Nursing homes (NHs) have been identified as a target for healthcare-associated infection (HAI) surveillance and prevention efforts as part of the U.S. Department of Health and Human Services (HHS) National Action Plan to prevent HAIs. 1 On a given day, more than 1.4 million people are receiving care in the 15,643 NHs in the United States. 2 Crude estimates suggest that 1.8–13.5 million infections per 1,000 resident-care days occur among U.S. NH residents each year.Reference Strausbaugh and Joseph 3 However, these estimates are extrapolated from small studies conducted decades ago, which utilized diverse methods and a variety of infection definitions. Establishing current estimates of burden and types of HAIs that occur among residents of NHs is critical to developing and assessing future prevention targets.
Point-prevalence surveys are an effective way to measure the magnitude and types of HAI and antimicrobial usage in healthcare settings. In 2011, the Centers for Disease Control and Prevention (CDC) Emerging Infections Program (EIP) conducted a point-prevalence survey among 183 U.S. acute-care hospitals and determined that 4% of patients had HAIs and nearly 50% of all patients received antimicrobial drugs.Reference Magill, Edwards and Bamberg 4 , Reference Magill, Edwards and Beldavs 5 Because point prevalence surveys are feasible even when resources and infrastructure for sustained surveillance are inadequate or unavailable, this approach is well suited to assess the scope of the problem in the NH setting. To illustrate, the U.S. Department of Veteran’s Affairs conducted 3 single-day point prevalence surveys to assess HAI prevalence among approximately 130 NHs between 2005 and 2009, with HAI prevalence ranging between 4.2% and 5.3%.Reference Tsan, Davis and Langberg 6 , Reference Tsan, Langberg and Davis 7 , Reference Danko, Roselle and Tsan 8 In addition, the European Centre for Disease Prevention and Control (ECDC) supported the Healthcare-Associated Infections in European Long-Term Care Facilities Project (HALT) project, a multicountry point-prevalence survey initially conducted in 2010 among 722 facilities across 25 countries to assess HAI prevalence. 9 A larger follow-up Healthcare-Associated Infections and Antimicrobial Use in European Long-Term Care Facilities Project (HALT-2) survey was conducted in 2013 among 1,181 long-term care facilities across 28 countries. 9 HAI prevalences in these 2 HALT surveys were 2.4% and 3.4%, respectively.
We conducted a pilot point-prevalence survey among community-based NHs using data collection instruments and infection surveillance definitionsReference Stone, Ashraf and Calder 10 specifically designed for use in the NH setting. Our objectives were to assess the feasibility of conducting a prevalence survey using NH personnel and to compare the quality of resident-level data collected by NH personnel and record review by external staff. The experience gained from this survey will inform planning for future prevalence surveys in NHs, including data collection methods, types and content of data collection instruments, surveillance instructions, and staff training.
METHODS
Survey Participants
The survey was conducted within the CDC’s EIP network of state health departments and collaborators, which provides a national infrastructure for surveillance, prevention, and control of emerging infectious diseases.Reference Magill, Dumyati, Ray and Fridkin 11 Each participating EIP Site (Connecticut, Minnesota, New Mexico, and New York) identified a convenience sample of 2 or 3 NHs using the following eligibility criteria: certification by the Centers for Medicare & Medicaid Services (CMS), at least 120 beds, and voluntary participation in the pilot survey. Any resident present in the NH on the day of and the day before the prevalence survey date was eligible for inclusion. At each of the participating NHs, the prevalence survey was conducted from December 1, 2013, through May 30, 2014.
Data Collection
Two separate teams were established to collect data on eligible residents at each participating NH: (1) the NH team and (2) the EIP team. The NH team was headed by a team leader, who was required to be a registered nurse or licensed practical nurse and preferably involved in the facility’s infection prevention program. The NH team leader was primarily responsible for prevalence survey activities and data collection by the NH team. Other members of the NH team were identified by the NH team leader and were required to have permission to access and review resident care records but did not necessarily have specific infection surveillance experience. The EIP team included epidemiologists or surveillance officers whose usual activities involved medical record review and data analysis for other EIP surveillance projects (eg, the CDC’s acute care prevalence survey).Reference Magill, Edwards and Bamberg 4 , Reference Magill, Edwards and Beldavs 5 Prior to survey data collection, each NH team received 1.5 hours of webinar training conducted by CDC project staff outlining the goals of the project, data collection roles and responsibilities, and variable definitions to guide data collection using standardized forms, with the form used by the NH team containing only a subset of the variables collected by the EIP team. The EIP teams received similar webinar training plus instruction on the collection of additional data used to apply infection definitions.Reference Stone, Ashraf and Calder 10 A summary of the resident-level data elements for each team is provided in Table 1.
The NH team collected information on the survey date for each resident eligible for participation and was encouraged to collect resident data during routine resident care activities. NH personnel were permitted to use direct observation of residents, information obtained from resident caregivers, and information in facility documents (eg, resident medical records, infection logs, and medication administration records). The EIP team was instructed not to perform direct observation of residents; they collected data exclusively from facility documents (eg, resident medical records, infection logs, and/or medication administration records). EIP data collection was performed within 90 days of the prevalence survey date, which was the date as the NH team review.
HAI Screening Criteria
We created 3 HAI screening criteria based on findings from prior prevalence surveysReference Magill, Edwards and Bamberg 4 , Reference Magill, Edwards and Beldavs 5 , 9 and expert opinion to identify the subset of residents that would undergo a detailed chart review by the EIP team to collect comprehensive data regarding infection signs, symptoms, and related test results: (1) receipt of any systemic antimicrobial (for either prophylaxis or treatment), (2) signs or symptoms of an infection (eg, fever, new or worsening cough or diarrhea), or (3) a change in clinical status that required notification of a clinical provider. Data regarding the presence of any of the 3 HAI screening criteria were collected by both the NH team and EIP team. The presence of an HAI screening criterion was recorded as ‘yes’ (ie, any of the HAI screening criteria was present) or ‘no’ (ie, no HAI screening criteria was present); a ‘yes’ determination required ≥1 HAI screening criterion to be present on the day of the prevalence survey or the day prior to the survey date. For residents with ≥1 HAI screening criterion documented by either team, the EIP team subsequently conducted a detailed medical chart review using a structured chart review form (Online Supplementary Appendix I) to enable assessment for the presence of an HAI using the 2012 CDC/Society for Healthcare Epidemiology of America (SHEA) definitions of infection in long-term care facilities (revised McGeer criteria) which includes constitutional criteria and definitions for respiratory, urinary, gastrointestinal, skin, soft-tissue, mucosal and bloodstream infections.Reference Stone, Ashraf and Calder 10 Residents without any criteria documented as present by either team did not undergo a full chart review and were deemed not to have an HAI.
Data Analysis
EIP staff entered data into a customized database (Microsoft Access 2013, Redmond, WA) and transmitted data securely to CDC project staff for cleaning, application of the revised McGeer criteria, and analysis. The descriptive epidemiology of resident characteristics was assessed, and the prevalence of clinical risk factors and HAI screening criteria as determined by the NH team and the EIP team were compared using 2 measures: (1) the level of concordance (Cohen’s κ coefficient) between the NH team and EIP team regarding the presence or absence of a specific factor, and (2) positive agreement among the proportion of residents with the factor recorded as present by both teams. For the purposes of this evaluation, the EIP data collection based on review of facility documents and medical records was considered the referent standard. A κ coefficient of <0.41 was considered poor, 0.41–0.60 was considered moderate, and >0.60 was considered good.
The HAI prevalence rate per 100 residents was calculated by dividing the number of residents with ≥1 HAI by the total number of eligible residents and multiplying by 100. To evaluate the impact of using the NH team to collect the HAI screening criteria, we compared HAI prevalence between a subset of residents determined to have ≥1 HAI screening criterion by the NH team and a subset of residents identified by the EIP team. Data were analyzed using SAS software, version 9.3 (Cary, North Carolina).
Human Subjects Review
A protocol for this surveillance evaluation project was reviewed by the Office of the Director in the National Center for Emerging and Zoonotic Infectious Diseases at the CDC and was determined not to constitute human subjects research.
RESULTS
Participants
Among the 9 participating NHs (median beds 130, range 104–229), there were 1,272 eligible residents. The median age was 85 years (range, 21–91 years), and 30% were male. Most (86%) were considered long-stay residents (expected length of stay >100 days); 29% had diabetes; and 56% required the use of a wheelchair or were unable to get out of bed without assistance.
Clinical Risk Factors
Each team detected a similar proportion of NH residents with clinical risk factors, with the exception of vascular devices and pressure ulcers (Table 2). The κ coefficient measuring overall concordance for risk factors ranged from 0.50 to 0.93. The degree of positive agreement (residents identified with factor present by both teams) ranged between 39% and 87% for all clinical risk factors (Table 2).
HAI Screening Criteria and HAI Prevalence
The EIP team detected more residents with any of the HAI screening criteria (7%–20%) than the NH team (5%–12%) (Table 3). In particular, the EIP team identified nearly twice as many residents receiving antimicrobial drugs (11%) than did the NH team (5%). The EIP team identified 253 of the 1,272 residents (20%) as having ≥1 HAI screening criterion present, compared to just 152 of 1,272 residents (12%) by the NH team.
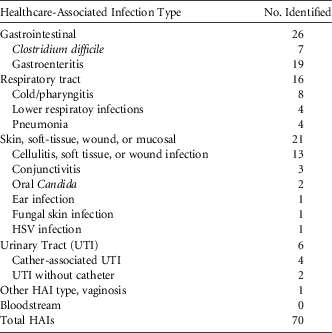
Among resident charts the EIP team identified to have ≥1 HAI screening criteria, 67 residents had at least 1 HAI (Table 3) as defined by revised McGeer criteria,Reference Stone, Ashraf and Calder 10 for an HAI prevalence of 5.3 per 100 residents. Among the 70 HAIs identified (3 residents had 2 HAIs), the most common infections were gastrointestinal tract (n = 26; 37%), skin, soft-tissue, or mucosal (n = 21; 30%) infection, and respiratory tract infection (n = 16; 23%) (Table 4). From resident charts the NH team identified with ≥1 HAI screening criteria, 45 residents were identified that had at least 1 HAI (Table 3), yielding a prevalence of 3.5 per 100 residents; this prevalence was significantly lower than that from EIP data collection (P > .05).
DISCUSSION
Currently, data regarding the prevalence of HAIs among U.S. NH residents are limited. To better understand the practicality of conducting a prevalence survey among NHs, we implemented a dual data-collection method to assess the feasibility and quality of surveillance data collection for measuring the prevalence of HAIs using revised McGeer criteria in a population of nearly 1,300 NH residents from 4 different geographic areas. Overall concordance among the data collected by the NH and EIP teams was moderate for vascular devices, pressure ulcers, and other wounds and was good for all other risk factors (urinary catheters, percutaneous endoscopic gastrostomy /jejunostomy tube). However, our findings illustrate significant differences in the estimates of HAI prevalence generated using data collected by the EIP team compared to data collected by the NH team; this difference is likely related to methods of data collection. Specifically, we found that the proportion of NH residents determined to have ≥1 HAI screening criterion was lower when using data collected by the NH teams (ie, 12%) than when data collected by the EIP teams was used (ie, 20%). These findings have important implications for the design and implementation of future HAI prevalence surveys among NHs. For example, future surveys will restrict data collection of the HAI screening criteria and antimicrobials to only the NH team leader, and all antimicrobial use will be reviewed by the EIP team. Further changes have also been made to simplify and clarify the elements of NH resident data collection.
Some discrepancies between NH and EIP team estimates are likely attributable to differences in the methods of data collection, which were intentionally permitted. We initially hypothesized that estimates obtained from data collected exclusively from medical records would result in lower HAI prevalence due to inadequate or limited documentation of information required to identify infections and satisfy surveillance criteria.Reference Schnelle, Bates-Jensen, Chu and Simmons 12 , Reference Laurin, Voyer, Verreault and Durand 13 In addition, we anticipated that data collected on the presence of certain HAI risk factors (eg, ventilator, indwelling urinary catheter, or PEG/J tube use) might be more complete via direct observation in the NH setting as opposed to exclusive medical record review. However, despite access to a variety of data sources including direct observations and verbal reports from caretakers, the NH team identified 8% fewer residents with any HAI screening criteria than the EIP team, who relied exclusively on facility documentation and medical record review. The identification of antimicrobial use as an HAI screening criterion revealed the largest discrepancy between the NH team and EIP team data collection; the EIP team detected twice as many patients receiving antimicrobial drugs as the NH team. While some factors might be more amenable to direct observation, others, such as antimicrobial usage, require verification through documentation like the medication administration record. Furthermore, multiple data sources, including some data that may not have been available in ‘real time’ on the survey date during NH team review, may have further complicated data collection for the NH teams. In addition, the level of familiarity and experience among the EIP surveillance officers regarding infection surveillance data collection using standardized definitions may also have contributed to discrepancies. To illustrate, while the EIP team identified all residents for whom a provider was notified of a change in clinical status for any reason, NH personnel may not have identified these same residents if the perceived reason for notification among NH personnel was not directly related to an infection. Additionally, while the EIP team identified all NH residents receiving antimicrobials regardless of indication, the NH team may have only identified patients receiving certain antimicrobials indicated for treatment only.
Use of surveillance criteria for systematic identification of HAIs is novel for NH personnel, therefore, it is possible that NH personnel may have misunderstood the surveillance instructions provided or applied their own interpretations of the data elements using clinical judgement. To illustrate, CDC instructed both teams to collect data regarding all specified antimicrobials, regardless of indication (ie, prophylaxis vs treatment). However, a large proportion of NH residents receiving antimicrobials indicated for prophylaxis by the EIP team were absent from the NH team data collection. Additionally, the NH team members may have been reluctant to report the presence of signs or symptoms of a possible infection to avoid scrutiny from surveyors or if the NH resident did not also have a specific infection diagnosis by a clinical provider. Finally, it is possible that some members of the NH team did not participate in the CDC webinar training prior to the survey date. We have conducted a thorough evaluation of data collection tools and the training materials for the NH team members to improve data collection for future NH prevalence surveys.
We are aware of several limitations of our study. First, we did not formally assess which method(s) (eg, chart review, direct observation, etc.) the NH team used to collect specific types of information. This knowledge could improve our understanding of the differences we observed in the data collected by the 2 teams. Second, we used the EIP team as the referent standard, but we cannot be certain that the information they collected is absolutely accurate. Accurate and complete documentation is necessary for the delivery of safe, quality resident care. Therefore, in the absence of an official gold standard, we believe that the use of facility documents and medical charts as the referent standard is appropriate. Furthermore, most of the NHs did not have a centralized electronic medical record system, making the record review process less efficient, and differences in the use of electronic or paper medical records could have impacted the quality of data collection. Finally, we used a convenience sample of NHs and these results may not be generalizable.
The experience gained in this pilot survey will contribute to refinements in the methods, data collection instruments, instructions, roles and responsibilities, as well as training to improve the quality of data collected in future NH prevalence surveys and other surveillance activities conducted by the CDC. Point-prevalence surveys are a practical approach to obtaining HAI surveillance data, and they can generate important information using public health data to describe the burden and spectrum of HAIs and antimicrobial usage.Reference Magill, Edwards and Bamberg 4 , Reference Magill, Edwards and Beldavs 5 , Reference Humphreys and Smyth 14 However, we identified several modifications that are necessary to improve the quality of data collection in NHs. Improvements in methods in response to the lessons learned from this pilot survey should help key stakeholders to acquire the data needed to inform development of NH surveillance, infection prevention programs, and antibiotic stewardship activities.
TABLE 1 Comparison of Data Collection by the Nursing Home (NH) Team and Emerging Infection Program (EIP) Team

a Data collected on the date of the survey. Documented information from the medical chart review and/or direct observations of residents or caregivers
b Data collected by retrospect review of resident medical chart or other nursing home records
c Only performed if a HAI screening criterion was noted to be present by either the EIP team or NH team.
TABLE 2 Prevalence of Clinical Risk Factors and Comparison of Nursing Home (NH) Team and Emerging Infection Program (EIP) Team (N = 1,272)

a Collected information on the actual date of the survey only; documented information from the medical chart review and/or direct observations of residents or caregivers.
b Collected resident information retrospectively from medical chart review only.
c The κ coefficient showing overall agreement between the NH team and EIP team.
d Determined by calculating the percent of NH residents that were positively identified by both teams/total number of residents identified by either team as having the specified variable.
TABLE 3 Prevalence of Healthcare-Associated Infections (HAIs) by Screening Criterion (N=1,272)

a Collected information on the actual date of the survey only; documented information from the medical chart review and/or direct observations of residents or caregivers.
b Percentage is no. of nursing home residents specified indicator of infection/total number of nursing home residents; no. is number of nursing home residents with the specified indicator of infection and confirmed HAI.
c Collected resident information retrospectively from medical chart review only.
TABLE 4 Description of Healthcare-Associated Infection Types (N = 70)
ACKNOWLEDGMENTS
The authors would like to acknowledge Barbara Mooney, BSMB, BSMT (ASCP), CIC of Infection Control Consultants of NM, LLC for her assistance with facility recruitment and expertise.
Financial support. The survey was supported through a cooperative agreement with the Emerging Infections Program.
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/ice.2016.200






