Enterobacteriaceae, namely Escherichia coli and Klebsiella pneumoniae, are the most common human pathogens causing a range of infections that include urinary tract infections,pyelonephritis, pneumonia, meningitis, and bloodstream infections (BSIs).Reference Nordmann, Dortet and Poirel 1 , Reference Tzouvelekis, Markogiannakis, Psichogiou, Tassios and Daikos 2 Carbapenemase-producing Enterobacteriaceae (CPE) are Enterobacteriaceae that are resistant to carbapenem antimicrobials through the production of carbapenemase enzymes. Carbapenems are the most broad-spectrum β-lactam antimicrobials active against gram-negative organisms, including Enterobacteriaceae, and they have been used successfully as the last form of treatment since their introduction in the early 1980s.Reference Peleg and Hooper 3 – Reference Naas, Vandel, Sougakoff, Livermore and Nordmann 5 The emergence of carbapenem-resistant Enterobacteriaceae (CRE), therefore, severely limits the available treatment options for patients with these infections. Furthermore, the production of carbapenemase enzymes is found on mobile genetic elements, which increases the transmission potential of CPE.Reference Queenan, Torres-Viera and Gold 6 , Reference Watanabe, Iyobe, Inoue and Mitsuhashi 7 The widespread influence of Enterobacteriaceae-related infections coupled with the limited antimicrobial therapy options for carbapenem-resistant (CR) organisms, and the transmission potential of carbapenemase enzymes, further emphasizes the significant threat of CPE not only to infected patients but also to public health.
CPE infections are associated with considerable mortality, approaching 60% in the published literature.Reference Navarro-San Francisco, Mora-Rillo and Romero-Gómez 8 Although the development of antimicrobial resistance (AMR) has been documented for decades, the extensive rise in the number of immunocompromising conditions, like diabetic patients and individuals who have undergone organ transplantation, makes the body an easy target for hospital-acquired infections, thereby contributing to further spread of AMR. The impact of AMR, therefore, extends into all aspects of medicine and threatens the significant progress that has been made in managing patients with complex conditions including transplantation, oncology, and surgery. In addition to deleterious implications for infected individuals, there are implications for the healthcare system. In the United States, the additional annual costs of managing infections caused by resistant organisms as compared to susceptible organisms are estimated between US$21 and 34 billion.Reference Roberts, Hota and Ahmad 9 , Reference Mauldin, Salgado, Hansen, Durup and Bosso 10
Due to the relatively recent emergence, the long-term health outcomes associated with CPE-related illnesses are not well described. There currently exist only two CPE health outcome-related systematic reviews that describe CPE mortality rates.Reference Xu, Sun and Ma 11 , Reference Tzouvelekis, Markogiannakis, Piperaki, Souli and Daikos 12 However, the review by Tzouvelekis et alReference Tzouvelekis, Markogiannakis, Piperaki, Souli and Daikos 12 did not include a control group and therefore, it is not possible to determine the attributable mortality of CPE infection. The study by Xu et alReference Xu, Sun and Ma 11 only compared crude mortality rates between CRE-infected patients and a carbapenem-suseptible control group. They reported mortality due to CPE in a subgroup analysis, but it was limited to the production of only two carbapenemase enzymes Klebsiella pneumoniae Carbapenemase (KPC) and Verona integron-encoded metallo-β-lactamase (VIM) in only K. pneumoniae. Furthermore, no systematic reviews report the impact of CPE infections on patient outcomes other than mortality. An understanding of other CPE-related health outcomes, including health-related quality of life (HRQoL), can highlight the severity of these infections in the medical community and support physicians with tertiary prevention efforts. Therefore, we conducted a systematic scientific literature review to synthesize current evidence on short and long-term health outcomes and HRQoL attributable to CPE infections.
Methods
Search strategy, information sources, and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in the conduct of our systematic review (Appendix 1 online).Reference Moher, Liberati and Tetzlaff 13 A protocol was developed a priori and was published on PROSPERO (CRD42018097357). Eligible studies were identified through a systematic comprehensive search of five electronic databases from January 2008 until May 2018: MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCO), HTA Database Canadian Repository, and Cochrane Library (Wiley).
The search strategy was designed by a medical research librarian (J.B.) following the Cochrane systematic review methodology and included published search filters.Reference Mackintosh, Casañas i Comabella, Hadi, Gibbons, Fitzpatrick and Roberts 14 – Reference Wilczynski, Haynes, Lavis, Ramkissoonsingh and Arnold-Oatley 16 The initial search was designed for MEDLINE and translated into the syntax of the other databases. Our search included terms related to the concepts of CPE, health outcomes, long-term sequelae, HRQoL, and utilities (Appendix 2 online).
All screenings (title and abstract, full-text) were independently completed in duplicate by 2 reviewers (D.B. and S.M.) in Distiller SR software (Evidence Partners, Ottawa, Ontario, Canada). Conflicts were resolved through consensus or were discussed with a third reviewer (B.S.). The study selection process is documented in Fig. 1. Studies included in our review were required to meet the following criteria: (1) published in English; (2) a primary study (ie, randomized control trials, cross-sectional, case-control, and cohort studies); (3) published after January 1, 2008; (4) assessed health outcomes or HRQoL for case patients (≥1 month of age) with confirmed CPE infection, and (5) included a control group infected with carbapenemase-susceptible Enterobacteriaceae. We limited the publication date to January 1, 2008, because this was the year that New Delhi metallo-lactamase was first detected.Reference Yong, Toleman and Giske 17 This CPE pathogen was subsequently detected throughout the world, in many of the 36 Organization for Economic Co-operation and Development (OECD) countries. The included studies each had a control group of patients without CRE infections. We included studies investigating patients with coinfections to ensure that our findings are generalizable to a wide range of clinical settings. Furthermore, the underlying conditions of patients were documented to better understand the mortality that was attributable to CPE infection.
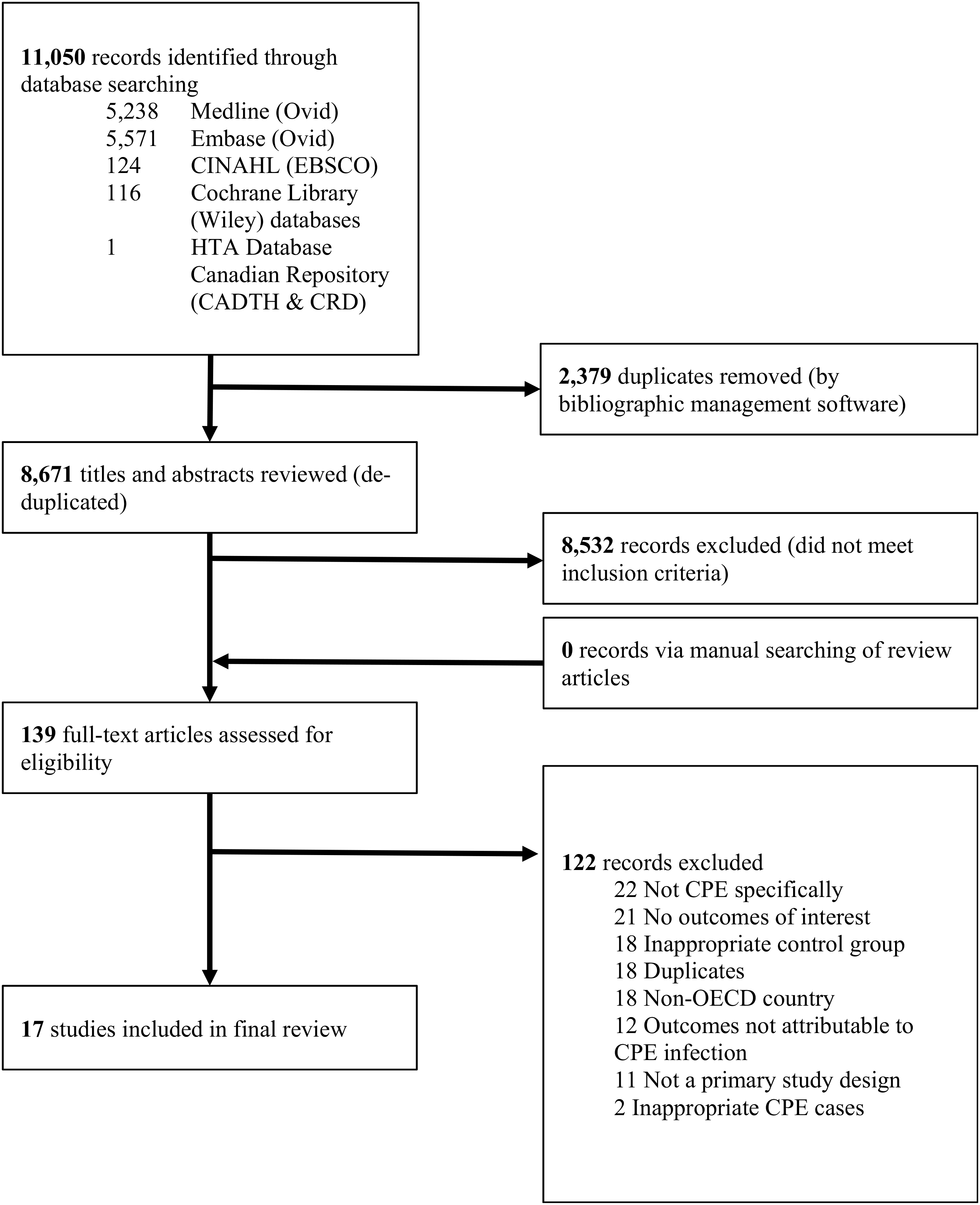
Fig. 1. PRISMA Flow Diagram of Study Selection.
We excluded studies without control groups such as case reports and case series. We also excluded studies with unconfirmed CPE infection or CPE-colonized case groups. We limited our study population to any of the 36 OECD countries to control for the baseline health status of the general population and quality and type of health-care systems. In addition, we excluded animal studies and publications such as editorials, letters and news articles. Systematic reviews and meta-analyses were included into the full-text stage to manually search for primary literature.
Data extraction and quality assessment
Data extraction and quality appraisal were completed in duplicate (D.B. and S.M.), and conflicts were resolved through consensus or discussion with a third reviewer (B.S.). We extracted the following study characteristics: authors, year of publication, study design, setting, sample size, age for both groups, sex (percentage of females), study location/setting, definition of cases and controls, underlying conditions, enzyme type, bacteria strain, and most common infections. If reported, we further extracted, outcomes examined in each of the studies: mortality, sequelae, length of hospitalization, and HRQoL. In certain instances, we extracted data from subgroups of the case cohorts, if only those patients met our inclusion criteria (Appendix 3 online).
Quality appraisal was completed for all studies using their respective Joanna Briggs Institute (JBI) Critical Appraisal checklist tools. 18 , 19 We summarized quality appraisals, and studies were deemed to be good quality if at least 50% of the quality assessment criteria were met and high quality if at least 80% of the quality assessment criteria were met for the given study design.Reference Patel, Sander and Nelder 20 We did not calculate an overall quality score, according to PRISMA guidelines.Reference Moher, Liberati and Tetzlaff 13
Data synthesis and analysis
We reported data on each included study for one or more of the outcomes and summarized them. We extracted the point estimates (e.g. odds ratio) from each study and summarized that data. In addition, we conducted a meta-analysis using a random-effects model in Review Manager (RevMan) version 5.3 software (Nordic Cochrane Centre, Copenhagen) to generate pooled estimates, if applicable.
Results
In total, we identified 8,671 unique studies, of which 17 met our eligibility criteria and were included in our review (Fig. 1).Reference Daikos, Petrikkos and Psichogiou 21 – Reference Sánchez-Romero, Asensio and Oteo 37 Descriptive details of the 17 included studies are provided in Appendix 3 online. All of the included studies focus on health outcomes; no studies assessed HRQoL associated with CPE infection. Most studies were conducted in Europe (n = 11), followed by Israel (n = 3), the United States (n = 2), and Mexico (n = 1). Study designs included case-control (n = 9) and cohort (n = 8) studies. Of the nine case-control studies, eight conducted a prospective evaluation for health outcomes. Therefore, we assessed those eight studies as cohort studies in our quality appraisal. Sample sizes ranged from 26 (8 cases and 18 controls)Reference Lübbert, Becker-Rux and Rodloff 32 to 657 patients (426 cases and 231 controls).Reference Tumbarello, Trecarichi and Tumietto 27 Most studies were conducted in teaching or university-affiliated hospitals (n = 13). The most common infection for cases were BSI (n = 8), followed by urinary tract infections (n = 4). KPC was the most common carbapenemase (n = 13), followed by VIM (n = 4), and oxacillinase (OXA, n = 2). Klebsiella pneumoniae was reported in most studies (n = 16) with only 2 studies reporting Enterobacter cloacae and 1 reporting E. coli. All studies included control groups without CRE infections; 4 studies used control groups comprised of CPE-colonized patients.
Quality appraisal results are shown in Appendix 4 online. Overall, 14 of 17 studies (82%) met >80% of their respective criteria; three studies met 70% of the criteria and five studies met 100%. From the JBI checklist for cohort studies (n = 16), strategies to deal with confounding factors was most often not reported (Appendix 5 online).
All primary study outcomes are presented in Table 1. We organized study outcomes into four categories: (1) mortality (in hospital, intensive care unit (ICU), attributable to infection by accounting for mortality due to underlying comorbidities, mortality associated with inappropriate antibiotic therapy, and 14–90 day), (2) antibiotic therapy (duration, appropriate antibiotics used), (3) sequelae (relapse rate, BSI secondary to initial infection, and functional status), and (4) length of hospital stays (total, after infection). Our meta-analysis generated a pooled estimate of in-hospital mortality attributable to initial CPE BSI between the cases and carbapenem-suseptible control group. We did not conduct meta-analyses for the other types of infections or outcomes due to the limited data on other infections coupled with the heterogeneity of the outcome measurements and/or units. Proportions of cases and controls with mortality and sequelae outcomes, however, were described.
Table 1. Summary of Data Extraction Categorized by Health Outcomes and Sequelae a

Note. CI, confidence interval; NA, not available; SD, standard deviation; ESBL, extended-spectrum β-lactamase; KP, Klebsiella pneumoniae; CS, carbapenem susceptible; BSI, bloodstream infection; IQR, interquartile range.
a See Appendix 6 online for details.
Mortality outcomes
In our study, eight different measures of mortality were reported, with the most common being in-hospital mortality (n = 8), followed by mortality in ICU (n = 4), and attributable to infection by accounting for mortality due to other underlying comorbidities (n = 3). Studies that reported in-hospital mortality may include patients from the ICU, and we have reported mortality outcomes as specified in the included studies. Across all studies that reported mortality outcomes, 63.5% of all 494 deaths were reported as in-hospital mortality in CPE patients, followed by 12.8% in the ICU. The least commonly reported mortality rates were 14-day to 90-day mortality rates, at <5%. Reported mortality rates ranged from 11.1% in 27 case patientsReference Mouloudi, Massa and Papadopoulos 34 to 82.4% in 17 ICU case patients.Reference Fraenkel-Wandel, Raveh-Brawer, Wiener-Well, Yinnon and Assous 30
Results from the meta-analysis (n = 5 studies)Reference Tumbarello, Trecarichi and Tumietto 27 – Reference Fraenkel-Wandel, Raveh-Brawer, Wiener-Well, Yinnon and Assous 30 , Reference Mouloudi, Protonotariou and Zagorianou 33 are displayed in Fig. 2. In total, 266 in-hospital deaths occurred in 588 CPE-infected patients, and 158 in-hospital deaths occurred among 599 patients with a carbapenem-suseptible BSI. Each of the included studies reported a positive absolute difference between cases and controls, ranging from 0.20 (95% confidence interval [CI], 0.13–0.26)Reference Tumbarello, Trecarichi and Tumietto 27 to 0.39 (95% CI, 0.23–0.55).Reference Ben-David, Kordevani and Keller 28 Appendix 3 online shows that the most common underlying conditions in the study with the highest absolute difference is renal failure,Reference Ben-David, Kordevani and Keller 28 whereas cardiovascular disease is most common in the study with the smallest absolute difference, which was the only study to include a control group enrolling patients colonized with CPE.Reference Tumbarello, Trecarichi and Tumietto 27 On the other hand, Ben-David et alReference Shilo, Assous and Lachish 24 (highest risk difference) and Fraenkel-Wandel et al,Reference Torres-Gonzalez, Ortiz-Brizuela and Cervera-Hernandez 26 case groups of carbapenem-suseptible extended-spectrum β-lactamase–producing (ESBL) Enterobacteriaceae. Overall, we calculated a pooled absolute risk difference between 588 case and 599 control patients for in-hospital mortality of 0.25 (95% CI, 0.17–0.32), which was statistically significant (P < .00001). The I2 for this pooling was 21%, demonstrating low heterogeneity in this analysis.Reference Higgins, Thompson, Deeks and Altman 38

Fig. 2. Meta-analysis of in-hospital mortality attributable to initial carbapenemase-producing Enterobacteriaceae bloodstream infections between the cases and carbapenem-susceptible control group.
Sequelae
Moreover, five studies reported four different types of sequelae due to CPE infection with the most common being BSI secondary to pneumonia and urinary tract infections (n = 4). Only three of these studies reported the rate of secondary BSI outcomes for both cases and controls. Rates of secondary BSI reported for cases and controls were between 22.2% of 27 patients and 71.4% of 7 patients,Reference Falcone, Mezzatesta and Perilli 22 , Reference Torres-Gonzalez, Ortiz-Brizuela and Cervera-Hernandez 26 and between 11.1% of 18 patients and 40.9% of 22 patients, 18 , Reference Ben-David, Kordevani and Keller 28 respectively. The absolute risk difference of secondary BSI was highly variable between these studies, ranging from 2.8% of 135 patients to 30.5% of 29 patients between cases and controls.Reference Falcone, Mezzatesta and Perilli 22 , Reference Torres-Gonzalez, Ortiz-Brizuela and Cervera-Hernandez 26 Data for relapse,Reference Falcone, Mezzatesta and Perilli 22 functional status dependence,Reference Fraenkel-Wandel, Raveh-Brawer, Wiener-Well, Yinnon and Assous 30 and length of infection in days,Reference McLaughlin, Advincula, Malczynski, Barajas, Qi and Scheetz 29 were limited; each was reported in only a single study (Table 1). The highest proportion of 54 case patients had secondary BSI (ncases = 66.7%), whereas the most common sequelae in the 71 controls was functional dependence (ncontrols = 54.9%).
Antibiotic therapy
Five studies reported three types of outcomes related to antibiotic therapy; the most common was duration of antibiotic therapy (n = 3). In 2009, Falcone et alReference Falcone, Mezzatesta and Perilli 22 report the longest mean duration of antibiotic therapy for cases and controls at 29.7 days (95% CI, 21.5–37.8) and 23.6 days (95% CI, 10.3–36.8), respectively. On the other hand, in 2016, Sbrana et alReference Sbrana, Malacarne and Bassetti 23 reported the shortest median (interquartile range) duration of antibiotic therapy for cases and controls of 4 days (2–5) and 1 day (0–3), respectively. Full course of antibiotics completed,Reference Lopez-Gonzalez, Candel and Vinuela-Prieto 31 and appropriate antibioticsReference Fraenkel-Wandel, Raveh-Brawer, Wiener-Well, Yinnon and Assous 30 were the other types of outcomes, but each was reported in a single study.
Length of stay
In addition, seven studies reported length of stay outcomes, mostly length of hospital stay (LOHS) in days (n = 6). In liver transplant recipients, infected with K. pneumoniae carrying the KPC gene following admission to the ICU, Lubbert et alReference Lübbert, Becker-Rux and Rodloff 32 reported the longest mean LOHS in cases and controls of 87 (standard deviation [SD], 47.3) and 42.7 (SD, 23.7) days, respectively. On the other hand, in patients with OXA-producing Enterobacteriaceae infections in a tertiary-care hospital, Torres-Gonzalez et al,Reference Torres-Gonzalez, Ortiz-Brizuela and Cervera-Hernandez 26 reported the shortest median LOHS in cases, carbapenem-suseptible control group, and carbapenem-suseptible extended spectrum β-lactamase control group of 21 (interquartile range [IQR], 8–15), 15 (IQR, 7–32) and 15 (IQR, 11–35) days, respectively. The other type of hospitalization outcome reported was postinfection hospital stay in days, but this was reported in one study.Reference Ben-David, Kordevani and Keller 28
Discussion
We systematically reviewed 17 studies for health outcomes associated with CPE infections. Mortality was the most commonly reported outcome, followed by sequelae, antibiotic therapy, and length of stay. No studies reported HRQoL associated with CPE infection or its sequelae, which is likely due to what is considered short-term sequelae since follow-up was typically conducted until discharge or in-hospital death. Nevertheless, a knowledge gap exists for CPE infections, which pose an urgent public health threat. 39 The duration of antibiotic therapy (4–29.7 compared to 1–23.6 days) and length of hospital stay (21–87 compared to 15–42.7 days) seemed to increase between CPE and non-CPE infected patients, respectively.
Based on the limited number of sequelae post-hospitalization, in-hospital mortality seemed to be of greatest concern for CPE-infected patients. In-hospital mortality varied from 28.9% to 75% in CPE-infected patients, likely due to the differences in study designs, comorbidities of patients, and hospital types (eg, tertiary-care hospital vs teaching hospital) between studies. The development of novel antibiotics and changing prevalence rates of AMR organisms over time may affect mortality rates, but in this review, we did not observe differences for in-hospital mortality rates from 2009 to 2016 (n = 8 studies) or ICU mortality rates from 2010 to 2016 (n = 4 studies).
In our meta-analysis of five studies, we estimated that CPE infection can increase the risk of in-hospital BSI mortality by 25%. Different comorbidities and inappropriate definitive and empirical antibiotic therapy for patients infected with AMR organisms may explain the increased mortality rate.Reference Vardakas, Rafailidis, Konstantelias and Falagas 40 One included study reported mortality due to inappropriate antibiotic use, which was similar to our pooled estimate for in-hospital BSI mortality (28.6% and 25%, respectively).Reference Fraenkel-Wandel, Raveh-Brawer, Wiener-Well, Yinnon and Assous 30 Of the studies in our meta-analysis, the highest mortality difference (39%) occurred in case patients with chronic renal failure (39%) and control patients with malignancy (24%),Reference Ben-David, Kordevani and Keller 28 and the lowest mortality difference (20%) occurred in case and control patients with cardiovascular disease (39.2% and 45.5%, respectively).Reference Tumbarello, Trecarichi and Tumietto 27 Furthermore, in three included studies, mortality attributable to infection by accounting for mortality due to underlying comorbidities clustered within a relatively smaller range (3.7%–29.0%) compared to in-hospital mortality (3.7%–63.9%). Due to the small sample size and range of comorbidities reported between these studies, however, we were not able to examine the association of specific comorbidities and mortality.
Other reviews have reported mortality associated with CPE infections.Reference Xu, Sun and Ma 11 , Reference Tzouvelekis, Markogiannakis, Piperaki, Souli and Daikos 12 In a 2014 review, Tzouvelekis et alReference Tzouvelekis, Markogiannakis, Piperaki, Souli and Daikos 12 found that in-hospital mortality rates were highly variable (17.6%–61.1%, 43.5% difference), which was similar to what we observed in our review for CPE-infected patients (28.9%–75%, 46.5% difference). However, Tzouvelekis et al did not include a carbapenem-suseptible control group in their review and did not conduct a meta-analysis. In 2017, Xu et alReference Xu, Sun and Ma 11 conducted a systematic review and meta-analysis focused on carbapenem-resistant K. pneumoniae. Their study only examined crude mortality due to KPC and VIM-producing K. pneumoniae infection, whereas our study classified mortality into eight measures (e.g. in-hospital, ICU, 14-day mortality rates) for VIM, KPC, and OXA-producing Enterobactericace, which included K. pneumoniae, Enterobacter cloacae, and E. coli. Although our study populations varied and our pooled estimates were specific for in-hospital mortality attributable to CPE-BSI, we reported a trend similar to that reported by Xu et alReference Xu, Sun and Ma 11 of increased number of deaths with CPE-infected patients compared to CS controls (25% compared with 25.59%–26.54%).Reference Xu, Sun and Ma 11
Our analysis has several limitations. We limited our search to the English language because of resource constraints, which may have introduced language bias, and thus we ultimately excluded studies from countries with higher CPE prevalence where English is not the official language. To a similar effect, limiting studies to the OECD countries may underestimate the frequency of sequelae and mortality rates from a global point of view, given that OECD countries are considered to have more developed healthcare systems to provide adequate care. Furthermore, it was difficult to complete a rigorous meta-analysis with a larger subset of studies due to the heterogeneity across studies (eg, different types of infections, groups of patients, geographic setting, outcome definition and measurements).
To our knowledge, this study is the first systematic review to characterize the literature on a broad range of clinical outcomes (sequelae attributable to CPE infections and perform a meta-analysis on in-hospital mortality). This review also serves as a validation study for the increased risk of mortality attributable to CRE infections. Our methods of selecting studies with a carbapenem-susceptible group allowed us to collect outcomes attributable to CPE infection and limit the number of potential cofounders. In addition, conclusions from this review are supported by high-quality studies; we did not have to exclude studies for low quality scores. Most included studies focused on mortality outcomes and future studies of CPE infection should address a broader range of sequalae relating to the initial infection.
Acknowledgements
None
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.282





