Clostridioides difficile infection (CDI) is a major cause of morbidity and excess healthcare costs worldwide.Reference McDonald, Gerding and Johnson1 In 2011, C. difficile was estimated to cause nearly half a million infections annually in the United States.Reference Lessa, Mu and Bamberg2, 3 Although CDI is now common, false-positive C. difficile test results have been observed among patients who are colonized and asymptomatic. Screening for C. difficile in asymptomatic patients is not recommended by consensus guidelines.Reference McDonald, Gerding and Johnson1 Preventing testing and treatment of asymptomatic patients is supported by multiple studies.Reference Gerding, Olson and Peterson4–Reference Loo, Bourgault and Poirier6 Most antibiotic treatments targeting C. difficile are nonselective and may exacerbate pre-existing dysbiosis among carriers.Reference Lamendella, Wright and Hackman7, Reference Cannon, Byrne and Happe8 Treatment with commonly prescribed C. difficile–directed antibiotic agents (eg, metronidazole and oral vancomycin) has been shown to reduce gut microbial diversity,Reference Lamendella, Wright and Hackman7 delaying restoration of a eubiotic state. Thus, avoiding antibiotic treatment of asymptomatic carriers is expected to benefit patients, lower CDI rates, and decrease antibiotic consumption by curtailing unnecessary prescribing.
Inappropriate testing of asymptomatic C. difficile carriers can falsely elevate the CDI standardized infection ratio (SIR) 9 and promote unnecessary antibiotic treatment leading to patient harms. Highly sensitive diagnostic technologies, such as nucleic acids amplification tests (NAATs), can accurately detect the presence of toxigenic C. difficile but have been shown to yield clinical false positives.Reference Dubberke, Han and Bobo10 The 2017 IDSA and Society for Healthcare and Epidemiology of America (SHEA) guidelinesReference McDonald, Gerding and Johnson1 offer 2 strategies to reduce clinical false positive results: (1) using the NAAT alone, establish an institutional diagnostic stewardship guideline that reduces inappropriate testing or (2) pair the NAAT with a second test to enhance specificity. We hypothesized that (when using an NAAT alone) a multidisciplinary, multifaceted antimicrobial stewardship program (ASP)-driven intervention paired with education would lead to a decrease in CDI burden.
Methods
Setting and population
We performed a single-center, quasi-experimental study to evaluate the impact of an antimicrobial stewardship program (ASP) intervention on hospitalized patients for whom a computerized provider order entry (CPOE) order for an NAAT was placed and a specimen was sent for testing to the clinical microbiology laboratory. The study took place at Northwestern Memorial Hospital (NMH), an 897-bed tertiary-care academic medical center in Chicago, Illinois. Adult inpatients admitted to the facility from January 2014 to April 2018 (52 months of data available) were eligible for C. difficile inclusion in the time-series analysis. Patients admitted to the stem cell transplant (SCT) unit were not included in the intervention and were therefore considered as contemporaneous noninterventional control in the time-series analysis from August 2014 to April 2018 (45 months of data available). Only aggregate data were obtained, and all study investigators were affiliated with NMH during their participation in the ASP initiative. The study was reviewed and determined to be non-human subjects’ research by the Northwestern University IRB (study no.: STU00207892).
Clostridioides difficile specimen processing, NAAT methods, and CDI reporting
During the study period, C. difficile specimens were evaluated using NAATs. Briefly, liquid stool specimens were screened and processed twice daily at 8 a.m. and 4 p.m. by the clinical microbiology laboratory. Orders occurring after 4 p.m. were processed during the following morning batch. Formed stool were rejected by the clinical microbiology laboratory. Specimens sent from patients with a previous NAAT result on record within the prior 7 days were rejected by the electronic health record (EHR) based on hospital protocol.
Clinical specimens underwent extraction and polymerase chain reaction (PCR) amplification for the toxin B gene (ie, tcdB). PCR was performed using the BD GeneOhm Cdiff assay kit (BD Diagnostics, Germany) on SmartCyclers between 2012 and 2015 and the BD Max (Becton Dickinson) Cdiff assay kit (BD Diagnostics, Germany) between 2015 and 2018. NAATs were performed according to the manufacturers’ protocols.
Positive NAAT results occurring on or after the fourth calendar day of hospitalization were reported to NHSN as incident hospital-onset (HO) CDI laboratory identified events. Aggregate reports of HO-CDI NAAT results were available from the NHSN portal and reported as the HO-CDI incidence rate (IR) per 10,000 patient days. The CDC negative binomial regression equations were also utilized to calculate the CDI SIR monthly across the study period by the Northwestern Healthcare Epidemiology and Infection Prevention team ^9. Patient days were aggregated according to CDC Multidrug-Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module ^9 using data from the Northwestern Enterprise Data Warehouse. Aggregate oral vancomycin consumption was downloaded from the NHSN AU Module, where antibiotic days (AD) per 1,000 days present (DP) were assessed at the facility-wide level ^9.
ASP provider education on NAAT test utilization
In January 2016, within the pre-intervention phase, ASP providers at our center initiated widespread education on appropriate use of NAAT, avoidance of inappropriate testing, clinical false positives, and predisposing risk factors for CDI. Formal educational materials and NAAT testing algorithm (Fig. 1) were disseminated in person during patient care rounds, were sent by email to specialist groups, and were posted as an intranet resource. In addition, the CPOE C. difficile PCR order was modified to include an information box containing the CDC definition of C. difficile test-worthy diarrhea.
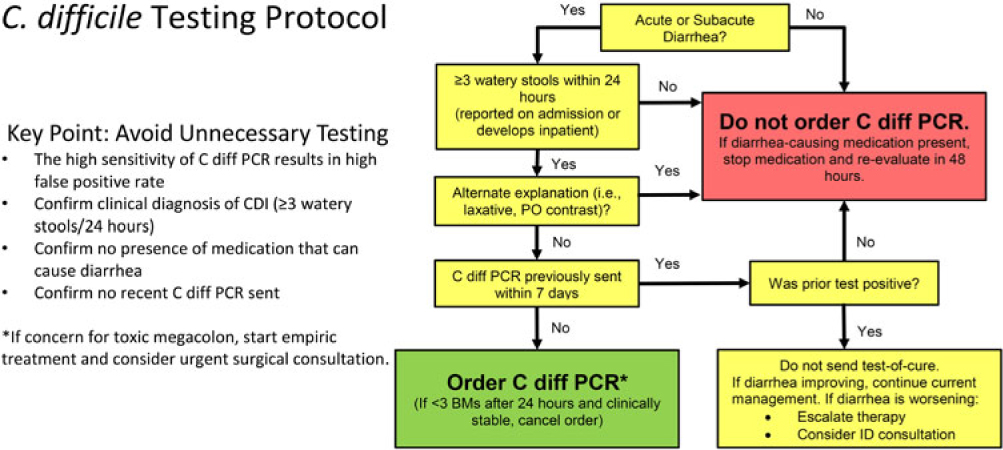
Fig. 1. Institutionally approved Clostridioides difficile NAAT algorithm and protocol. Note. BM, bowel movement; C. diff, C. difficile; CDI, C. difficile infection; ID, infectious diseases; HO, hospital-onset; PO, oral; PCR, polymerase chain reaction.
ASP prospective clinical review and preauthorization
In October 2016, an ASP clinical review with NAAT preauthorization was implemented within our center. All inpatient C. difficile NAAT orders generated on or after the fourth calendar day of hospital admission triggered a prospective clinical review by an ASP pharmacist as part of routine weekday ASP operations. All NAAT tests ordered during weekend hours were not subject to review and were processed as ordered. Briefly, a line list of NAAT orders received by the microbiology laboratory were forwarded to the ASP team twice daily during the day shift for review. The EHR from those patients on the line list were reviewed for clinical signs and documented symptoms consistent with C. difficile infections based on an institutionally approved algorithm (Fig. 1). Analysis of each case was based primarily on documentation of 3 or more liquid stools per day. Documentation or presence of clinical factors evaluated by the ASP team included the following: presence of leukocytosis (eg, WBC >15,000 cells/mm3), fever, recent NAAT results, administration of stool softeners or laxatives within the preceding 24 hours, receipt of oral contrast, tube feeding initiation, imaging results consistent with colitis or ileus, as well as other alternative explanations of diarrhea for each patient.Reference McDonald, Gerding and Johnson1 ASP team members evaluated whether CDI symptoms were absent or could be explained by plausible alternatives (eg, laxative use), which would classify the patient as not meeting test criteria. In cases where NAAT orders failed to meet preauthorization criteria, a recommendation to cancel the test was discussed with the ordering provider. ASP recommendations to cancel NAAT could be accepted or rejected after discussion. If ordering or covering providers did not follow-up with initial communications, orders not meeting criteria were cancelled and documented. Discrepancies in clinical assessment were adjudicated by the medical director of ASP. NAAT orders meeting preauthorization criteria were processed as ordered.
In February 2017, education on appropriate use of NAAT was reinforced using computer decision support which was embedded within the test order set (Cerner, Millennium). Briefly, providers were prompted at the point of order entry to complete a two-step assessment. In step 1, providers had to attest to new onset of patient diarrhea (3 or more large watery stools within prior 24 hours), presence of ileus on exam, verbal history of continued diarrhea beginning prior to arrival (many large watery stools within prior 24 hours), or admission to the SCT unit. In step 2, the order was cross-referenced against existing NAAT to determine whether a test had been sent within the prior 7 days. ASP pre-authorization continued throughout this period. ASP team members continued to alert providers if clinical and/or laboratory criteria were not met and notified them of cancellation of tests failing to meet the established criteria. Discussion with the ASP medical director was offered if providers felt an exception should be made.
Statistical analyses
In the present quasi-experimental study, the primary outcome was the change in the incident rate (IR) of HO-CDI per 10,000 patient days. The change in the monthly CDI SIR and consumption of oral vancomycin days of therapy (DOT) per 1,000 patient days were also evaluated. The preintervention period was defined as January 1, 2014, through September 30, 2016, and the postintervention period was defined as October 1, 2016, through April 30, 2018. The overall study period, including before and after the implementation of the ASP preauthorization program, was 52 months.
Univariate differences in HO-CDI-IR, SIR, and vancomycin consumption before and after protocol implementation were evaluated using the Student t test or Wilcoxon rank-sum test, as appropriate. Segmented regression models were constructed to evaluate changes in the dependent variable level, slope, or both as a function of time-dependent predictors. Regressions followed the generalized form:
where f[Y(t)] is a function of the dependent variable Y, time(t=1,52) is increasing months from 1 to 52, intervention is a binary classifier of the preauthorization intervention, and time(t=34,52) is increasing months from 34 to 52 used to define the postintervention step and slope change interaction.
Generalized linear models were fit to observed data using the Stats package within R version 3.2.4 software.11 Poisson or log-linked gamma regression models were utilized according to the distribution of the dependent variable, as appropriate. Models incorporating step-change (ie, intercept P value; P step), step and slope change (ie, intercept P value; P step and slope P value; P slope), and each of the study periods (ie, education only versus pre-authorization plus education) were iteratively constructed and compared using goodness of fit analyses. Significant differences in model fitness were considered P < .05 in model comparisons. The simplest most explanatory models were selected as final. Data plots were constructed in R, as previously described.Reference Bernal, Cummins and Gasparrini12 Relationships between antibiotic use and HO-CDI-IR measures were evaluated using least-squares regressions. To explore the durability of the intervention, the proportion of NAAT classified as HO-CDI that were placed on weekdays (ie, when ASP preauthorization was in effect) compared weekends (when ASP preauthorization was not in effect), which was calculated as a percent change from baseline.
Results
Overall, 743 HO-CDI NAAT results were documented during the 52 months between January 1, 2014, and April 1, 2018. The mean (± standard deviation [SD]) monthly number of HO-CDI NAAT results were 14.3±4.2, the mean HO-CDI-IR was 7.8 ± 2.3 per 10,000 patient days, the mean SIR was 0.9 ± 0.25, and the mean oral vancomycin was 10.8 ± 2.4 DOT per 1,000 DP at the facility-wide level. The mean monthly number of positive NAAT results decreased after versus before implementation (12.4 vs 15.4; P = .018) as did the HO-CDI-IR (6.5 vs 8.5 per 10,000 patient days; P = .0036) and the SIR (0.78 vs 0.97; P = .015). Mean vancomycin consumption was similar before and after implementation at the univariate level (10.8 vs 10.7 DOT per 1,000 DP; P = .91). Within the SCT population, the mean HO-CDI-IR was 32.8±19.8 per 10,000 patient days, and the mean HO-CDI-IR was similar after and before implementation (36.5 vs 30.3 per 10,000 patient days; P = .34) at the univariate level.
A summary of the Facility-wide HO-CDI-IR per 10,000 patient days is displayed in Fig. 2. The segmented regression analysis identified significant time-dependent decrease in the HO-CDI-IR trend (P step = .06; P trend = .008) after implementation of the ASP clinical review and preauthorization intervention: mean rate change −3.4% (95% confidence interval [CI], −0.90% to −6.1%) per month postimplementation. Concurrently, the HO-CDI-IR within the noninterventional control unit (ie, SCT unit over 45 months) did not change after protocol implementation (P step = .125; P trend = .115). A summary of the Facility-wide SIR is displayed in Fig. 3. Segmented regression analysis identified significant time-dependent decrease in the monthly facility-wide SIR trend (P step = .10; P trend = .017) with a mean rate change of −2.9% (95% CI, −0.52% to −5.4%) per month postimplementation. Reductions in NAAT and HO-CDI-IR per 10,000 patient days and SIR were accompanied by decreases in oral vancomycin consumption, as summarized in Fig. 4. Segmented regression analysis identified significant time-dependent decreases in oral vancomycin consumption (P step < .001; P trend < .001) with a mean rate change of −3.1% (95% CI, −2.6% to −3.7%) per month postimplementation. Vancomycin consumption was significantly (P = .002) positively correlated with HO-CDI-IR at the facility-wide level (Fig. 5). Time-series models of HO-CDI-IR parameterized with step-change only versus both step and slope changes produced inferior data fits by likelihood ratio testing (P = .008), whereas time-series models of HO-CDI-IR parameterized with education (starting at month 25) plus preauthorization intervention (starting at month 34) did not improve model fitness versus parameterization with preauthorization intervention alone according to likelihood ratio testing (P = .34). Alternative time-series models of SIR were also considered but did not improve model fitness by likelihood ratio testing (data not shown).
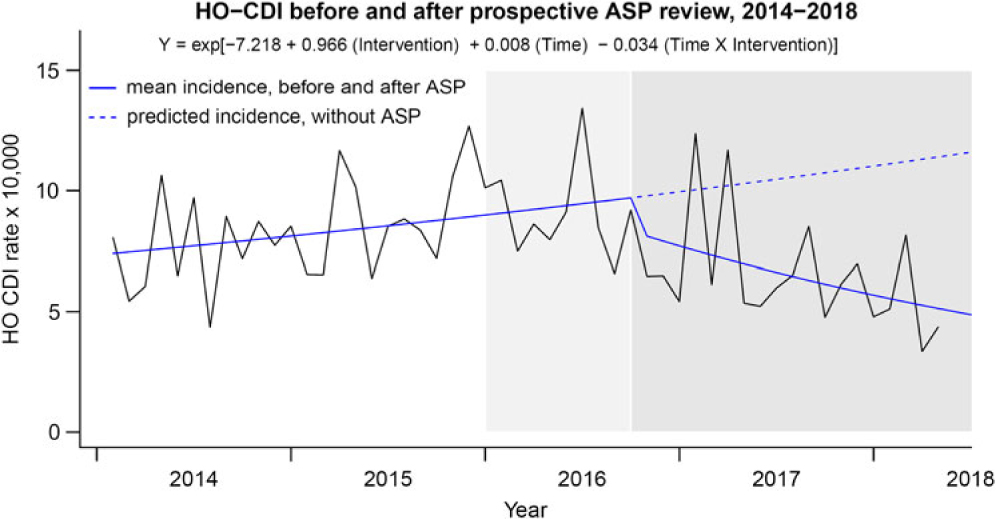
Fig. 2. HO-CDI incidence rates before and after implementation of ASP clinical review of NAAT. HO-CDI incidence rate per 10,000 patient days observed and predicted mean fits displayed (Y-axis). Transformed coefficients from Poisson regression were used to generate incident rate predictions per 10,000 patient days. Time was modeled as increasing months from 1 to 52 with Intervention as a binary classifier. Note. Light grey shading: provider education disseminated. Dark grey shading: ASP driven preauthorization paired with education. ASP, antimicrobial stewardship; CDI, Clostridioides difficile infection; HO, hospital-onset.
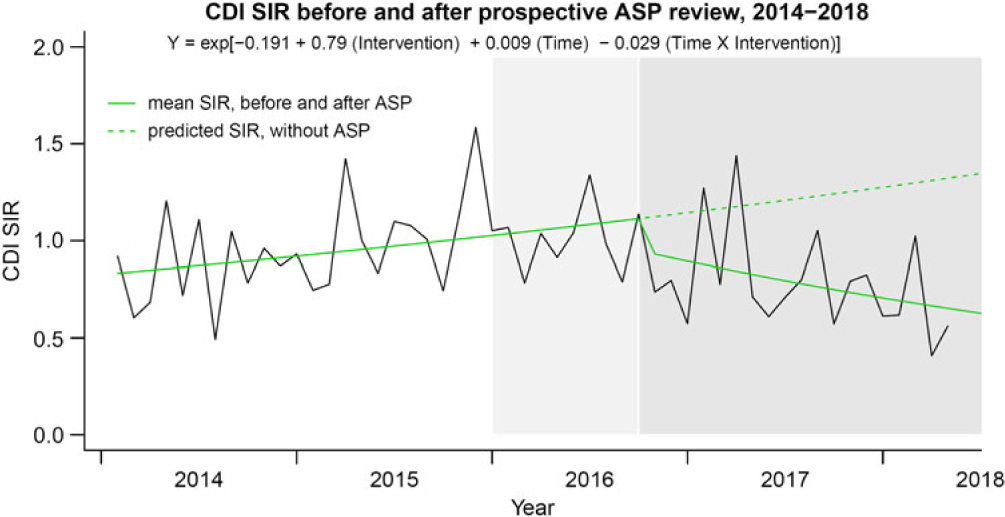
Fig. 3. Facility-wide CDI SIR before and after implementation of ASP clinical review of NAAT. HO-CDI SIR observed and predicted mean fits displayed (Y-axis). Transformed model coefficients from Gamma log-linked regression were used to generate CDI SIR predictions. Time was modeled as increasing months from 1–52 with intervention as a binary classifier. Note. Light grey shading: provider education disseminated. Dark grey shading: ASP driven pre-authorization paired with education. ASP, antimicrobial stewardship; CDI, Clostridioides difficile infection; SIR, standardized infection ratio.
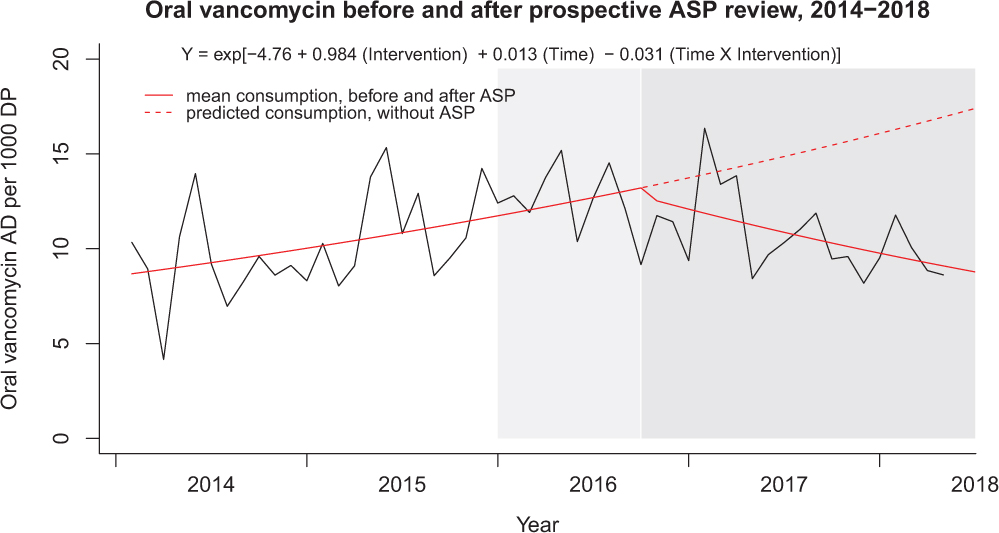
Fig. 4. Facility-wide oral vancomycin before and after implementation of ASP clinical review of NAAT. Oral vancomycin consumption (AD) per 1,000 days present (DP) observed and predicted mean fits displayed (Y-axis). Transformed coefficients from Poisson regression were used to generate consumption rate predictions per 1,000 patient days. Time was modeled as increasing months from 1–52 with Intervention as a binary classifier. Note. Light grey shading: provider education disseminated. Dark grey shading: ASP driven pre-authorization paired with education. AD, antimicrobial days; ASP, antimicrobial stewardship; PO, orally administered.

Fig. 5. Relationship between facility-wide HO-CDI incidence rate and oral vancomycin consumption. Note. AD, antimicrobial days; CDI, Clostridioides difficile infection; days present (DP); HO, hospital-onset; patient days (PD).
The stratification of HO-CDI NAAT orders by whether the order was placed during a weekday or on the weekend is shown in Fig. 6. There was no significant difference in the proportion of test positivity on weekdays versus weekends within the interventional units after implementation of the stewardship initiative (−29% vs −39% change from baseline; P = .27). Likewise, control units demonstrated similar proportions of test positivity on weekdays versus weekends after implementation of the stewardship initiative (−14% vs −18% change from baseline; P = .99).
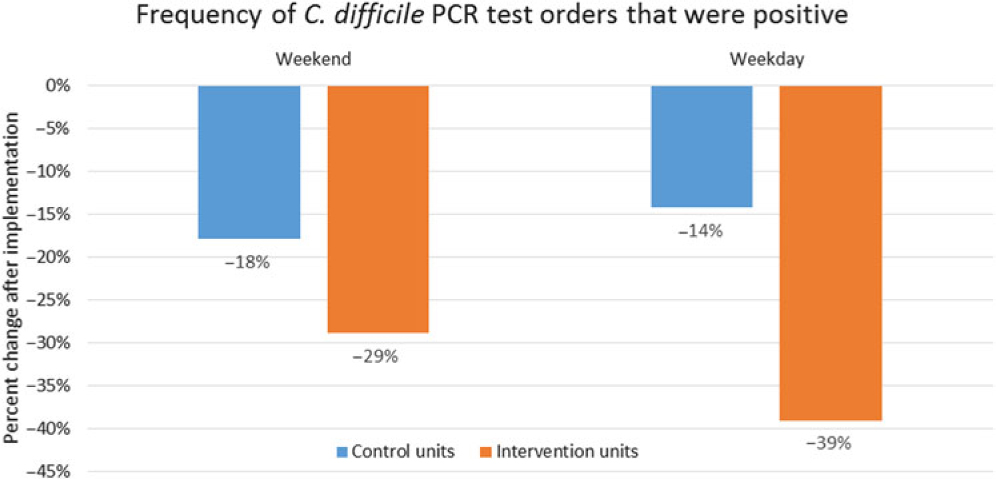
Fig. 6. Percent change in NAAT classified HO-CDI from baseline according to ASP coverage. The proportional change in positive NAAT considered to be HO-CDI were calculated as a percent change from baseline and stratified according to weekday or weekend day of ordering. ASP pre-authorization was in effect only during weekdays during the study. Control units were comprised of stem-cell transplant and were excluded from the intervention. Note. CDI, Clostridioides difficile infection; NAAT, nucleic acid amplification test.
Discussion
We observed reductions in HO-CDI NAAT, HO-CDI incident rates, CDI SIR, and oral vancomycin consumption within our center after implementation of a clinical review and preauthorization protocol led by ASP to decrease inappropriate testing. Noninterventional control unit (ie, SCT) NAAT positivity and HO-CDI-IR were similar before and after protocol implementation, strengthening the association between the intervention and the outcome. Practice guidelines call for institutional guidance and protocols to improve appropriate detection and treatment of CDI.Reference McDonald, Gerding and Johnson1 The high sensitivity of NAAT testing makes it difficult to differentiate carriers of the toxigenic C. difficile strain from CDI based on test results alone, yet NHSN classifies HO-CDI-IR solely based on laboratory event reporting. Our center previously examined the appropriateness of NAAT among patients who met laboratory criteria for hospital-onset C. difficile infection.Reference Kelly, Yarrington and Zembower13 It was determined that 14.8% of NAAT sent did not meet criteria. In 65.5% of cases, documentation of symptoms was not adequate, further underscoring the need for improved NAAT utilization. Our findings support the feasibility of ASP-driven initiatives to reduce inappropriate testing and treatment of C. difficile colonization.
Although decreases in HO-CDI can be obtained through improving antibiotic use and optimizing the hygiene practices, Reference Price, Cheek and Lippett14–Reference Barker, Alagoz and Safdar16 the impact of ASP interventions aimed at reducing inappropriate NAAT is beginning to emerge. Kociolek et alReference Kociolek, Bovee and Carter17 implemented an educational intervention to improve CDI testing in pediatric patients and observed improved trends in testing rates and test positivity without altering the HO-CDI incidence density. Khoury et alReference Khoury, Sistrunk and Hixson18 developed an EHR tool to identify patents at high risk for CDI, yielding a significant decrease in HO-CDI-IR by increasing appropriateness of testing. Others have taken a multifaceted approach to decreasing HO-CDI-IR and improving patient outcomes. Mermel et alReference Mermel, Jefferson and Blanchard19 demonstrated reduced HO-CDI IR and lower mortality after implementing a protocol that included daily monitoring, improved environmental cleaning, and implementation of a recommended CDI treatment plan. Quan et alReference Quan, Yim and Merrill20 demonstrated that a computerized physician order entry (CPOE) alert could improve testing conditions, including decreasing concomitant laxative use, while also lowering HO-CDI IR. White et alReference White, Hamilton, Pegues, Hanish and Umscheid21 utilized a similar clinical decision support tool and demonstrated decreased inappropriate testing and avoidance of testing among patients with concomitant laxative use. Our approach was similar to the aforementioned studies in that we optimized education targeting appropriate C. difficile NAAT testing and implemented EHR reinforcement; however, we added preauthorization to this milieu to address inappropriate testing, all of which led to reductions in HO-CDI-IR and SIR.
Our study has several limitations. First, our analysis was a retrospective, single-center epidemiological analysis subject to inherent limitations. Patients may have been misclassified as asymptomatic carriers who had active CDI. However, individual cases were prospectively assessed in consultation with the primary treatment teams as a standard of care. Providers were encouraged to re-send NAAT if patients continued to report diarrhea after confounding factors were addressed (eg, discontinuation of laxatives). Second, while our present analysis does not address patient-level outcomes, we evaluated the need for repeat CDI testing and treatment in a 30-day follow-up of patients in a pilot study.Reference Christensen, Gibson, Martin and Rhodes22 We only identified a single case (2% of the intervention group) in which persistent diarrhea prompted repeat C. difficile testing which was positive and required treatment. Third, certain aspects of the intervention were somewhat unique to our center (eg, test batching), limiting generalizability. However, our daily clinical review of NAAT orders demonstrated efficiency that may be similar to the experience at other centers. Daily work by a single ASP team member, with or without pharmacy trainees, required only 1–2 hours per day and was sustainable throughout the intervention period. Fourth, concurrent provider education and stewardship interventions existed during the study period; however, this is reflective of real-world practice and increases generalizability. Fifth, while we were not powered to compare weekday versus weekend NAAT orders, we observed similar proportions of positive tests classified as HO-CDI, irrespective of stewardship coverage. Although this suggests that the intervention maintained effectiveness during off-shifts, further improvements could potentially be realized if 7-day-a-week coverage were instituted. Lastly, we are unable to discern the impact attributed to the ASP preauthorization from the clinical decision support alert given the short time period between implementation. Our findings demonstrate that ASP preauthorization coupled with individual provider education can meaningfully reduce clinical false-positive NAAT results while also decreasing antibiotic overuse.
In summary, our multifaceted, multidisciplinary, antimicrobial stewardship-led approach to decreasing clinical false-positive C. difficile NAAT led to reductions in HO-CDI-IR. Our findings support the guideline-recommended strategy of linking established institutional testing criteria with C. difficile NAAT testing. Interventions similar to ours may be useful for ASPs seeking to reduce inappropriate CDI testing and treatment which may lead to favorable reductions in the SIR.
Financial support
No financial support for the present study was received. The project was completed as part of our normal work.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.








