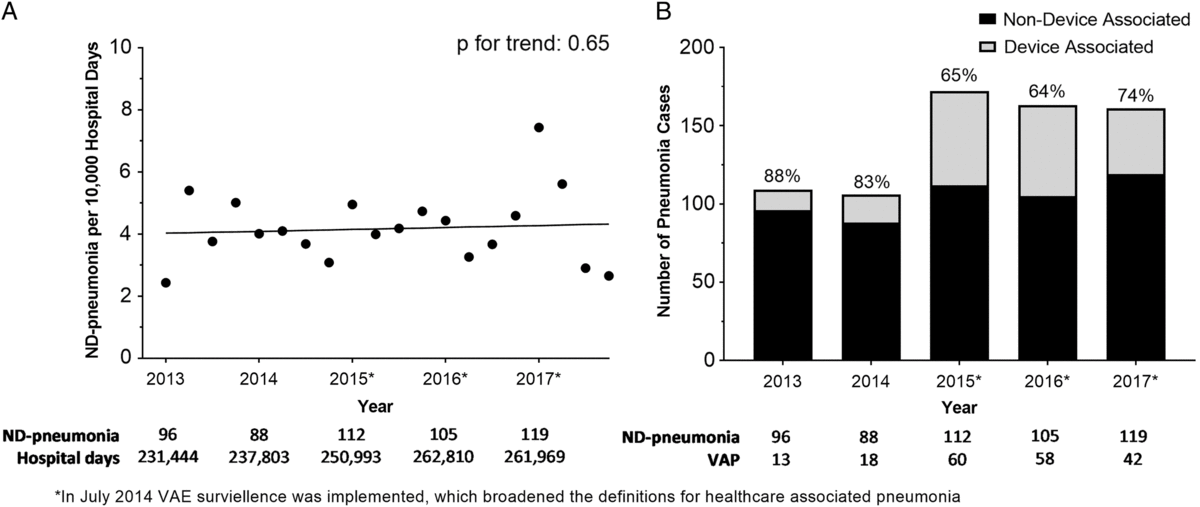
Fig. 1. (A) Quarterly rates of non–device-associated pneumonia (ND pneumonia), per 10,000 hospitalization days. (B) Proportion of hospital-associated pneumonia that are device associated and non–device associated, stratified by year.
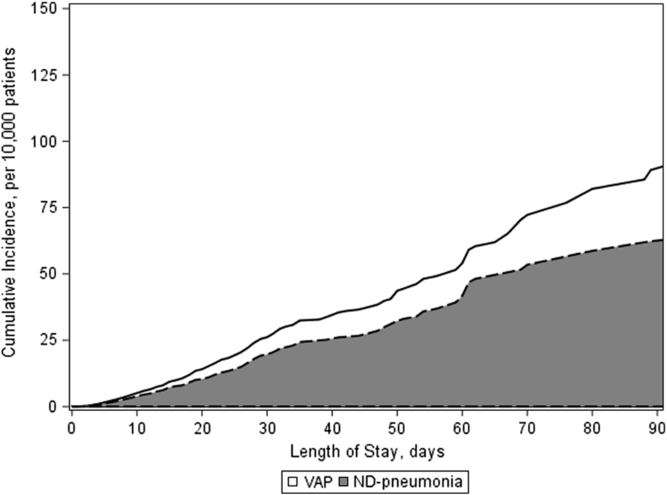
Fig. 2. Stacked cumulative incidences of non–device-associated pneumonia (ND pneumonia, gray) and ventilator-associated (VAP, white) pneumonia.
There were 88,487 hospitalizations between 2015 and 2017 with an LOS >2 days included in the risk factor analysis. Median IPMW was 1.07 (IQR, 1.03–1.24; range 1.00–21.38), and only 27 hospitalizations had a weight >10. After adjustment, male sex and older age were associated with increased incidence of ND pneumonia (Table 2). Additional risk factors included diagnoses for bronchitis or emphysema (hazard ratio [HR], 2.07; 95% confidence interval [CI], 1.40–3.06), congestive heart failure (HR, 1.48; 95% CI, 1.07–2.05), paralysis (HR, 1.72; 95% CI, 1.09–2.73), and immunosuppression (HR, 1.54; 95% CI, 1.18–2.00). Being in an ICU was also associated with increased ND pneumonia (HR, 1.49; 95% CI, 1.06–2.09). Conversely, patients with dementia were at lower risk of infection (HR, 0.41; 95% CI, 0.18–0.95). We did not detect a change in ND pneumonia risk for the following variables: use of chlorhexidine mouthwash, TPN, illness severity measures, all medications of interest, and prior ventilation variables. For chlorhexidine mouthwash, for example, although the hypothesis test of “HR = 1” was inconclusive, the point and interval estimates (HR, 0.90; 95% CI, 0.54–1.52) indicated that the data were compatible with hazard ratios as small as 0.54 and as large as 1.52 in the target population.Reference Wasserstein, Schirm and Lazar29
Table 2. Prevalence and Effect of Potential Factors for ND Pneumonia, Among Adults Hospitalized for >2 Days Between 2015 and 2017
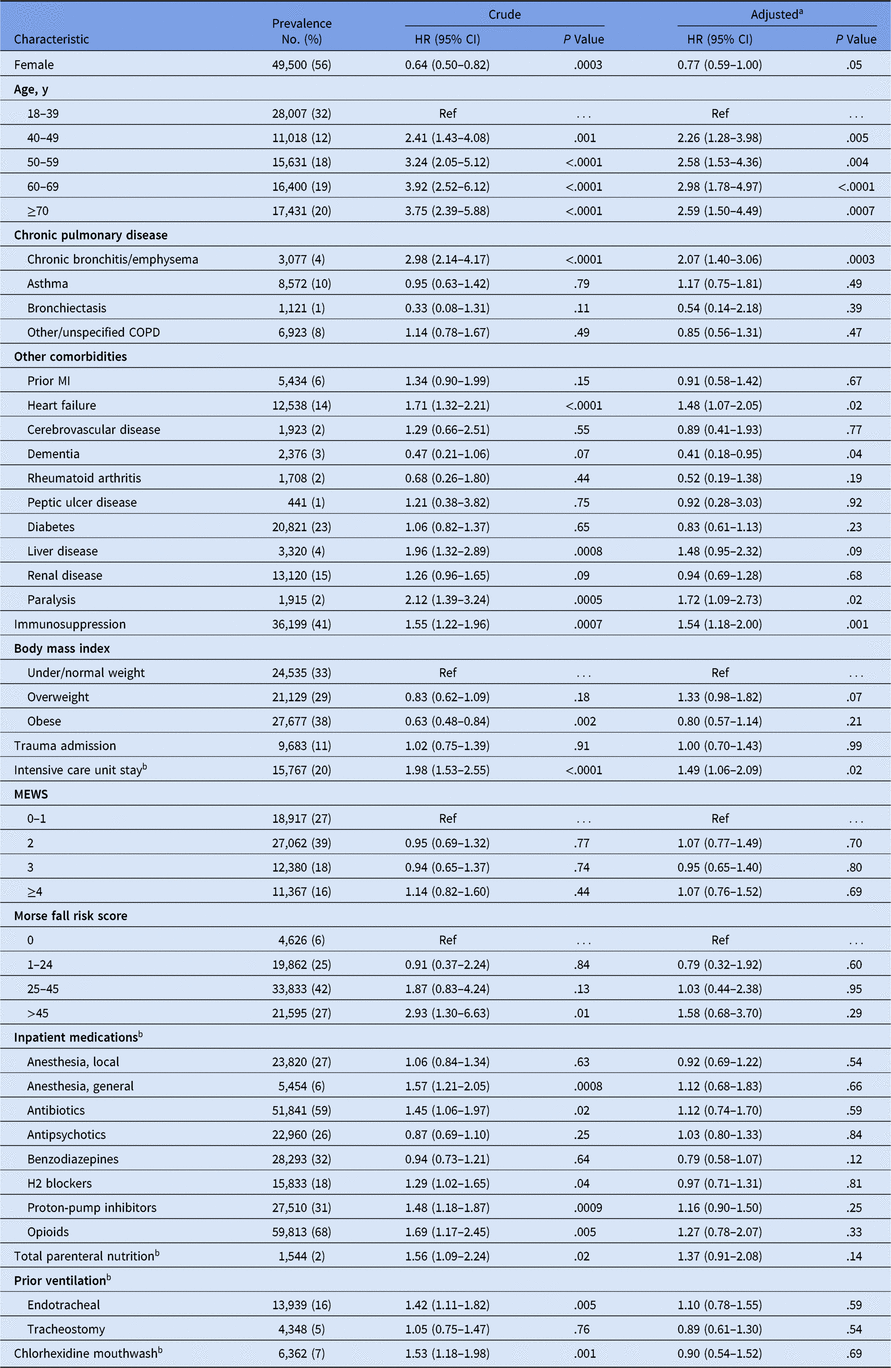
Note. HR, hazard ratio; CI, confidence interval; Ref, reference; MI, myocardial infarction; MEWS, modified early warning system; H2 blockers, histamine-type 2 receptor blockers.
a Adjusted for all risk factors in the table above; correlation between repeat hospitalizations of the same patients were taken into account using a robust sandwich covariance matrix and inpatient mortality was treated as a competing risk using the Fine and Gray model. Inverse probability of missingness weights were used to account for missing data.
b Treated as a time-varying exposure; patients were considered exposed for the remainder of the hospitalization.
Discussion
Between 2013 and 2017, the rate of ND pneumonia cases remained constant in UNC Hospitals, and non–device infections continue to account for most hospital-associated pneumonia cases. Risk factors for ND pneumonia included male sex, older age, ICU admission, and chronic bronchitis/emphysema, congestive heart failure, paralysis, and immunosuppression. To the best of our knowledge, this is the most comprehensive analysis of ND pneumonia rates and risk factors in an acute-care setting.
The incidence and proportion of ND pneumonia cases in our study are similar to rates reported by other centers. Davis and Finley,Reference Davis and Finley9 for example, utilized state-mandated comprehensive surveillance data from Pennsylvania and found that 71% of cases of pneumonia between 2009 and 2011 were non–device associated (5,597 ND pneumonia, 2,299 VAP). In a 2014 convenience sample of 21 hospitals across the United States, the rate of ND pneumonia ranged from 0.12 to 2.28 cases per 1,000 patient days.Reference Baker and Quinn11 Our findings are also consistent with a prior report from our institution. Between 2008 and 2012, the proportion of non–device-associated pneumonia cases increased from roughly 40% to 60%, predominantly due a decrease in device-associated infections without concomitant change in the incidence of non–device-associated infections.Reference DiBiase, Weber, Sickbert-Bennett, Anderson and Rutala8
Older age, pulmonary disease, and ICU stays have been associated with increased risk of VAP.Reference Chastre and Fagon30 In our study, these same risk factors extended to ND pneumonia as well, albeit with some slight differences. Middle-aged adults (40–49 and 50–59 years old) were at higher risk than patients 18–39 years old, and only specific types of pulmonary disease (bronchitis and emphysema) had an increased risk (differences in ND pneumonia risk among patients with asthma, bronchiectasis, and other/unspecified COPD were not detected). In our study, paralysis was associated with increased incidence of ND pneumonia. Paralytic agents and coma/stupor have been associated with VAP, suggesting that limited mobility may increase a patient’s risk.Reference Chastre and Fagon30, Reference Rello, Ollendorf and Oster31 Patients with paralysis may also have other neurological issues (eg, impaired swallowing or decreased level of consciousness) that could also account for the increased pneumonia risk. Male sex, chronic pulmonary disease, and congestive heart failure have also been shown to increase the risk of community-acquired pneumonia in older adults.Reference Jackson, Neuzil and Thompson32 Overall, these finding suggest that certain characteristics may predispose some adults to healthcare-associated pneumonia.
Interestingly, we did not detect an association between acid-suppressing medication (ie, histamine-2 agonists and proton pump inhibitors [PPIs]), although they have been associated with increased risk of VAP.Reference Chastre and Fagon30 However, the literature for VAP is mixed. Additionally, evidence-based guidelines and awareness of potential adverse effects have narrowed indications for acid suppression therapies in the acute-care setting, and UNC Hospitals implemented changes to the clinical guidelines for stress ulcer prophylaxis prior to the study period. Similarly, antipsychotics have also been associated with aspiration pneumonia in both the hospitalReference Herzig, LaSalvia and Naidus33 and community setting,Reference Dzahini, Singh, Taylor and Haddad34 but we did not detect an association between antipsychotic use and ND pneumonia.
A few recent studies have reported that the use of oral care prevented ND pneumonia in high-risk patients (eg, neurologic injury patients, older adults).Reference Warren, Medei, Wood and Schutte35–Reference Munro, Haile-Mariam, Greenwell, Demirci, Farooqi and Vasudeva37 Although we did not detect an association between chlorhexidine mouthwash and ND pneumonia rates, this discrepancy could be due to targeted use of oral care with chlorhexidine at UNC Hospitals. In contrast to its universal use in prior studies, only 8% of hospitalized patients included in this analysis received this treatment. These individuals may have been selected to receive oral care with chlorhexidine specifically due to perceived higher risk for respiratory infections (although indication for chlorhexidine could not be determined), which could explain our inconclusive finding. Chlorhexidine mouthwash has also been associated with increased inpatient mortality.Reference Deschepper, Waegeman, Eeckloo, Vogelaers and Blot38 Studies assessing the utility of chlorhexidine mouthwash and oral care with VAP are mixed, and currently there are no recommendations on its use for device-associated infections.Reference Klompas, Branson and Eichenwald6
This study has several limitations. First, we were unable to assess causality, and the risk factors we identified may only be indicators for the true underlying mechanisms. Second, this was a single-center study and our results may not generalize to other hospitals, particularly if the patient population is different. We also did not account for duration, dose, or underlying indications for medication use. Additionally, ICD-9-CM and ICD-10-CM codes were used to identify comorbidities. Using these codes likely underestimates the prevalence of comorbidities, although we expect this misclassification to be nondifferential and, if anything, to bias results toward the null. Similarly, ND pneumonia and VAP were captured using CDC definitions, some of which require laboratory confirmation, and patients who are treated for suspected pneumonia but are not cultured would be missed. Finally, although we had a large sample size, the incidence of ND pneumonia and prevalence of some risk factors were low, resulting in low levels of precision of the estimators as indicated by the widths of the observed confidence intervals.
In conclusion, between 2013 and 2017, the incidence rate of ND pneumonia was unchanged, and non–device infections represent the majority of healthcare-associated pneumonia cases in our acute-care hospital. Our study and similar findings by others suggest that hospital infection prevention programs should consider expanding the scope of surveillance and prevention programs to include nonventilated patients. Male sex, older age, bronchitis/emphysema, congestive heart failure, paralysis, immunosuppression, and ICU stays were all associated with increased risk of ND pneumonia. Future research should continue to look for modifiable risk factors and assess potential prevention strategies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.300
Acknowledgments
The authors would also like to thank Adam M. Lee at the NC TraCS Institute for his help querying and obtaining EMR data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support
This project was supported by the National Center for Advancing Translational Sciences (NCATS), NIH (grant no. UL1TR002489).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.






