Urinary tract infections (UTIs) account for an estimated 14%–23% of healthcare-associated infections (HAIs) in the United States, with most of these infections occurring in individuals with indwelling urinary catheters.Reference Klevens, Edwards and Richards1–Reference Magill, Hellinger and Cohen3 Although surveillance activities focus on catheter-associated UTIs (CAUTIs), this surveillance does not fully capture the incidence of HA-UTI overall. Current knowledge of hospital-associated UTIs is also largely based on surveillance and epidemiologic studies in hospitalized patients. For instance, the National Healthcare Safety Network (NHSN) routinely monitors healthcare-associated (HA), hospital-onset (HO) UTIs but does not require facilities to report infections with onset after discharge.4 Recently discharged patients are theoretically still at risk for hospital-associated UTI. As facilities encourage shorter hospital stays to meet efficiency and quality measures, these HAIs may be more likely to become symptomatic after discharge.
Scant data exist to describe the incidence or risk factors associated with hospital-associated UTIs following discharge. This absence represents a critical barrier to identifying patients at high risk for hospital-associated, community-onset (CO) UTIs for targeted infection prevention measures. In our study, we sought to estimate the incidence of HA-CO UTIs and to identify potential risk factors.
Methods
Study design and patient population
This retrospective cohort study was conducted at Oregon Health and Science University, a 556-bed academic, quaternary-care healthcare center in Portland, Oregon, that also serves as a regional referral center. Patients age 18 years and older admitted to the Department of Family Medicine service between May 2009 and December 2011 with a primary care provider in the Department of Family Medicine were eligible for inclusion. The Department of Family Medicine includes an active inpatient service that predominantly manages patients receiving primary care at 1 of 4 family medicine outpatient clinics in the greater Portland area. The department aims to schedule follow-up visits with their inpatients within 2 weeks of discharge.
We excluded patients with a history of UTI in the 30 days before admission, identified as (1) an acute UTI diagnosis (based on International Classification of Diseases, Ninth Revision (ICD-9) codes 590.1x, 590.8x, 590.9, 595.0, 595.4, 595.89, 595.9, 597.80, 597.81, 599.0, or 996.64; (2) positive urine microbiology culture (ie, urine cultures with growth of ≥ 10,000 CFU/mL, < 3 pathogenic bacteria isolated, and taxonomy identified to the genus level; and (3) nitrofurantoin prescription in the 30 days before admission. Additionally, patients with a chronic UTI diagnosis (ICD-9 code 590.0, 590.3, 595.1, 595.2, 595.81, or 595.82) in the year preceding admission or during hospitalization were excluded. Only the first eligible admission was included for patients with repeat hospitalizations during the study period. The study was approved by the Oregon Health and Science University Institutional Review Board.
Data collection
Patient data were abstracted from the Pharmacy Research Repository (PHARR), a repository developed and maintained in collaboration with the Oregon Clinical and Translational Research Institute. PHARR includes electronic health record (EHR) data and associated databases. Abstracted data include patient characteristics and demographics as well as information related to encounters, diagnoses, and symptoms based on ICD-9 codes; laboratory tests including microbiology cultures; medication orders; and surgeries for admissions and outpatient visits to OHSU. Data were collected during patients’ hospitalization, the preceding year (medical history), and during the 30 days after discharge.
Variable definitions
We identified hospital-associated UTI using ICD codes, urinary antibiotic prescriptions (ie, nitrofurantoin, ertapenem, imipenem, amoxicillin-clavulanate, ampicillin-sulbactam, moxifloxacin, ciprofloxacin, levofloxacin, trimethoprim-sulfamethoxazole, or trimethoprim alone), and positive urine microbiology culture results. Patients with HA-HO UTI were excluded from primary analyses, but their data were quantified for descriptive purposes. Patients meeting one of the following criteria during their hospitalization and at least 48 hours after admission were defined to have a HA-HO UTI: (1) nitrofurantoin prescription, (2) acute UTI diagnosis and other non-nitrofurantoin urinary antibiotic prescription, (3) positive urine culture and other non-nitrofurantoin urinary antibiotic prescription, or (4) acute UTI diagnosis and positive urine culture.
Our primary outcome was HA-CO UTI in the 30 days following hospital discharge. Individuals without a UTI during their index hospitalization were considered to be at risk. HA-CO UTI outcome definitions were dependent on treatment setting (ie, infections treated at inpatient versus outpatient encounters) and are summarized in Table 1. For readmitted patients, HA-CO UTI was defined as patients with hospital-associated UTI criteria in the first 48 hours of that subsequent inpatient admission. Readmitted patients were censored from follow-up after the first 48 hours of that subsequent inpatient admission. For patients with outpatient visits during the follow-up period, HA-CO UTI was defined as patients’ first incident UTI within 30 days meeting any of the following criteria: (1) a nitrofurantoin prescription, (2) acute UTI diagnosis, (3) positive urine culture or positive urinalysis with any urinary antibiotic prescription, or (4) positive urine culture, positive urinalysis, and dysuria (ICD-9 code 781.1). Chart review was performed to validate our definition of HA-CO UTI. Our case definition performed with 100.0% (95% confidence interval [CI]: 96.0%–100.0%) sensitivity and 88.1% (95% CI%–92.8%) specificity compared to chart review for identifying true symptomatic UTI. Each patient was included only once in our analyses. Illustrative examples of outcome classification are provided in Fig. 1.

Fig. 1. Illustrative examples of identification of cases of healthcare-associated, community-onset urinary tract infections. Abbreviations: UTI, urinary tract infection; HA-CO, healthcare-associated, community-onset UTI; HA-HO, healthcare-associated, hospital-onset UTI.
Table 1. Criteria for Identifying Cases of Healthcare-Associated, Community-Onset Urinary Tract Infection by Healthcare Setting

a Within 3 days of admission.
b Between −1 and 3 days of visit.
Potential predictors of HA-CO UTI were identified from patient characteristics, social history, and medical history from the qualifying hospitalization and medical history from the preceding year including prior antibiotic prescriptions and comorbidities. A weighted summary score for the Elixhauser comorbidity measure was calculated for each patient’s index hospitalization.Reference Elixhauser, Steiner, Harris and Coffey5–Reference Thompson, Fan and Dalton7 To explore the urban versus rural place of residence as a potential predictor, patient zip code was combined with zip code–based rural–urban commuting area codes, then aggregated into urban and rural categories as recommended by the Washington–Wyoming–Alaska–Montana–Idaho Rural Health Research Center.Reference Hart and Cromartie8,9
Statistical analyses
Patient characteristics were summarized with descriptive statistics. The 30-day cumulative incidence was calculated and expressed per 1,000 patients for HA-HO and for HA-CO UTI. We performed a Kaplan-Meier analysis based upon time to HA-CO UTI diagnosis following hospital discharge. Multivariable logistic regression was performed to identify potential risk factors for HA-CO UTI using patient data from the year prior to admission and during hospitalization. Variables significantly associated with the outcome (P < .05) and those with a confounding effect were retained in the model. Variables were considered confounders if their inclusion in the final model resulted in a ≥ 20% change in the odds ratio between covariates and outcome. We also tested for the presence of interaction between sex and indwelling catheterization, which had been hypothesized a priori. Adjusted odds ratios (aORs) and 95% CIs were calculated from the final model. Data management and statistical analyses were conducted in SAS statistical software version 9.2 software (SAS Institute, Cary, NC) and R version 3.1.2 software (R Foundation for Statistical Computing, Vienna, Austria).10
Results
Overall, 3,617 patients were evaluated for inclusion in our study. Of these, 309 (8.5%) were excluded due to chronic or acute UTI, positive urine culture, or nitrofurantoin prescription in the 30 days preceding hospitalization or presence of UTI criteria within 48 hours of admission, leaving 3,308 patients at risk of HA-UTI. Also, 35 patients had an acute UTI diagnosis between 48 hours post admission and discharge from their initial hospitalization and were categorized as having a HA-HO UTI. These individuals, and the 221 patients with no documented follow up with the OHSU healthcare system in the 30 days following discharge, were removed from analysis. After these exclusions, 3,052 individuals remained at risk for HA-CO UTI. Of these patients, 91 (3%) met the case definition of HA-CO UTI in the 30-day follow-up period (Fig. 2).
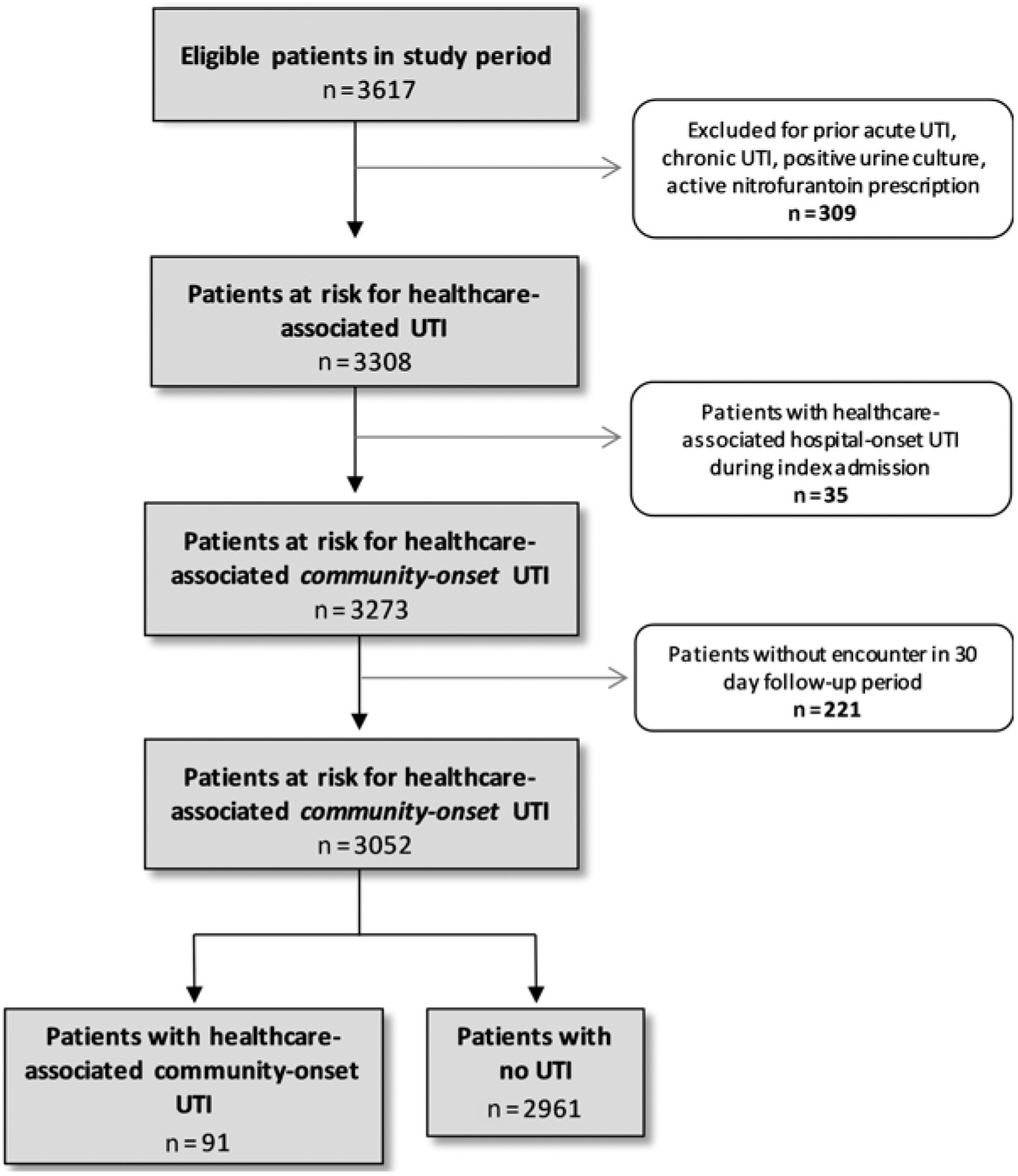
Fig. 2. Identification of cohort and healthcare-associated urinary tract infections (UTIs).
Patient characteristics are described in Table 2. Most patients were female (68.6%), white (87.0%), and non-Hispanic (95.1%). More than half of patients were hospitalized for 3 or more days during their initial admission. Based upon Medicare Severity-Diagnosis Related Groups (MS-DRGs), the 3 primary reasons for admission were diseases or conditions related to the female reproductive system (29.6%), surgeries and procedures (16.4%), and cancer (11.3%).
Table 2. Cohort Demographics and Characteristics

Note. UTI, urinary tract infection; HIV, human immunodeficiency virus; STI, sexually transmitted infection; OHP, Oregon Health Plan; MRSA, methicillin-resistant Staphylococcus aureus.
a Assessed during index admission.
b Assessed in the year preceding index admission.
c Assessed in the year preceding index admission except for acute UTI, which was evaluated in the preceding year up to 30 d before index admission.
The incidence of HA-HO UTI was 10.6 per 1,000 patients. In the postdischarge period, 91 patients were diagnosed with a UTI, yielding an incidence rate of 29.8 per 1,000 patients for HA-CO UTI. Thus, most (72.2%) hospital-associated UTIs were diagnosed after patients were discharged. We used a Kaplan-Meier survival curve to better understand the relationship between time after discharge and UTI diagnosis for patients at risk for HA-CO UTI (Fig. 3). The rate of UTI diagnosis was fairly consistent across the 30-day period; 33% were diagnosed within the first week post discharge and within 2 weeks, 60% of patients were diagnosed (Fig. 3).

Fig. 3. Kaplan-Meier Curve depicting time to Healthcare-associated UTI post hospital discharge among those patients with this outcome.
Among the HA-CO UTI patients with available positive culture data, Escherichia coli was isolated in 43.4% of positive cultures, followed by Enterococcus spp (15.1%), and Klebsiella spp (11.3%). The resistance profiles among E. coli isolated from the HA-CO cases were comparable to those isolated from those patients who had been excluded due to HA-UTI with onset during the index hospitalization (Table 3).
Table 3. Antimicrobial Susceptibilities of Escherichia coli Isolated From Patients With Healthcare-Associated Urinary Tract Infections

Note. TMP/SMX, trimethoprim/sulfamethoxazole.
Table 4 presents the results of the multivariable risk factor model. Paraplegia or quadriplegia was a strong independent predictor (aOR, 4.6; 95% CI, 1.2–18.0) of HA-CO UTI in the 30 days following hospital discharge in the multivariable logistic regression model. Other risk factors of HA-CO UTI included history of urine retention (aOR, 3.7; 95% CI, 1.9–67.5), history of uropathy or other urinary tract abnormalities (aOR, 3.1; 95% CI, 1.3–6.9), female sex (aOR, 2.9; 95% CI, 1.6–5.2), history of acute UTI (aOR, 2.0; 95% CI, 1.2–3.4), prior piperacillin-tazobactam prescription (aOR, 2.3; 95% CI, 1.1–4.5), indwelling catheter at index hospitalization (aOR, 1.5; 95% CI, 1.0–2.3), and prior penicillin/penicillin combination prescription (aOR, 1.7; 95% CI, 1.0–2.8). Private insurance was found to be protective of HA-CO UTI (aOR, 0.6; 95% CI, 0.4–0.9).
Table 4. Independent Predictors of Potentially Healthcare-Associated Urinary Tract Infections (UTIs) Diagnosed Within 30 Days Post Hospital Discharge

Note. aOR, adjusted odds ratio; CI, confidence interval.
a Penicillin-class antibiotics were classified to included penicillin, oxacillin, nafcillin, and dicloxacillin.
Discussion
The results of our single-center, retrospective cohort study suggest that current surveillance strategies that focus on HO CAUTI may not capture the full patient burden of HA-UTI, particularly those infections with onset post discharge. Among UTI cases with positive culture results, we observed that HA-CO UTI are similar to HA-HO UTI with respect to uropathogen and antibiotic susceptibility distributions, which indicates that patients developing a UTI following hospital discharge may necessitate different treatment strategies than patients with typical community-onset infection. Although these data cannot conclusively establish that the post-discharge infections were acquired in hospital, the evidence is sufficient to warrant further study.
Many of the risk factors we identified for HA-CO UTI (eg, multiple sclerosis, para/quadriplegia, history of urine retention) are known risk factors for UTI, even in the absence of hospitalization. Thus, it is unclear whether these predictors are contributing to any added risk during hospitalization. Other significant predictors are associated with increased healthcare exposure (eg, history of antibiotic use, urinary catheterization), which makes identifying their role in the causal pathway more challenging. We also identified private insurance as protective for HA-CO UTI, which may indicate that patients with lower socioeconomic status are at increased risk of HA-CO UTI. This potential disparity in patient outcomes warrants further investigation.
A primary limitation this study is the misclassification of cases of HA-CO UTI. Although our algorithm for identifying UTI cases was validated via chart review, the possibility of misclassification remains. Approximately one-quarter of HA-CO UTI cases that were cultured yielded negative cultures results. Because the treating provider in these cases chose to initiate treatment based on symptom presentation, we classified these cases as UTIs. We selected the 30-day follow-up window to allow for a more sensitive strategy for outcome ascertainment; this likely sacrifices some specificity to our definition, as the likelihood that the case was truly hospital-acquired would diminish over time. Although we used pathogen characteristics to help provide evidence for the setting of acquisition, our geographic region has a lower prevalence of multidrug-resistant Enterobacteriaceae than other regions of the United States, and the difference in uropathogen and antibiotic susceptibilities between healthcare-associated and community-associated UTIs may be more similar than in other areas. Further work is needed to assess the validity of our results in regions with higher rates of resistance in uropathogens.
Although the risk of device-associated infections such as catheter-associated UTI may, at least in theory, be easier to reduce than some other HAIs, all HAIs represent an undesirable patient outcome. Thus, if HAI risk persists post discharge, HAI research should focus more broadly than current surveillance definitions. Without a broader evidence base, it may be difficult to stimulate innovation within HAI prevention. Because HA-UTIs represent a large proportion of overall HAIs, further effort is warranted to better capture the burden of HA-UTI to better inform patient care and infection prevention efforts.
Author ORCIDs
Jessina C McGregor, 0000-0002-6093-5444
Acknowledgments
We thank Robin Singh, Christiane Winter, and the data analysts at OHSU Family Medicine for their assistance with data collection.
Financial support
This work was supported by the Agency for Healthcare Research and Quality (grant no. 1 R03 HS020970) to J.C.M. and the Oregon Clinical and Translational Research Institute, which is funded by the National Institutes of Health National Center for Advancing Translational Sciences (grant no. UL1TR000128).
Conflicts of interest
J.C.M. has received research funding from Merck & Co (Kenilworth, NJ). All other authors report no conflicts of interest relevant to this article.









